Abstract
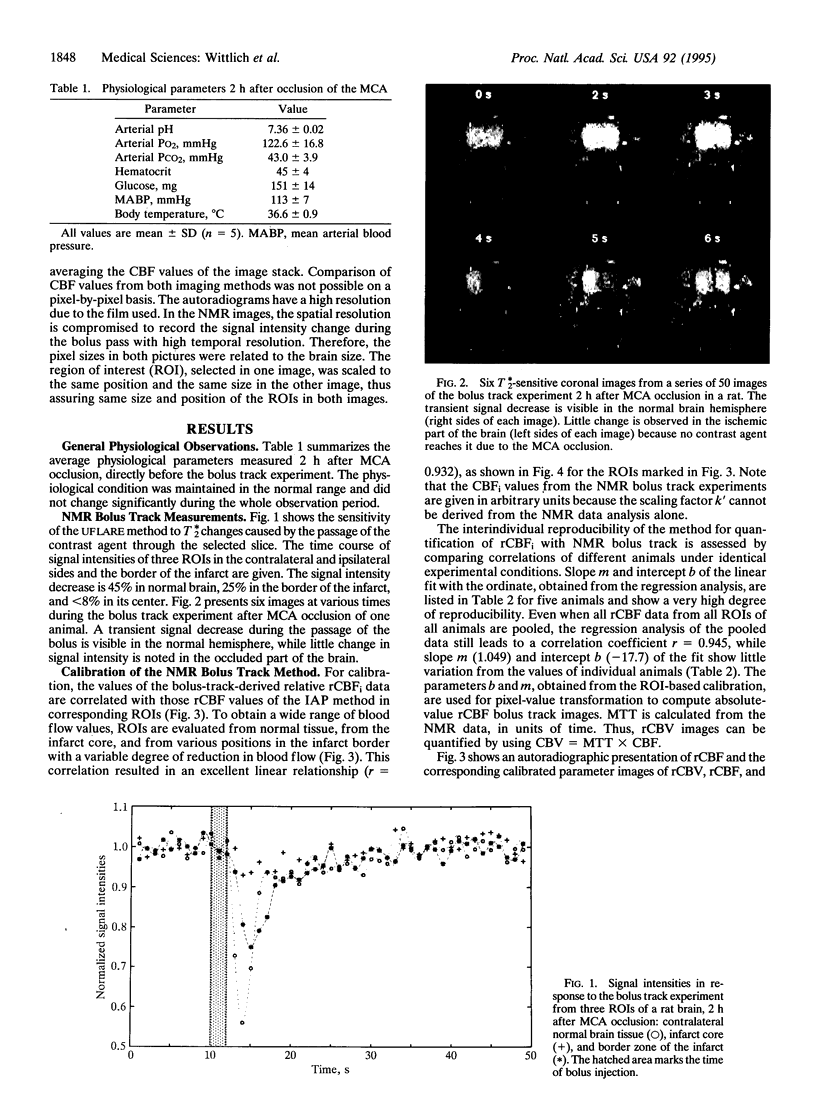
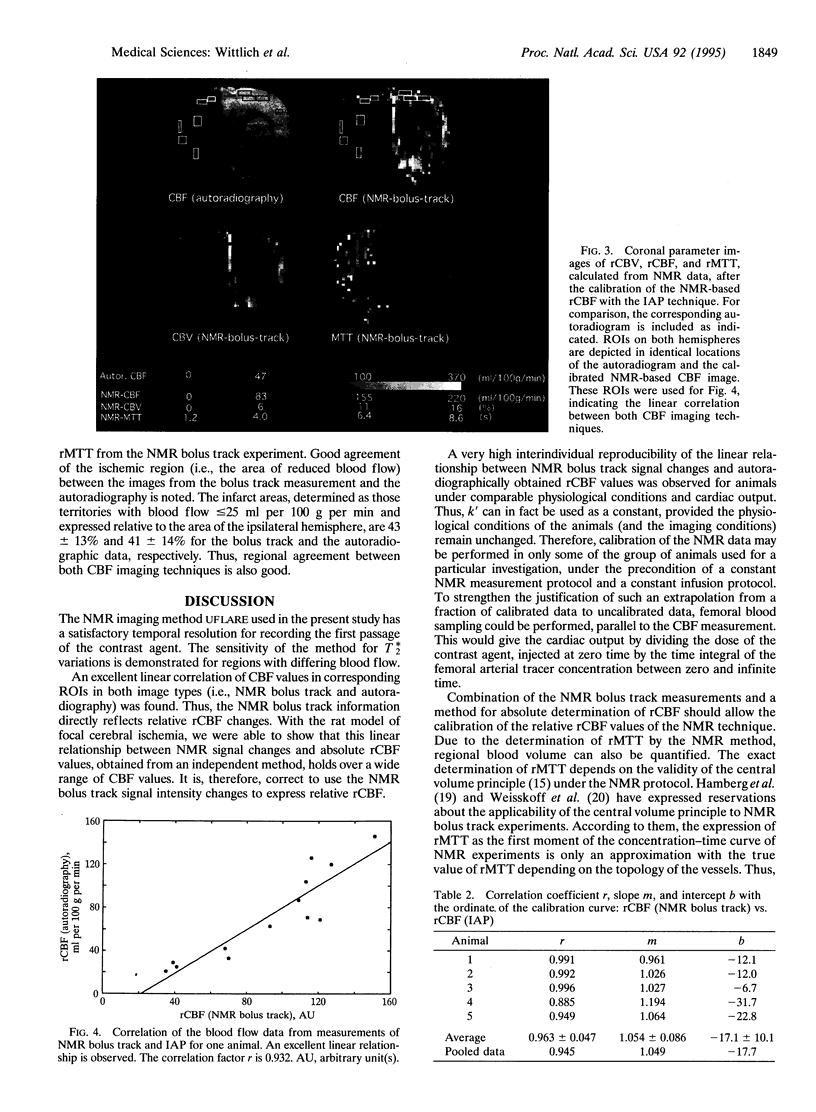
NMR bolus track measurements were correlated with autoradiographically determined regional cerebral blood flow (rCBF). The NMR method is based on bolus infusion of the contrast agent gadolinium diethylenetriaminepentaacetate and high-speed T*2-sensitive NMR imaging. The first pass of the contrast agent through the image plane causes a transient decrease of the signal intensity. This time course of the signal intensity is transformed into relative concentrations of the contrast agent in each pixel. The mean transit time and relative blood flow and volume are calculated from such indicator dilution curves. We investigated whether this NMR technique correctly expresses the relative rCBF. The relative blood flow data, calculated from NMR bolus track experiments, and the absolute values of iodo[14C]antipyrine autoradiography were compared. A linear relationship was observed, indicating the proportionality of the transient NMR signal change with CBF. Excellent interindividual reproducibility of calibration constants is observed (r = 0.963). For a given NMR protocol, bolus track measurements calibrated with autoradiography after the experiment allow determination of absolute values for rCBF and regional blood volume.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrup J., Symon L., Branston N. M., Lassen N. A. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke. 1977 Jan-Feb;8(1):51–57. doi: 10.1161/01.str.8.1.51. [DOI] [PubMed] [Google Scholar]
- Belliveau J. W., Rosen B. R., Kantor H. L., Rzedzian R. R., Kennedy D. N., McKinstry R. C., Vevea J. M., Cohen M. S., Pykett I. L., Brady T. J. Functional cerebral imaging by susceptibility-contrast NMR. Magn Reson Med. 1990 Jun;14(3):538–546. doi: 10.1002/mrm.1910140311. [DOI] [PubMed] [Google Scholar]
- Böck J. C., Sander B., Hierholzer J., Cordes M., Haustein J., Schörner W., Felix R. Regionale Gehirndurchblutung bei Infarktpatienten. Schnelle dynamische T2*-gewichtete MRT nach Gadolinium-DTPA-Bolusinjektion. Rofo. 1992 Apr;156(4):382–387. doi: 10.1055/s-2008-1032905. [DOI] [PubMed] [Google Scholar]
- Fisel C. R., Ackerman J. L., Buxton R. B., Garrido L., Belliveau J. W., Rosen B. R., Brady T. J. MR contrast due to microscopically heterogeneous magnetic susceptibility: numerical simulations and applications to cerebral physiology. Magn Reson Med. 1991 Feb;17(2):336–347. doi: 10.1002/mrm.1910170206. [DOI] [PubMed] [Google Scholar]
- Hamberg L. M., Macfarlane R., Tasdemiroglu E., Boccalini P., Hunter G. J., Belliveau J. W., Moskowitz M. A., Rosen B. R. Measurement of cerebrovascular changes in cats after transient ischemia using dynamic magnetic resonance imaging. Stroke. 1993 Mar;24(3):444–451. doi: 10.1161/01.str.24.3.444. [DOI] [PubMed] [Google Scholar]
- Kent T. A., Quast M. J., Kaplan B. J., Najafi A., Amparo E. G., Gevedon R. M., Salinas F., Suttle A. D., DiPette D. J., Eisenberg H. M. Cerebral blood volume in a rat model of ischemia by MR imaging at 4.7 T. AJNR Am J Neuroradiol. 1989 Mar-Apr;10(2):335–338. [PMC free article] [PubMed] [Google Scholar]
- Kety S. S. Regional cerebral blood flow: estimation by means of nonmetabolized diffusible tracers--an overview. Semin Nucl Med. 1985 Oct;15(4):324–328. doi: 10.1016/s0001-2998(85)80010-6. [DOI] [PubMed] [Google Scholar]
- Kohno K., Back T., Hoehn-Berlage M., Hossmann K. A. A modified rat model of middle cerebral artery thread occlusion under electrophysiological control for magnetic resonance investigations. Magn Reson Imaging. 1995;13(1):65–71. doi: 10.1016/0730-725x(94)00081-d. [DOI] [PubMed] [Google Scholar]
- Kucharczyk J., Mintorovitch J., Asgari H., Tsuura M., Moseley M. In vivo diffusion-perfusion magnetic resonance imaging of acute cerebral ischemia. Can J Physiol Pharmacol. 1991 Nov;69(11):1719–1725. doi: 10.1139/y91-255. [DOI] [PubMed] [Google Scholar]
- MEIER P., ZIERLER K. L. On the theory of the indicator-dilution method for measurement of blood flow and volume. J Appl Physiol. 1954 Jun;6(12):731–744. doi: 10.1152/jappl.1954.6.12.731. [DOI] [PubMed] [Google Scholar]
- Mies G., Ishimaru S., Xie Y., Seo K., Hossmann K. A. Ischemic thresholds of cerebral protein synthesis and energy state following middle cerebral artery occlusion in rat. J Cereb Blood Flow Metab. 1991 Sep;11(5):753–761. doi: 10.1038/jcbfm.1991.132. [DOI] [PubMed] [Google Scholar]
- Moseley M. E., Chew W. M., White D. L., Kucharczyk J., Litt L., Derugin N., Dupon J., Brasch R. C., Norman D. Hypercarbia-induced changes in cerebral blood volume in the cat: a 1H MRI and intravascular contrast agent study. Magn Reson Med. 1992 Jan;23(1):21–30. doi: 10.1002/mrm.1910230104. [DOI] [PubMed] [Google Scholar]
- Moseley M. E., Vexler Z., Asgari H. S., Mintorovitch J., Derugin N., Rocklage S., Kucharczyk J. Comparison of Gd- and Dy-chelates for T2 contrast-enhanced imaging. Magn Reson Med. 1991 Dec;22(2):259–267. doi: 10.1002/mrm.1910220220. [DOI] [PubMed] [Google Scholar]
- Norris D. G., Hoehn-Berlage M., Wittlich F., Back T., Leibfritz D. Dynamic imaging with T2* contrast using U-FLARE. Magn Reson Imaging. 1993;11(7):921–924. doi: 10.1016/0730-725x(93)90210-5. [DOI] [PubMed] [Google Scholar]
- Reivich M., Jehle J., Sokoloff L., Kety S. S. Measurement of regional cerebral blood flow with antipyrine-14C in awake cats. J Appl Physiol. 1969 Aug;27(2):296–300. doi: 10.1152/jappl.1969.27.2.296. [DOI] [PubMed] [Google Scholar]
- Rudin M., Sauter A. Noninvasive determination of regional cerebral blood flow in rats using dynamic imaging with Gd(DTPA) Magn Reson Med. 1991 Nov;22(1):32–46. doi: 10.1002/mrm.1910220105. [DOI] [PubMed] [Google Scholar]
- Sakurada O., Kennedy C., Jehle J., Brown J. D., Carbin G. L., Sokoloff L. Measurement of local cerebral blood flow with iodo [14C] antipyrine. Am J Physiol. 1978 Jan;234(1):H59–H66. doi: 10.1152/ajpheart.1978.234.1.H59. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer C., Syha J., Haase A. Quantification of regional blood volumes by rapid T1 mapping. Magn Reson Med. 1993 May;29(5):709–712. doi: 10.1002/mrm.1910290521. [DOI] [PubMed] [Google Scholar]
- Shockley R. P., LaManna J. C. Determination of rat cerebral cortical blood volume changes by capillary mean transit time analysis during hypoxia, hypercapnia and hyperventilation. Brain Res. 1988 Jun 28;454(1-2):170–178. doi: 10.1016/0006-8993(88)90816-5. [DOI] [PubMed] [Google Scholar]
- THOMPSON H. K., Jr, STARMER C. F., WHALEN R. E., MCINTOSH H. D. INDICATOR TRANSIT TIME CONSIDERED AS A GAMMA VARIATE. Circ Res. 1964 Jun;14:502–515. doi: 10.1161/01.res.14.6.502. [DOI] [PubMed] [Google Scholar]
- Villringer A., Rosen B. R., Belliveau J. W., Ackerman J. L., Lauffer R. B., Buxton R. B., Chao Y. S., Wedeen V. J., Brady T. J. Dynamic imaging with lanthanide chelates in normal brain: contrast due to magnetic susceptibility effects. Magn Reson Med. 1988 Feb;6(2):164–174. doi: 10.1002/mrm.1910060205. [DOI] [PubMed] [Google Scholar]
- Weiss H. R., Buchweitz E., Murtha T. J., Auletta M. Quantitative regional determination of morphometric indices of the total and perfused capillary network in the rat brain. Circ Res. 1982 Oct;51(4):494–503. doi: 10.1161/01.res.51.4.494. [DOI] [PubMed] [Google Scholar]
- Weisskoff R. M., Chesler D., Boxerman J. L., Rosen B. R. Pitfalls in MR measurement of tissue blood flow with intravascular tracers: which mean transit time? Magn Reson Med. 1993 Apr;29(4):553–558. doi: 10.1002/mrm.1910290420. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L. EQUATIONS FOR MEASURING BLOOD FLOW BY EXTERNAL MONITORING OF RADIOISOTOPES. Circ Res. 1965 Apr;16:309–321. doi: 10.1161/01.res.16.4.309. [DOI] [PubMed] [Google Scholar]