Abstract
OBJECTIVE
To assess associations between dietary intake and rates of change in insulin resistance and β-cell function in Hispanic women with prior gestational diabetes mellitus (GDM).
RESEARCH DESIGN AND METHODS
Sixty-two nondiabetic Hispanic women with pregnancies complicated by GDM completed oral and intravenous glucose tolerance tests and bioelectrical impedance measurements of body fat every 12–15 months postpartum for up to 12 years. Self-reported dietary intake was collected at all visits by structured food frequency questionnaires developed for Hispanics. Mixed-effects models were used to assess the relationship between dietary intake and rates of change in metabolic outcomes during follow-up.
RESULTS
The median length of follow-up from the first postpartum evaluation was 8.0 years (interquartile range 4.5–10.8 years). At baseline, women were 32 ± 5.7 years old and had a median calorie intake of 2,091 kcal/day. Over the course of follow-up, dietary intake did not change significantly. Higher baseline calorie intake was associated with a faster decline in insulin sensitivity, measured by the insulin sensitivity index (SI) (P = 0.029), and β-cell compensation, measured by the disposition index (DI) (P = 0.027), over time. These associations remained after adjustment for baseline characteristics; changes in BMI, calorie intake, levels of physical activity; and additional pregnancies during the follow-up period. The median rates were −0.06 vs. −0.02 units/year for SI and −810 vs. −692 units/year for DI for women with baseline calorie intake above versus below the cohort median.
CONCLUSIONS
High calorie intake is associated with a faster decline in insulin sensitivity and β-cell compensation in Hispanic women who are at high risk for type 2 diabetes, independent of adiposity.
Introduction
The development of type 2 diabetes is characterized by chronic insulin resistance and a progressive fall in β-cell compensation for insulin resistance (1–4). Previous studies (5–10) have demonstrated that calorie restriction reduces insulin resistance, improves glucose tolerance, and delays or prevents the onset of type 2 diabetes.
Severe, short-term calorie restriction has been shown to improve insulin sensitivity and enhance β-cell function (11,12). However, little is known about the relationship between spontaneous calorie intake and long-term changes in β-cell function under free-living conditions. In this report, we examine this relationship using data from a cohort of nondiabetic Hispanic women with recent gestational diabetes mellitus (GDM) who were prospectively observed postpartum for up to 12 years in the University of Southern California (USC) GDM Cohort Study.
Research Design and Methods
Study Participants
Selection of the original cohort has been described in detail previously (13,14). Briefly, pregnant Hispanic women referred to the Los Angeles County Women’s Hospital for management of GDM between August 1993 and March 1995 were asked to participate in the USC GDM Cohort Study if they met all of the following criteria: 1) gestational age between 28 and 34 weeks; 2) no current or prior insulin therapy; 3) all fasting serum glucose concentrations <130 mg/dL (7.2 mmol/L) during pregnancy; 4) otherwise uncomplicated singleton pregnancy; 5) negative for anti-islet cell antibodies; and 6) both parents and at least three of four grandparents born in Mexico, Guatemala, or El Salvador. All women underwent detailed metabolic testing during the third trimester (13). They were asked to return to undergo a 75-g oral glucose tolerance test (OGTT) 6 months postpartum, and then for more detailed metabolic testing at intervals of 12–15 months starting at 15 months postpartum. This metabolic testing included measurement of body morphometry and composition by bioelectrical impedance assessment (BIA) of diet and physical activity (PA) by questionnaire and performance of OGTTs and intravenous glucose tolerance tests (IVGTTs) as described below. Women who had abnormal fasting or postchallenge glucose levels at a follow-up visit were referred to a dietitian for advice on nutrition and daily walking; no other formal intervention was performed. Participants remained in follow-up until they withdrew consent, were lost to follow-up, reached the final scheduled study visit 12 years postpartum, or developed a fasting plasma glucose concentration of >140 mg/dL (>7.8 mmol/L), at which time they were referred for pharmacological treatment. Women who were pregnant at the time of a scheduled follow-up visit were studied at least 4 months after pregnancy and at least 1 month after the completion of breast-feeding. A family history of diabetes was not assessed for this cohort.
All participants gave written informed consent for participation in the study, which was approved by the Institutional Review Board of the USC and the Los Angeles County+USC Medical Center.
For the present report, we analyzed data from the 62 members of the cohort who had baseline OGTT and IVGTT results without an indication of diabetes and returned to undergo at least one additional OGTT and IVGTT.
Testing Procedures and Assays
For each follow-up assessment, women came to the Clinical Research Center on two separate days, at least 48 h apart, after 8–12 h of overnight fasting and at least 3 days on an unrestricted diet. On day 1, a BIA and OGTT were performed (15). On day 2, a frequently sampled IVGTT was performed (15). Glucose was measured by glucose oxidase (Beckman Glucose Analyzer II; Beckman Coulter, Brea, CA). Insulin was measured by a radioimmunoassay (Novo Pharmaceuticals, Danbury, CT) that measured insulin and proinsulin with intraobserver and interobserver coefficients of variation of <2.3% and 4.4%.
Dietary and PA Assessment
Trained bilingual interviewers administered both dietary and PA questionnaires. Dietary intake was assessed by the structured food frequency questionnaires developed for Hispanics by the Hawaii-Los Angeles Multiethnic Cohort Study (MEC) (16,17). The questionnaire consists of a list of food items including Hispanic-specific foods such as tamales and tortillas. Three choices of portion sizes, eight frequency categories for each food item, and nine frequency categories for each beverage item were used to record food intake during the past year. Total calorie and nutrient intake were computed from the percent contribution of the individual food items by the MEC team. The questionnaire was validated and calibrated in multiethnic populations by comparing the diet reported from a single questionnaire with three structured telephone interviews eliciting all food intake in the last 24 h (17).
The amount and intensity of PA were assessed by the questionnaires developed by the MEC (16,18). The questionnaire was composed of a list of structured questions describing various types of activity (sitting, strenuous sports, vigorous work, and moderate activities, including sports and work) during the past year. Responses were then used to estimate the total number of minutes of moderate and vigorous activity per week. The total number of minutes for all activities was calculated as the number of minutes of moderate plus vigorous activities. The questionnaire was validated against doubly labeled water by the Energetics Study (19). We previously showed that PA assessed by these questionnaires was associated with β-cell function in a separate cohort of Mexican American adults (20).
Data Analysis
Insulin response to oral glucose was assessed using the 30-min change in insulin level computed as the 30-min insulin concentration minus the fasting insulin concentration. Insulin sensitivity index (SI) and the acute insulin response to glucose (AIRg) from the first 10 min of an IVGTT were calculated from the IVGTT glucose and insulin concentrations using the MIMMOD program (21). Disposition index (DI), a measure of β-cell compensation for insulin resistance, was computed as the product of AIRg and SI. The percent of body fat and fat mass were calculated using the formula of Kotler et al. (22) using height, weight, and BIA measurements. Nutrient densities were calculated as the percentage of calorie intake attributable to the main macronutrients based on the calories in each nutrient (23). PA levels were compared with the U.S. Department of Health and Human Services recommendation of at least 75 min/week vigorous activity or 150 min/week moderate activity for adults 18–64 years of age (24).
Fasting and postchallenge glucose and insulin concentrations, incremental insulin responses, SI, DI, and total number of calories were log transformed to approximate normal distribution prior to analysis. Rates of change were estimated by regressing follow-up data of each subject against the follow-up time for that subject. Mixed-effects models with random intercepts and random slopes were used to test for significant changes over time for SI, DI, body composition, dietary intake, PA levels, and other metabolic traits from results of the OGTTs and IVGTTs. An additional quadratic time effect was tested for evidence against the linear trend. If no significant quadratic time effect was observed, data analyses were conducted using linear rates of change over time. Mixed-effects models were used to assess whether baseline calorie intake significantly predicted the rates of change in SI and DI for primary outcomes, and the rates of change in anthropometric and other metabolic traits from the results of the OGTTs and IVGTTs for secondary outcomes. Results are presented as unadjusted; adjusted for baseline age, BMI, levels of PA, parity, and baseline value of the trait (adjusted-1); and further adjusted for the covariates in adjusted-1, and for changes in BMI, total calorie intake, levels of PA, and additional pregnancies during follow-up as time-dependent variables (adjusted-2). Since calories accounted for by different nutrients have been shown to differentially affect body composition and glucose metabolism (25,26), we performed additional analyses exploring associations between dietary macronutrient densities (fats, carbohydrates, and proteins) and rates of change in metabolic traits. All statistical tests were two-sided. SAS version 9.2 (SAS Institute Inc., Cary, NC) was used for data analysis.
Results
At baseline (Table 1), the median age was 32 years and the median BMI was 30.3 kg/m2; 36 participants (58%) had impaired glucose tolerance. The median calorie intake was 2,091 kcal/day, 33% from fat, 53% from carbohydrates, and 14% from protein. The fraction of calories derived from fat (33%) and saturated fat (11%) were slightly above the upper limits of 30% and 10%, respectively, recommended by the World Health Organization (WHO) for preventing diet-related chronic diseases (27). The fraction of calories derived from carbohydrates was below the lower limit of 55% recommended by the WHO. Very few subjects were physically active; the median duration of total activity was 0 min/week. Only six (<10%) of the women met the WHO recommendation of >75 min/week vigorous activity for adults (24), and no women met the recommendation of >150 min/week moderate activity (24). Correlations between baseline total calorie intake and baseline anthropometric and metabolic outcomes were weak and were not statistically significant.
Table 1.
Baseline characteristics and rates of change during a median of 8.0 years of follow-up for the 62 women with GDM
| Baseline | Annualized rates of change† | |
|---|---|---|
| Anthropometrics | ||
| Age (years) | 32 (28, 36) | |
| BMI (kg/m2) | 30.3 (28.0, 32.6) | 0.30 (0.06, 0.59)‡ |
| Body fat (%) | 44.3 (40.6, 48.4) | 0.36 (−0.06, 0.66)‡ |
| Weight (kg) | 70.0 (64.1, 78.9) | 0.82 (0.20, 1.38)‡ |
| Fat mass (kg) | 31.9 (26.8, 37.0) | 0.59 (0.04, 1.08)‡ |
| Waist-to-hip ratio × 100 | 0.83 (0.79, 0.85) | 0.006 (0.0004, 0.011)‡ |
| OGTT-derived measures | ||
| Fasting glucose (mmol/L)*,§ | 5.3 (5.0, 5.7) | 0.02 (0.003, 0.04)‡ |
| 2-h glucose (mmol/L)*,§ | 8.0 (6.8, 9.5) | 0.03 (0.01, 0.09)‡ |
| Fasting insulin (pmol/L)* | 115 (87, 167) | 0.06 (−0.03, 0.09)‡ |
| 30-min ∆ insulin (pmol/L)* | 639 (483, 906) | −0.04 (−0.11, 0.02) |
| 2-h insulin (pmol/L)* | 695 (514, 1,285) | 0.002 (−0.117, 0.063) |
| IVGTT-derived measures | ||
| SI (×10−3 min−1/pmol/L)* | 1.4 (1.0, 2.2) | −0.03 (−0.10, 0.04)§ |
| AIRg (pmol/L × 10 min)* | 3,167 (1,854, 5,372) | −0.05 (−0.11, −0.01)‡ |
| DI (SI × AIRg)* | 5,172 (2,374, 8,214) | −0.04 (−0.09, −0.01)‡ |
| Dietary intake | ||
| Calorie intake (kcal/day)* | 2,091 (1,526, 3,013) | −0.02 (−0.07, 0.03) |
| Total fat (%)* | 32.8 (28.9, 37.6) | 0.04 (−0.64, 0.70) |
| Saturated fatty acids (%)* | 10.9 (9.3, 12.7) | −0.01 (−0.37, 0.23) |
| Polyunsaturated fatty acids (%)* | 6.8 (6.0, 7.7) | −0.01 (−0.15, 0.12) |
| Monounsaturated fatty acids (%)* | 11.8 (10.4, 13.5) | 0.02 (−0.29, 0.26) |
| Total carbohydrate (%)* | 53.1 (46.3, 57.8) | −0.14 (−0.98, 0.74) |
| Protein (%)* | 14.0 (12.5, 17.0) | 0.01 (−0.02, 0.03) |
| PA | ||
| Vigorous activity (min/week) | 0 (0, 0) | 0.0 (−3.1, 13.9) |
| Moderate activity (min/week) | 0 (0, 44.9) | 3.5 (0, 28.2)‡ |
| Total activity (min/week) | 0 (0, 44.9) | 8.0 (0, 70.5)‡ |
| Meets WHO guidelines | 6 (10%) |
Data are median (25th, 75th percentiles), unless otherwise indicated.
*Log transformation was applied prior to calculating annualized rates of change.
†Calculated as (follow-up – baseline)/years of follow-up.
‡P < 0.001.
§P < 0.05.
The median duration of follow-up was 8.0 years (interquartile range 4.5–10.8 years) with a median of five sets of OGTTs, IVGTTs, and BIAs per participant over the follow-up period. During follow-up, 41 women in the cohort (66%) experienced a deterioration of glucose tolerance over time, and diabetes developed in 27 of the women (44%), as determined by American Diabetes Association criteria (28). Fourteen of the women (23%) experienced one or more additional pregnancies during follow-up, and 5 of them had GDM during these pregnancies.
No significant quadratic trends of change over follow-up time were observed for any metabolic traits (P > 0.14). Thus, only linear rates of change are presented (Table 1). Measures of adiposity, fasting glucose, 2-h glucose, fasting insulin, and moderate and total PA increased significantly over time (all P < 0.001), while SI, AIRg, and DI decreased significantly over time (P = 0.0003, P = 0.001, P < 0.0001, respectively). No significant change over time was found for the 30-min change in insulin or in 2-h insulin on the OGTT, dietary intake, macronutrient densities, or vigorous activity (all P > 0.10).
Table 2 presents associations between baseline total calorie intake and rates of change in metabolic traits over time. In the unadjusted analysis, higher total calorie intake at baseline was significantly associated with a faster decline in SI and DI (P = 0.029 and P = 0.027, respectively). Although statistically insignificant, higher total calorie intake at baseline was associated with a faster increase in adiposity, and fasting and 2-h glucose levels, and a faster decrease in 2-h insulin levels and AIRg. Adjustment for baseline age, BMI, parity, levels of PA, and baseline value of the trait had little impact on the results; higher baseline calorie intake remained significantly associated with a faster decline in SI (P = 0.035) and DI (P = 0.021). Although calorie intake did not change significantly over time among the entire cohort, an increase in calorie intake over time was positively associated with an increase in BMI, weight, and total body fat mass over time (P = 0.032, P = 0.024, and P = 0.025, respectively) after adjusting for baseline characteristics. Change in calorie intake was not significantly associated with rates of change in metabolic outcomes (all P > 0.11). Higher baseline calorie intake remained significantly associated with a faster decline in SI (P = 0.05) and DI (P = 0.034) after further adjusting for changes in calorie intake, BMI, levels of PA, and additional pregnancies during follow-up (adjusted-2). Further adjustment for GDM status in subsequent pregnancies did not impact the results. Sensitivity analysis excluding the six women who met the WHO recommended level of PA did not change the conclusion.
Table 2.
Associations between baseline calorie intake and rates of change in morphometric and metabolic traits during a median of 8.0 years of follow-up for the 62 women with GDM
| Unadjusted |
Adjusted-1 |
Adjusted-2 |
||||
|---|---|---|---|---|---|---|
| β (SEM) | P | β (SEM) | P | β (SEM) | P | |
| BMI (kg/m2/year) | 0.111 (0.125) | 0.37 | 0.069 (0.110) | 0.53 | NA | NA |
| Body fat (%/year) | 0.100 (0.147) | 0.50 | 0.083 (0.128) | 0.52 | NA | NA |
| Weight (kg/year) | 0.268 (0.313) | 0.39 | 0.152 (0.264) | 0.57 | NA | NA |
| Fat mass (kg/year) | 0.253 (0.249) | 0.31 | 0.147 (0.214) | 0.49 | NA | NA |
| Waist-to-hip ratio × 100 (units/year) | 0.159 (0.197) | 0.42 | 0.140 (0.200) | 0.48 | NA | NA |
| OGTT-derived measures | ||||||
| Fasting glucose (mmol/L/year)‡ | 0.016 (0.011) | 0.13 | 0.010 (0.010) | 0.34 | 0.009 (0.010) | 0.37 |
| 2-h glucose (mmol/L/year)‡ | 0.021 (0.013) | 0.12 | 0.020 (0.012) | 0.08 | 0.018 (0.011) | 0.12 |
| Fasting insulin (pmol/L/year)‡ | 0.011 (0.023) | 0.62 | 0.011 (0.022) | 0.62 | 0.007 (0.021) | 0.74 |
| 30-min ∆ insulin (pmol/L/year)‡ | 0.008 (0.020) | 0.69 | 0.006 (0.021) | 0.77 | 0.003 (0.022) | 0.88 |
| 2-h insulin (pmol/L/year)‡ | −0.021 (0.027) | 0.44 | −0.019 (0.026) | 0.46 | −0.026 (0.025) | 0.30 |
| IVGTT-derived measures | ||||||
| SI (×10−3 min−1/pmol/L/year)‡ | −0.045 (0.020) | 0.029 | −0.043 (0.020) | 0.035 | −0.038 (0.020) | 0.05 |
| AIRg (pmol/L × 10 min/year)‡ | −0.015 (0.019) | 0.43 | −0.010 (0.016) | 0.54 | −0.009 (0.017) | 0.57 |
| DI (SI × AIRg/year)‡ | −0.026 (0.012) | 0.027 | −0.025 (0.011) | 0.021 | −0.021 (0.010) | 0.034 |
NA, not applicable.
‡Log transformation was applied prior to calculating annualized rates of change.
We tested whether the association between baseline calorie intake and rates of decline in SI and DI differed by whether or not 1) women experienced a deterioration in glucose tolerance, 2) diabetes developed, or 3) women had additional pregnancies during follow-up, using interaction tests in the mixed-effects models. Interaction test results were not significant for deterioration of glucose tolerance and additional pregnancies (all P > 0.55) and were of only borderline significance for the development of diabetes during follow-up for SI (P = 0.06 for SI, P = 0.79 for DI). Further stratified analysis by follow-up diabetes status showed that the inverse association between baseline calorie intake and rates of decline in SI and DI was a little stronger for women in whom diabetes developed at follow-up (β = −0.066 for SI, β = −0.035 for DI) than for women who did not developed diabetes (β = −0.033 for SI, and β = −0.016 for DI).
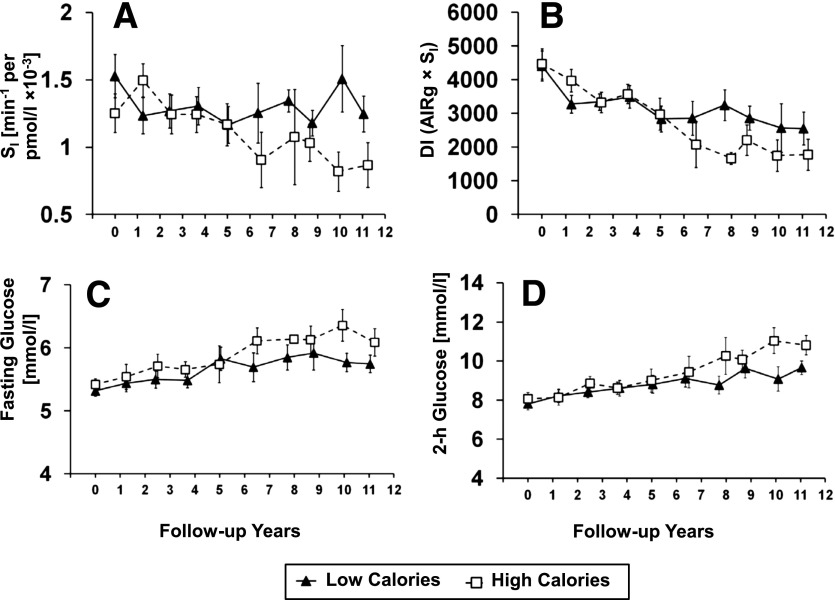
Figure 1 depicts the covariate (from adjusted-2) adjusted geometric means for SI, DI, and fasting and 2-h glucose levels over the follow-up years when the cohort was divided at the median of the total baseline calorie intake (2,091 kcal/day). On average, the high-calorie group (median calorie intake 3,013 kcal/day) had a threefold faster decline in SI (−0.06 vs. −0.02 units/year, P = 0.10) and a 17% faster decline in DI (−810 vs. −692 units/year, P = 0.042) compared with the low-calorie group (median calorie intake 1,526 kcal/day). The high-calorie group also tended to have a faster increase in fasting glucose levels (0.08 vs. 0.06 units/year) and 2-h glucose levels (0.35 vs. 0.16 units/year) than the low-calorie group, although these differences did not reach statistical significance (P > 0.21).
Figure 1.
Adjusted geometric means (SEM) of SI (A), β-cell function (DI) (B), OGTT fasting glucose level (C), and OGTT 2-h glucose level (D) over the follow-up years for women who had baseline total calorie intake above (high calories, open square, n = 31) and below (low calories, filled triangle, n = 31) the cohort median calorie intake (2,091 kcal/day) at baseline.
For the macronutrient density analyses, we did not observe significant associations between specific densities from fat, carbohydrates, or protein and rates of change in SI (P > 0.10) or DI (P > 0.36) with or without adjustment for covariates. High fat density at baseline was significantly associated with rates of increase in BMI, percent of body fat and fat mass (P < 0.036 for each), and the rate of decrease in 30-min change in insulin (P = 0.031) after adjusting for covariates. High fat density at baseline was marginally associated with the rate of increase in fasting glucose levels (P = 0.07).
Conclusions
In this long-term, prospective, observational study of Hispanic women with a history of GDM, we made a novel observation that higher daily calorie intake at baseline under free-living conditions predicted a long-term decline in insulin sensitivity and β-cell compensation. The predictive value was independent of baseline characteristics and changes in adiposity, calorie intake, levels of PA and additional pregnancies during follow-up. On average, the women in this cohort consumed a diet at baseline containing a slightly higher number of calories than recommended; the total calorie intake did not increase over time. On this background, adiposity, insulin sensitivity, β-cell compensation, and glucose levels all deteriorated significantly over time. Thus, our findings indicate that a prolonged high calorie intake may contribute to rising insulin resistance and falling β-cell function, independent of obesity.
A few short-term clinical trials have evaluated the impact of calorie intake on insulin sensitivity (9,29) and β-cell function (11,12). A 25% restriction in calories for 6 months caused a significant decrease in fasting insulin levels in nonobese subjects (29). A caloric restriction to 800 kcal/day for 3 months reduced hepatic glucose production, and increased insulin sensitivity and insulin secretion in seven obese subjects with type 2 diabetes (9). A 3-month trial (11) showed that 74% of subjects increased both insulin sensitivity and β-cell function after a diet intervention of 1,200 kcal/day. A recent study (12) showed that very low calorie intake (600 kcal/day) could reverse declines in β-cell function and hepatic insulin sensitivity in 11 type 2 diabetes patients. In the current study, we showed that long-term high calorie intake was significantly associated with deteriorating insulin sensitivity and β-cell compensation, independent of changes in obesity, which we previously found to be associated with declines in insulin sensitivity and β-cell function in this cohort (15). The association with caloric intake was a little stronger among women in whom diabetes developed during follow-up than among women in whom diabetes did not develop. One possible mechanism linking calorie intake to β-cell function decline is insulin resistance. Further adjustment for the rate of change in insulin sensitivity explained 46% of the association between baseline calorie intake and the rate of change in β-cell compensation, but did not completely eliminate the association. It is also possible that high calorie intake contributes to lipotoxicity (30) and inflammation (15), which further contribute to β-cell decline.
High-fat diets have been shown to be associated with increasing adiposity, and impaired glucose tolerance and insulin action (25,31–34). We found that higher baseline fat density was related with a faster increase in adiposity and fasting glucose levels. Our results support the concept that high fat consumption contributes to obesity and rising fasting glucose levels. The fact that we did not observe significant associations between dietary fat density and the decline in insulin sensitivity and β-cell function could be due to the relatively narrow range of consumption in our cohort, which limits the ability to detect associations. It is also possible that it is the total calorie intake that drives the decline in insulin sensitivity and β-cell function, since foods are not consumed in isolation in a free-living condition. Declining insulin sensitivity and β-cell function lead to an increase in glucose; however, glucose levels rise slowly in the process of declining β-cell function prior to the onset of diabetes (1). This explains why the deleterious effect of high calorie intake was less manifested on changes in fasting and 2-h glucose levels than on changes in insulin sensitivity and β-cell function in this study.
The strength of this study is the long time of follow-up with detailed OGTT, IVGTT, diet, and PA assessments at multiple times. Insulin sensitivity and β-cell function were measured directly by IVGTTs every 12–15 months. The dietary questionnaire was validated and calibrated for the Hispanic population (17) and was administered at the same time as IVGTTs. Unlike previous studies that investigated the impact of short-term calorie restrictions, we provided more realistic information about long-term dietary intake in a free-living environment. We also recognize several weaknesses. First, the relatively small sample size only allowed us to detect relatively large effect sizes. Second, only Hispanic women with a history of GDM were included. These women are at a high risk for the development of diabetes, and they were generally inactive. The variation in calorie intake and metabolic profiles in this cohort may be smaller than in the general population, which precluded us from detecting some important associations. The special cohort also limits the generalizability of the results. Third, although we considered weight, BMI, total body fat, and waist-to-hip ratio, we do not have a direct measure of abdominal obesity. Last, causal inferences may not be drawn since this is an observational study, and it is possible that there are other unmeasured factors that may explain some of the associations.
In summary, we found that high baseline calorie intake in a free-living environment predicted declining insulin sensitivity and β-cell compensation over time in Hispanic women who are at high risk for type 2 diabetes. The associations were independent of baseline characteristics, rates of change in calorie intake, adiposity, levels of PA, and additional pregnancies during follow-up. Our results shed light on the importance of controlling daily calorie intake. We recommend controlling calorie intake to preserve insulin sensitivity and β-cell function, and to prevent type 2 diabetes.
Article Information
Funding. This work was supported by grant R01-DK-046374 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH); grant M01-RR-000043 from the Division of Clinical Research, National Center for Research Resources, NIH; grant UL1-TR-000130 from the National Center for Advancing Translational Sciences, NIH; and a Distinguished Clinical Scientist Award from the American Diabetes Association.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Z.C. and A.H.X. researched the data and wrote and reviewed the manuscript. R.M.W. and T.A.B. contributed to the data collection and discussion and reviewed and edited the manuscript. D.O.S. contributed to the discussion and reviewed and edited the manuscript. Z.C. and A.H.X. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, 21–25 June 2013.
References
- 1.Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA. Coordinate changes in plasma glucose and pancreatic β-cell function in Latino women at high risk for type 2 diabetes. Diabetes 2006;55:1074–1079 [DOI] [PubMed] [Google Scholar]
- 2.Festa A, Williams K, D’Agostino R, Jr, Wagenknecht LE, Haffner SM. The natural course of β-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes 2006;55:1114–1120 [DOI] [PubMed] [Google Scholar]
- 3.Cnop M, Vidal J, Hull RL, et al. Progressive loss of β-cell function leads to worsening glucose tolerance in first-degree relatives of subjects with type 2 diabetes. Diabetes Care 2007;30:677–682 [DOI] [PubMed] [Google Scholar]
- 4.Weyer C, Tataranni PA, Bogardus C, Pratley RE. Insulin resistance and insulin secretory dysfunction are independent predictors of worsening of glucose tolerance during each stage of type 2 diabetes development. Diabetes Care 2001;24:89–94 [DOI] [PubMed] [Google Scholar]
- 5.Lindström J, Louheranta A, Mannelin M, et al. Finnish Diabetes Prevention Study Group . The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003;26:3230–3236 [DOI] [PubMed] [Google Scholar]
- 6.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larson-Meyer DE, Heilbronn LK, Redman LM, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, β-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care 2006;29:1337–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Després JP, Pouliot MC, Moorjani S, et al. Loss of abdominal fat and metabolic response to exercise training in obese women. Am J Physiol 1991;261:E159–E167 [DOI] [PubMed] [Google Scholar]
- 9.Kelley DE, Wing R, Buonocore C, Sturis J, Polonsky K, Fitzsimmons M. Relative effects of calorie restriction and weight loss in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1993;77:1287–1293 [DOI] [PubMed] [Google Scholar]
- 10.Henry RR, Scheaffer L, Olefsky JM. Glycemic effects of intensive caloric restriction and isocaloric refeeding in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1985;61:917–925 [DOI] [PubMed] [Google Scholar]
- 11.Utzschneider KM, Carr DB, Barsness SM, Kahn SE, Schwartz RS. Diet-induced weight loss is associated with an improvement in β-cell function in older men. J Clin Endocrinol Metab 2004;89:2704–2710 [DOI] [PubMed] [Google Scholar]
- 12.Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011;54:2506–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiang AH, Peters RK, Trigo E, Kjos SL, Lee WP, Buchanan TA. Multiple metabolic defects during late pregnancy in women at high risk for type 2 diabetes. Diabetes 1999;48:848–854 [DOI] [PubMed] [Google Scholar]
- 14.Buchanan TA, Xiang A, Kjos SL, et al. Gestational diabetes: antepartum characteristics that predict postpartum glucose intolerance and type 2 diabetes in Latino women. Diabetes 1998;47:1302–1310 [DOI] [PubMed] [Google Scholar]
- 15.Xiang AH, Kawakubo M, Trigo E, Kjos SL, Buchanan TA. Declining β-cell compensation for insulin resistance in Hispanic women with recent gestational diabetes mellitus: association with changes in weight, adiponectin, and C-reactive protein. Diabetes Care 2010;33:396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000;151:346–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stram DO, Hankin JH, Wilkens LR, et al. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol 2000;151:358–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nöthlings U, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Kolonel LN. Body mass index and physical activity as risk factors for pancreatic cancer: the Multiethnic Cohort Study. Cancer Causes Control 2007;18:165–175 [DOI] [PubMed] [Google Scholar]
- 19.Adams SA, Matthews CE, Ebbeling CB, et al. The effect of social desirability and social approval on self-reports of physical activity. Am J Epidemiol 2005;161:389–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Z, Black MH, Watanabe RM, et al. Self-reported physical activity is associated with β-cell function in Mexican American adults. Diabetes Care 2013;36:638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergman RN. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes 1989;38:1512–1527 [DOI] [PubMed] [Google Scholar]
- 22.Kotler DP, Burastero S, Wang J, Pierson RN, Jr. Prediction of body cell mass, fat-free mass, and total body water with bioelectrical impedance analysis: effects of race, sex, and disease. Am J Clin Nutr 1996;64(Suppl.):489S–497S [DOI] [PubMed] [Google Scholar]
- 23.Gibson RS. Principles of Nutritional Assessment. New York, Oxford University Press, 2005 [Google Scholar]
- 24.U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans [article online], 2008. Available from http://www.health.gov/PAGuidelines/guidelines/default.aspx Accessed May 1, 2011
- 25.Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology 2009;136:1552–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petro AE, Cotter J, Cooper DA, Peters JC, Surwit SJ, Surwit RS. Fat, carbohydrate, and calories in the development of diabetes and obesity in the C57BL/6J mouse. Metabolism 2004;53:454–457 [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Diet, Nutrition and Prevention of Chronic Diseases. In Report of the Joint WHO/FAO Expert Consultation. Geneva, Switzerland, World Health Organization, 2003 (WHO Technical Report Series No. 916; TRS 916)
- 28.Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–3167 [DOI] [PubMed] [Google Scholar]
- 29.Heilbronn LK, de Jonge L, Frisard MI, et al. Pennington CALERIE Team Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 2006;295:1539–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unger RH, Zhou YT. Lipotoxicity of β-cells in obesity and in other causes of fatty acid spillover. Diabetes 2001;50(Suppl. 1):S118–S121 [DOI] [PubMed] [Google Scholar]
- 31.Tuomilehto J, Lindström J, Eriksson JG, et al. Finnish Diabetes Prevention Study Group . Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 32.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 33.van den Berg SA, Guigas B, Bijland S, et al. High levels of dietary stearate promote adiposity and deteriorate hepatic insulin sensitivity. Nutr Metab (Lond) 2010;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Black MH, Watanabe RM, Trigo E, et al. High-fat diet is associated with obesity-mediated insulin resistance and β-cell dysfunction in Mexican Americans. J Nutr 2013;143:479–485 [DOI] [PMC free article] [PubMed] [Google Scholar]