Abstract
OBJECTIVE
To investigate the relationship between HDL cholesterol (HDL-C) and cancer risk among type 2 diabetic patients.
RESEARCH DESIGN AND METHODS
We performed a retrospective cohort study of 14,169 men and 23,176 women with type 2 diabetes. Cox proportional hazards regression models were used to estimate the association of various levels of HDL cholesterol (HDL-C) with cancer risk.
RESULTS
During a mean follow-up period of 6.4 years, 3,711 type 2 diabetic patients had a cancer diagnosis. A significant inverse association between HDL-C and the risk of cancer was found among men and women. The multivariable-adjusted hazard ratios (HRs) of cancer at various levels of HDL-C at baseline (<30, 30–39.9, 40–49.9, 50–59.9, 60–69.9, 70–79.9, and ≥80 mg/dL) were 1.00, 0.87, 0.95, 1.01, 0.61, 0.45, and 0.37, respectively, in men (Ptrend = 0.027) and 1.00, 0.98, 0.88, 0.85, 0.84, 0.86, and 0.84, respectively, in women (Ptrend = 0.025). When stratified by race, BMI, smoking status, or medication use, the inverse association was still present. With an updated mean of HDL-C used in the analysis, the inverse association of HDL-C with cancer risk did not change. The inverse association substantially attenuated after excluding patients who died of or were diagnosed with cancer during the first 2 years of follow-up.
CONCLUSIONS
The study suggests an inverse association of HDL-C with cancer risk among men and women with type 2 diabetes, whereas the effect of HDL-C was partially mediated by reverse causation.
Introduction
Emerging data suggest that type 2 diabetes is associated with an increased risk of cancer (1–3). The most frequently cited reason is the potential effect of insulin (3). Lipid metabolism has also been suggested to be associated with cancer risk. Several studies found an inverse association of serum HDL cholesterol (HDL-C) with cancer risk in the general population (4–9), but few assessed this association among patients with type 2 diabetes, although diabetic patients are more likely to have dyslipidemia. One study found a U-shaped association between HDL-C and cancer risk in patients with type 2 diabetes (10). Another study suggested that the observed inverse association between HDL-C and cancer risk may be explained by confounding and reverse causation, such that HDL-C is not a risk factor for cancer among patients with type 2 diabetes (11).
Epidemiological studies found that women generally have higher levels of serum HDL-C than men in both general and diabetic populations, and sex differences in HDL-C might be associated with observed sex differences in cancer risk. The aim of the current study was to examine the sex-specific association between various levels of HDL at baseline and during follow-up and the risk of cancer among patients with type 2 diabetes in the Louisiana State University Hospital-Based Longitudinal Study (LSUHLS).
Research Design and Methods
Study Population
From 1997 through June 2013, the Louisiana State University Health Care Services Division (LSUHCSD) operated seven public hospitals and affiliated clinics in seven Louisiana population centers. The LSUHCSD delivered quality medical care to residents of Louisiana, regardless of their income or insurance coverage (12–20). Overall, LSUHCSD facilities served ∼1.6 million patients.
Administrative (name, address, date of birth, sex, race/ethnicity, types of insurance, family income, and smoking status), anthropometric (date of examination and measurements of body weight, height, and blood pressure for each visit), laboratory (test code, test collection date, test results, and abnormal flag), clinical diagnosis (date of diagnosis, diagnosis code, priority assigned to diagnosis, ICD-9 code, and Current Procedural Terminology code), and medication data (medication generic name, pharmacopeia dispensable drug identifier, medication strength-dose form, medication strength units, medication route code and description, medication form, etc.) collected at these facilities are available in electronic form for LSUHCSD inpatients and outpatients since 1997. These data were used to established the LSUHLS (12). Within the LSUHLS, ICD-9-CM code 250 was used to define a cohort of patients with diabetes and who used LSUHCSD primary care clinic services between 1 January 1999 and 31 December 2009. LSUHCSD internal diabetes disease management guidelines call for physician confirmation of diabetes diagnoses by applying American Diabetes Association criteria: fasting plasma glucose level ≥126 mg/dL, 2-h glucose level ≥200 mg/dL after a 75-g 2-h oral glucose tolerance test, and one or more classic symptoms plus a random plasma glucose level ≥200 mg/dL (21). We validated the diabetes diagnosis in LSUHCSD hospitals. The agreement of diabetes diagnosis was 97%; 20,919 of a sample of 21,566 hospital discharge diagnoses based on ICD codes also had physician-confirmed diabetes by American Diabetes Association diabetes diagnosis criteria (21).
The first ICD-9 diabetes diagnosis date was used to define the baseline date for each patient in the present analyses per the design of the cohort study. Before diabetes diagnosis, these patients had used the LSUHCSD system for an average of 5.0 years. After excluding patients with a history of cancer at baseline and patients with incomplete data on any required variable, the current study finally included 37,345 patients (14,169 men and 23,176 women) with type 2 diabetes who were 30–96 years of age. Of these patients, ∼76.8% qualified for free care (by virtue of being low income and uninsured [any individual or family unit with an income ≤200% of the Federal Poverty Level]), ∼5.2% were self-pay (uninsured but incomes not low enough to qualify for free care), ∼5.3% were covered by Medicaid, ∼10.4% had Medicare, and ∼2.3% were covered by commercial insurance. The study and analysis plan were approved by the institutional review boards of both the Pennington Biomedical Research Center and the Louisiana State University Health Sciences Center in New Orleans. We obtained waivers of informed consent and Health Insurance Portability and Accountability Act authorization from both institutional review boards because this retrospective research could not practicably be carried out without such waivers.
Baseline and Follow-up Measurements
The patient characteristics, including age at diabetes diagnosis, sex, race/ethnicity, family income, smoking status, type of health insurance, weight, height, BMI, blood pressure, total cholesterol, HDL-C, LDL cholesterol (LDL-C), triglycerides, HbA1c, and medication (antihypertensive drug, cholesterol-lowering drug, and antidiabetic drug) use within 0.5 year of the diabetes diagnosis date (baseline) and during follow-up after diabetes diagnosis (follow-up) were extracted from the computerized hospitalization records. The updated mean values of HDL-C, HbA1c, LDL-C, triglycerides, BMI, blood pressure, and estimated glomerular filtration rate over time were calculated for each patient from baseline to each year of follow-up. For example, at 1-year follow-up, the updated mean was the average of the baseline and 1-year values, and at 3-year follow-up, the updated mean was the average of baseline, 1-year, 2-year, and 3-year values. In the case of a new cancer diagnosis during follow-up, the period for estimating the updated mean value was from baseline to the year before this event occurred (22). The average number of HDL-C measurements during the follow-up period was 5.5.
Prospective Follow-up
Follow-up information was obtained from the LSUHLS database by using the unique number designated for each patient who visits the LSUHCSD hospitals each time. The diagnosis of the first cancer event was the primary end point of interest and was defined according to ICD-9 codes 140–208. Follow-up of each cohort member continued until the date of cancer diagnosis, the date of the last LSUHCSD encounter if the patient stopped using LSUHCSD hospitals, death, or 31 May 2012.
Statistical Analyses
The association of HDL-C at baseline and during follow-up with the risk of cancer was analyzed by using Cox proportional hazards models. HDL-C was included in these models in two alternative ways: 1) as seven categories (<30, 30–39.9, 40–49.9, 50–59.9, 60–69.9, 70–79.9, and ≥80 mg/dL) and 2) as a continuous variable. All multivariable analyses were adjusted for age and race and further for type of insurance, income, smoking status, BMI, LDL-C, triglycerides, HbA1c, systolic blood pressure, use of antihypertensive drugs, use of diabetes medications, and use of cholesterol-lowering agents. To avoid potential bias due to severe disease at baseline, additional analyses were carried out excluding the patients who died of or were diagnosed with cancer during the first 2 years of follow-up. All hypothesis tests were two-tailed, and the statistical significance level was considered to be P < 0.05. In the Cox models that treated HDL-C as a continuous variable, we used restricted cubic splines to produce a hazard ratio (HR) curve capable of capturing nonlinear and nonmonotonic associations between HDL-C and cancer risk. The splines used five knots at 0.05, 0.275, 0.50, 0.75, and 0.95. In such models, the HR between two points of a continuous variable can be estimated by exp(Y2 – Y1), where Y1 and Y2 are the corresponding spline function values of the two points (10). All statistical analyses were performed with PASW for Windows version 20.0 (IBM Corporation, Chicago, IL) and SAS for Windows version 9.3 (SAS Institute, Cary, NC).
Results
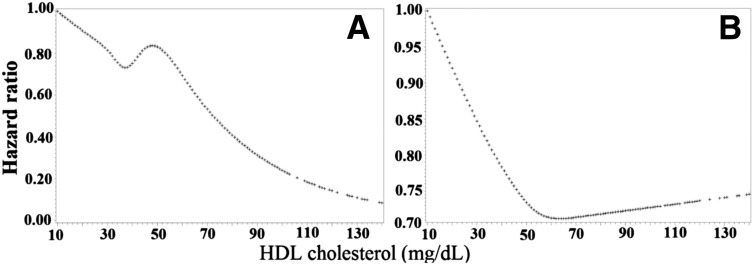
Table 1 shows characteristics of the study population at baseline. During a mean follow-up period of 6.4 years, cancer developed in 3,711 subjects (1,492 men and 2,219 women). Cancer risk was higher in men (17.6/1,000 person-years) than in women (14.4/1,000 person-years) with type 2 diabetes. The multivariable-adjusted (age, race, type of insurance, income, smoking status, BMI, LDL-C, triglycerides, HbA1c, systolic blood pressure, use of antihypertensive drugs, use of diabetes medications, and use of cholesterol-lowering agents) HRs of cancer at various levels of HDL-C at baseline (<30, 30–39.9, 40–49.9, 50–59.9, 60–69.9, 70–79.9, and ≥80 mg/dL) were 1.00, 0.87, 0.95, 1.01, 0.61, 0.45, and 0.37, respectively, in men (Ptrend = 0.027) and 1.00, 0.98, 0.88, 0.85, 0.84, 0.86, and 0.84, respectively, in women (Ptrend = 0.025) (Table 2). When HDL-C was considered as a continuous variable by using restricted cubic splines, a linear association of HDL-C with cancer risk was observed in men and a nonlinear association with a nadir at ∼70 mg/dL in women (Fig. 1). Each 15 mg/dL increase in baseline HDL-C was associated with a 9% (HR 0.91, 95% CI 0.84–0.98) decreased risk of cancer in men and a 6% (HR 0.94, 95% CI 0.89–0.99) decreased risk of cancer in women with type 2 diabetes (Table 2). The associations of HDL-C at baseline with the risk of major types of cancer are presented in Supplementary Table 1.
Table 1.
Baseline characteristics among patients with type 2 diabetes by various levels of serum HDL-C
| HDL-C (mg/dL) |
P value | |||||||
|---|---|---|---|---|---|---|---|---|
| <30 | 30–39.9 | 40–49.9 | 50–59.9 | 60–69.9 | 70–79.9 | ≥80 | ||
| Men | ||||||||
| Participants (n) | 3,243 | 5,466 | 3,348 | 1,279 | 471 | 185 | 177 | |
| Age (years) | 50.7 (0.2) | 52.1 (0.1) | 52.5 (0.2) | 52.9 (0.3) | 53.1 (0.5) | 52.2 (0.8) | 51.2 (0.8) | <0.001 |
| Black | 1,455 (44.9) | 2,668 (48.8) | 1,926 (57.5) | 871 (68.1) | 345 (73.2) | 151 (81.6) | 143 (80.8) | <0.001 |
| Income ($/family) | 20,545 (609) | 22,855 (464) | 23,216 (592) | 21,466 (988) | 24,147 (1,623) | 24,534 (2,654) | 25,366 (2,804) | 0.014 |
| BMI (kg/m2) | 33.3 (0.1) | 33.1 (0.1) | 32.3 (0.1) | 30.6 (0.2) | 28.3 (0.4) | 27.7 (0.6) | 25.6 (0.6) | <0.001 |
| Systolic blood pressure (mmHg) | 142 (0.4) | 143 (0.3) | 143 (0.4) | 143 (0.7) | 142 (1.2) | 143 (1.9) | 139 (2.0) | 0.358 |
| HbA1c [% (mmol/mol)] | 8.1 (65) | 8.0 (64) | 7.9 (63) | 7.7 (61) | 7.8 (62) | 7.9 (63) | 7.5 (58) | <0.001 |
| LDL-C (mg/dL) | 96 (0.7) | 108 (0.5) | 114 (0.7) | 112 (1.1) | 107 (1.8) | 105 (2.9) | 96 (3.0) | <0.001 |
| Triglycerides (mg/dL) | 173 (1.6) | 164 (1.3) | 142 (1.6) | 119 (2.7) | 106 (4.4) | 97 (7.1) | 92 (7.5) | <0.001 |
| Current smoker | 1,672 (51.6) | 2,244 (41.1) | 1,271 (38.0) | 502 (39.2) | 209 (44.4) | 94 (51.0) | 110 (62.0) | <0.001 |
| Type of insurance | <0.001 | |||||||
| Free | 2,253 (69.5) | 3,940 (72.1) | 2,340 (69.9) | 880 (68.8) | 338 (71.8) | 118 (63.8) | 121 (68.4) | |
| Self-pay | 301 (9.3) | 369 (6.8) | 232 (6.9) | 91 (7.1) | 33 (7.0) | 19 (10.3) | 22 (12.4) | |
| Medicaid | 180 (5.6) | 244 (4.5) | 142 (4.2) | 72 (5.6) | 27 (5.7) | 19 (10.3) | 14 (7.9) | |
| Medicare | 364 (11.2) | 716 (13.1) | 535 (16.0) | 196 (15.3) | 64 (13.6) | 27 (14.6) | 19 (10.7) | |
| Commercial | 117 (3.6) | 159 (2.9) | 81 (2.4) | 31 (2.4) | 4 (0.8) | 1 (0.5) | 1 (0.6) | |
| Use of medications | ||||||||
| Glucose lowering | 2,322 (71.6) | 3,844 (70.3) | 2,277 (68.0) | 812 (63.5) | 277 (58.8) | 104 (56.0) | 78 (44.1) | <0.001 |
| Lipid lowering | 1,987 (61.3) | 3,466 (63.4) | 2,001 (59.8) | 690 (54.0) | 227 (48.1) | 85 (45.9) | 58 (32.9) | <0.001 |
| Antihypertensive | 2,545 (78.5) | 4,385 (80.2) | 2,675 (79.9) | 1,024 (80.1) | 360 (76.3) | 142 (76.7) | 128 (72.4) | 0.144 |
| Women | ||||||||
| Participants (n) | 1,994 | 6,634 | 7,465 | 4,214 | 1,755 | 663 | 451 | |
| Age (years) | 49.1 (0.2) | 50.9 (0.1) | 52.3 (0.1) | 53.5 (0.2) | 54.2 (0.2) | 55.0 (0.4) | 54.8 (0.5) | <0.001 |
| Black | 996 (49.9) | 3,506 (52.8) | 4,327 (58.0) | 2,658 (63.1) | 1,212 (69.1) | 474 (71.5) | 343 (76.1) | <0.001 |
| Income ($/family) | 18,878 (682) | 20,220 (370) | 21,145 (350) | 20,813 (471) | 20,373 (741) | 19,119 (1,207) | 19,342 (1,514) | 0.062 |
| BMI (kg/m2) | 36.0 (0.2) | 35.9 (0.1) | 35.8 (0.1) | 35.0 (0.1) | 33.9 (0.2) | 32.4 (0.4) | 31.3 (0.4) | <0.001 |
| Systolic blood pressure (mmHg) | 145 (0.6) | 146 (0.3) | 146 (0.3) | 145 (0.4) | 144 (0.6) | 142 (1.0) | 142 (1.3) | 0.002 |
| HbA1c [% (mmol/mol)] | 7.9 (63) | 7.6 (60) | 7.6 (60) | 7.4 (57) | 7.4 (57) | 7.3 (56) | 7.3 (56) | <0.001 |
| LDL-C (mg/dL) | 107 (0.9) | 113 (0.5) | 118 (0.5) | 119 (0.6) | 117 (1.0) | 114 (1.5) | 113 (1.9) | <0.001 |
| Triglycerides (mg/dL) | 173 (1.8) | 160 (1.0) | 142 (0.9) | 124 (1.2) | 112 (2.0) | 101 (3.2) | 94 (4.0) | <0.001 |
| Current smoker | 944 (47.3) | 2,338 (35.2) | 2,052 (27.5) | 1,057 (25.1) | 423 (24.1) | 146 (22.1) | 144 (31.9) | <0.001 |
| Type of insurance | <0.001 | |||||||
| Free | 1,597 (80.1) | 5,382 (81.1) | 6,070 (81.3) | 3,370 (80.0) | 1,361 (77.5) | 503 (75.9) | 327 (72.5) | |
| Self-pay | 112 (5.6) | 271 (4.1) | 244 (3.3) | 137 (3.3) | 66 (3.8) | 21 (3.2) | 17 (3.8) | |
| Medicaid | 141 (7.1) | 377 (5.7) | 378 (5.1) | 209 (5.0) | 101 (5.8) | 37 (5.6) | 40 (8.9) | |
| Medicare | 100 (5.0) | 472 (7.1) | 634 (8.5) | 412 (9.8) | 187 (10.7) | 87 (13.1) | 56 (12.4) | |
| Commercial | 43 (2.2) | 126 (1.9) | 134 (1.8) | 83 (2.0) | 40 (2.3) | 14 (2.1) | 10 (2.2) | |
| Use of medications | ||||||||
| Glucose lowering | 1,484 (7.4) | 4,800 (7.2) | 5,149 (6.9) | 2,695 (6.4) | 1,069 (6.1) | 364 (5.5) | 241 (5.3) | <0.001 |
| Lipid lowering | 1,282 (6.4) | 4,405 (6.6) | 4,795 (6.4) | 2,658 (6.3) | 1,065 (6.1) | 362 (5.5) | 232 (5.2) | <0.001 |
| Antihypertensive | 1,657 (8.3) | 5,573 (8.4) | 6,265 (8.4) | 3,507 (8.3) | 1,439 (8.2) | 533 (8.0) | 364 (8.1) | <0.001 |
Data are mean (SE) or n (%) unless otherwise indicated. All continuous variables are adjusted for age and race, except for age (adjusted for race only). SE of HbA1c is 0.05%, 0.04%, 0.05%, 0.08%, 0.13%, 0.21%, and 0.22%, respectively, in men and 0.05%, 0.03%, 0.03%, 0.04%, 0.06%, 0.09%, and 0.12%, respectively, in women.
Table 2.
Risk of cancer according to various levels of serum HDL-C or HDL-C as a continuous variable at baseline and during follow-up among diabetic patients
| HDL-C (mg/dL) |
P value for trend | Each 15 mg/dL increase | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <30 | 30–39.9 | 40–49.9 | 50–59.9 | 60–69.9 | 70–79.9 | ≥80 | |||
| Baseline | |||||||||
| Men | 3,243 | 5,466 | 3,348 | 1,279 | 471 | 185 | 177 | ||
| Cases (n) | 312 | 567 | 404 | 156 | 34 | 12 | 7 | ||
| Person-years | 17,018 | 33,272 | 21,255 | 8,123 | 2,839 | 1,217 | 986 | ||
| Model 1 | 1.00 | 0.84 (0.74–0.97) | 0.92 (0.80–1.07) | 0.94 (0.78–1.14) | 0.60 (0.42–0.85) | 0.54 (0.30–0.96) | 0.43 (0.20–0.90) | 0.005 | 0.91 (0.85–0.97) |
| Model 2 | 1.00 | 0.87 (0.76–1.01) | 0.96 (0.81–1.12) | 1.00 (0.81–1.23) | 0.61 (0.42–0.90) | 0.45 (0.22–0.92) | 0.38 (0.16–0.93) | 0.028 | 0.91 (0.84–0.98) |
| Model 3 | 1.00 | 0.87 (0.75–1.01) | 0.95 (0.81–1.12) | 1.01 (0.81–1.24) | 0.61 (0.41–0.89) | 0.45 (0.22–0.91) | 0.37 (0.15–0.91) | 0.027 | 0.91 (0.84–0.98) |
| Women | 1,994 | 6,634 | 7,465 | 4,214 | 1,755 | 663 | 451 | ||
| Cases (n) | 161 | 646 | 734 | 409 | 164 | 63 | 42 | ||
| Person-years | 10,886 | 42,353 | 51,523 | 29,380 | 12,123 | 4,460 | 3,018 | ||
| Model 1 | 1.00 | 0.97 (0.82–1.16) | 0.87 (0.73–1.03) | 0.82 (0.68–0.99) | 0.78 (0.63–0.97) | 0.80 (0.59–1.07) | 0.80 (0.57–1.13) | 0.001 | 0.92 (0.87–0.97) |
| Model 2 | 1.00 | 0.98 (0.82–1.17) | 0.88 (0.74–1.06) | 0.85 (0.70–1.03) | 0.84 (0.67–1.07) | 0.86 (0.63–1.18) | 0.84 (0.57–1.23) | 0.024 | 0.94 (0.89–0.99) |
| Model 3 | 1.00 | 0.98 (0.81–1.17) | 0.88 (0.74–1.06) | 0.85 (0.70–1.03) | 0.84 (0.67–1.06) | 0.86 (0.63–1.18) | 0.84 (0.57–1.23) | 0.025 | 0.94 (0.89–0.99) |
| Follow-up | |||||||||
| Men | 2,999 | 5,854 | 3,378 | 1,178 | 448 | 154 | 158 | ||
| Cases (n) | 287 | 626 | 404 | 126 | 28 | 14 | 7 | ||
| Person-years | 15,803 | 35,823 | 21,283 | 7,167 | 2,830 | 973 | 825 | ||
| Model 1 | 1.00 | 0.88 (0.76–1.01) | 0.95 (0.82–1.11) | 0.88 (0.72–1.09) | 0.52 (0.35–0.76) | 0.82 (0.48–1.40) | 0.49 (0.24–1.04) | 0.008 | 0.90 (0.84–0.96) |
| Model 2 | 1.00 | 0.95 (0.81–1.10) | 0.99 (0.84–1.18) | 0.95 (0.75–1.20) | 0.45 (0.29–0.70) | 0.73 (0.39–1.38) | 0.52 (0.23–1.18) | 0.017 | 0.89 (0.82–0.96) |
| Model 3 | 1.00 | 0.95 (0.82–1.10) | 0.99 (0.84–1.17) | 0.96 (0.76–1.21) | 0.45 (0.29–0.69) | 0.72 (0.38–1.37) | 0.51 (0.23–1.16) | 0.014 | 0.89 (0.82–0.96) |
| Women | 1,713 | 6,845 | 7,858 | 4,143 | 1,648 | 597 | 372 | ||
| Cases (n) | 143 | 637 | 811 | 393 | 143 | 56 | 36 | ||
| Person-years | 9,243 | 44,240 | 54,061 | 28,646 | 11,177 | 3,891 | 2,486 | ||
| Model 1 | 1.00 | 0.87 (0.72–1.04) | 0.87 (0.73–1.04) | 0.76 (0.63–0.92) | 0.70 (0.55–0.88) | 0.76 (0.55–1.03) | 0.79 (0.55–1.14) | 0.001 | 0.90 (0.86–0.95) |
| Model 2 | 1.00 | 0.88 (0.73–1.07) | 0.90 (0.74–1.09) | 0.81 (0.66–1.00) | 0.77 (0.60–0.99) | 0.87 (0.63–1.21) | 0.86 (0.57–1.29) | 0.067 | 0.93 (0.88–0.99) |
| Model 3 | 1.00 | 0.88 (0.73–1.07) | 0.89 (0.74–1.08) | 0.80 (0.65–0.99) | 0.76 (0.59–0.97) | 0.86 (0.62–1.20) | 0.85 (0.56–1.27) | 0.051 | 0.93 (0.88–0.98) |
Data are HR (95% CI) unless otherwise indicated. Model 1, adjusted for age. Model 2, adjusted for age, race, BMI, systolic blood pressure, HbA1c, LDL-C, triglycerides, type of insurance, family income, and smoking status. Model 3, adjusted for age, race, BMI, systolic blood pressure, HbA1c, LDL-C, triglycerides, type of insurance, family income, smoking status, use of antihypertensive drugs, use of diabetes medications, and use of cholesterol-lowering agents.
Figure 1.
HRs for incident cancer by baseline HDL-C level in men (A) and women (B). Adjusted for age, race, BMI, systolic blood pressure, LDL-C, triglycerides, HbA1c, type of insurance, family income, smoking status, use of antihypertensive drugs, use of diabetes medications, and use of cholesterol-lowering agents.
Additional analyses using an updated mean of HDL-C showed the same inverse association between HDL-C and cancer risk among men and women with type 2 diabetes (Table 2). When the mean value of HDL was updated to 2 years before cancer outcomes or the end of follow-up, each 15 mg/dL increase in baseline HDL-C was associated with a 10% (HR 0.90, 95% CI 0.83–0.98) decreased risk of cancer in men and a 7% (HR 0.93, 95% CI 0.88–0.99) decreased risk of cancer in women.
After excluding the subjects who died during the first 2 years of follow-up (n = 889), these inverse associations of HDL-C at baseline and during follow-up with cancer risk were still statistically significant among men (all Ptrend < 0.05) but no longer among women (all Ptrend > 0.05). Each 15 mg/dL increase in baseline HDL-C was associated with a 9% (HR 0.91, 95% CI 0.84–0.98) decreased risk of cancer in men and a 5% (HR 0.95, 95% CI 0.90–1.01) decreased risk of cancer in women. After excluding the 1,270 patients with cancer diagnosed within the first 2 years of follow-up, each 15 mg/dL increase in baseline HDL-C was associated with an 8% (HR 0.92, 95% CI 0.84–0.998) decreased risk of cancer in men and a 1% (HR 0.99, 95% CI 0.92–1.05) decreased risk of cancer in women.
In the multivariable analyses, the inverse association of HDL-C at baseline and during follow-up with cancer risk was present in African American and white patients with type 2 diabetes; obese and nonobese patients; smokers and nonsmokers; non–glucose-lowering medication users, metformin users, and other glucose-lowering medication users; and lipid-lowering medicine users and nonusers (Table 3 and Supplementary Table 2).
Table 3.
Risk of cancer according to various levels of serum HDL-C at baseline among subpopulations of diabetic patients
| HDL-C (mg/dL) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| <30 | 30–39.9 | 40–49.9 | 50–59.9 | 60–69.9 | 70–79.9 | ≥80 | P value for trend | P value for interaction | |
| Race | >0.90 | ||||||||
| Black | |||||||||
| Participants (n) | 2,451 | 6,174 | 6,253 | 3,529 | 1,557 | 625 | 486 | ||
| Adjusted HR (95% CI) | 1.00 | 1.00 (0.83–1.19) | 0.96 (0.80–1.15) | 0.92 (0.76–1.13) | 0.82 (0.65–1.05) | 0.83 (0.60–1.15) | 0.53 (0.33–0.84) | 0.003 | |
| White | |||||||||
| Participants (n) | 2,786 | 5,926 | 4,560 | 1,964 | 669 | 223 | 142 | ||
| Adjusted HR (95% CI) | 1.00 | 0.89 (0.77–1.03) | 0.86 (0.73–1.01) | 0.83 (0.69–1.01) | 0.72 (0.54–0.96) | 0.59 (0.35–1.00) | 1.27 (0.77–2.08) | 0.042 | |
| BMI | <0.05 | ||||||||
| <30 kg/m2 | |||||||||
| Participants (n) | 1,712 | 3,893 | 3,695 | 2,140 | 1,048 | 465 | 389 | ||
| Adjusted HR (95% CI) | 1.00 | 0.84 (0.70–1.00) | 0.82 (0.68–0.98) | 0.83 (0.67–1.03) | 0.69 (0.53–0.91) | 0.67 (0.46–0.97) | 0.71 (0.46–1.08) | 0.010 | |
| ≥30 kg/m2 | |||||||||
| Participants (n) | 3,525 | 8,207 | 7,118 | 3,353 | 1,178 | 383 | 239 | ||
| Adjusted HR (95% CI) | 1.00 | 0.99 (0.86–1.15) | 0.96 (0.82–1.12) | 0.90 (0.75–1.08) | 0.83 (0.65–1.06) | 0.81 (0.55–1.19) | 0.63 (0.36–1.11) | 0.016 | |
| Smoking status | >0.25 | ||||||||
| Never | |||||||||
| Participants (n) | 2,976 | 8,160 | 8,011 | 4,167 | 1,700 | 647 | 416 | ||
| Adjusted HR (95% CI) | 1.00 | 0.88 (0.77–1.02) | 0.84 (0.73–0.97) | 0.85 (0.72–1.00) | 0.74 (0.60–0.91) | 0.68 (0.49–0.93) | 0.69 (0.46–1.03) | 0.002 | |
| Current | |||||||||
| Participants (n) | 2,261 | 3,940 | 2,802 | 1,326 | 526 | 201 | 212 | ||
| Adjusted HR (95% CI) | 1.00 | 0.98 (0.81–1.18) | 1.00 (0.82–1.23) | 0.84 (0.65–1.08) | 0.79 (0.55–1.13) | 0.89 (0.54–1.48) | 0.65 (0.35–1.22) | 0.067 | |
| Glucose-lowering medication | >0.1 | ||||||||
| No use | |||||||||
| Participants (n) | 1,862 | 4,563 | 4,450 | 2,466 | 1,076 | 452 | 354 | ||
| Adjusted HR (95% CI) | 1.00 | 0.87 (0.72–1.06) | 0.80 (0.65–0.98) | 0.82 (0.65–1.03) | 0.58 (0.42–0.80) | 0.80 (0.54–1.20) | 0.82 (0.51–1.31) | 0.013 | |
| Metformin | |||||||||
| Participants (n) | 1,519 | 3,523 | 2,859 | 1,370 | 471 | 169 | 89 | ||
| Adjusted HR (95% CI) | 1.00 | 0.94 (0.76–1.16) | 0.96 (0.77–1.21) | 0.83 (0.64–1.09) | 0.64 (0.43–0.96) | 0.74 (0.42–1.31) | 0.73 (0.34–1.59) | 0.031 | |
| Other medications | |||||||||
| Participants (n) | 1,856 | 4,015 | 3,504 | 1,657 | 679 | 227 | 185 | ||
| Adjusted HR (95% CI) | 1.00 | 0.98 (0.82–1.17) | 0.96 (0.80–1.16) | 0.95 (0.77–1.18) | 1.05 (0.80–1.37) | 0.63 (0.39–1.02) | 0.51 (0.27–0.94) | 0.113 | |
| Lipid-lowering medication | >0.05 | ||||||||
| No use | |||||||||
| Participants (n) | 2,338 | 5,234 | 4,988 | 2,603 | 1,121 | 469 | 378 | ||
| Adjusted HR (95% CI) | 1.00 | 0.84 (0.71–1.00) | 0.76 (0.63–0.91) | 0.80 (0.65–0.99) | 0.65 (0.48–0.86) | 0.53 (0.34–0.82) | 0.67 (0.42–1.08) | <0.001 | |
| Use | |||||||||
| Participants (n) | 2,899 | 6,866 | 5,825 | 2,890 | 1,105 | 379 | 250 | ||
| Adjusted HR (95% CI) | 1.00 | 1.01 (0.87–1.17) | 1.01 (0.87–1.18) | 0.93 (0.77–1.11) | 0.87 (0.68–1.10) | 0.91 (0.65–1.28) | 0.67 (0.41–1.09) | 0.056 | |
Adjusted for age, sex, race, BMI, systolic blood pressure, HbA1c, LDL-C, triglycerides, type of insurance, income, smoking, use of antihypertensive drugs, use of diabetes medications, and use of cholesterol-lowering agents.
Conclusions
This study found a graded inverse association between HDL-C and the risk of cancer among men and women with type 2 diabetes. However, this inverse association was attenuated after excluding those who died of or were diagnosed with cancer during the first 2 years of follow-up, with the attenuation stronger among women.
Type 2 diabetes is associated with elevated plasma levels of glucose and insulin as well as with an increased risk of cancer (1–3). The most frequently hypothesized reason is the potential mitogenic effect of insulin (3). Substantial epidemiological studies support that low plasma levels of HDL represent a coronary heart disease risk (23,24). However, only a few studies explored the association of serum HDL-C levels and cancer risk, and the results are inconsistent. Some studies provided supportive information of the inverse associations in the general population (4–9). A meta-analysis took advantage of 24 large randomized controlled trials of lipid-modifying therapy to examine the relationship between HDL-C levels and the risk of cancer (9). After adjusting for baseline age, BMI, diabetes, sex, smoking status, and LDL-C level, the significant inverse relationship between baseline HDL-C and cancer risk persisted. Results from studies among patients with diabetes are inconsistent. One study reported that HDL-C was associated with cancer risk in a U-shaped manner, with the nadir at 1.22 mmol/L and a rapid increase above and below that level (10). However, a more recent study suggested that HDL-C is not a predictor of cancer risk in type 2 diabetes, and the inverse association of HDL-C and cancer risk may be attributable to confounding factors and reverse causation (11). Thus, more studies are warranted to confirm the observed associations.
Some researchers have speculated that observed relationships between cholesterol levels and cancer might be attributable to cholesterol-lowering action of preclinical cancer (25), that is, to reverse causation. Furthermore, several studies showed that tumor progression from localized to metastatic disease is associated with declining HDL-C levels (26,27). Thus, to determine whether HDL-C levels predict cancer risk, it is important to measure HDL-C levels before the diagnosis of cancer. The current study attempts to do this by defining a diabetes cohort and baseline time spans and observing cancer incidence during follow-up time spans. During a mean follow-up period of 6.4 years, 3,711 of 37,345 participants with diabetes were diagnosed with cancer. We found a graded inverse association both when treating baseline HDL-C levels as ordered categories and when using HDL-C level as a continuous variable with cancer risk among men and women with type 2 diabetes. This inverse association was also stable when we used HDL-C measurements during follow-up. A recent study suggested that this inverse association may be explained by confounding (11). In the current study, after further adjusting for other major confounding factors (type of insurance, income, smoking status, BMI, LDL-C, triglycerides, HbA1c, systolic blood pressure, use of antihypertensive drugs, use of diabetes medications, and use of cholesterol-lowering agents), this inverse association remained significant, and the HRs did not reduce significantly. In addition, we found that this graded inverse association was present in diabetic patients with differing race, smoking status, BMI, and lipid-lowering treatment. The use of metformin might confer stronger benefits in reducing cancer risk in diabetic patients with low HDL-C levels (28). Based on a subgroup analysis among non–glucose-lowering medication users, metformin users, and other glucose-lowering medication users, this graded inverse association was still present. However, it remains possible that the observed inverse associations are due to preclinical effects of malignancies (i.e., through metabolic depression or increased use of cholesterol during carcinogenesis) (8,11). Thus, we did another two sensitivity analyses that excluded the subjects who died of or were diagnosed with cancer within the first 2 years of follow-up. Those results showed an attenuated inverse association that was still statistically significant for men but not for women. The associations observed could be partially attributable to the influence of preclinical disease to lower cholesterol concentrations, but an additional etiologic role is consistent with the current results.
Biological mechanisms that might account for this inverse association between HDL-C and incident cancer risk are not well understood. Cancer is well-known to be a proinflammatory state in which inflammatory cells actively participate in the neoplastic process, allowing tumor cell proliferation, survival, and migration (29,30). It is plausible that HDL-C exerts its protective effects through the modulation of cytokine production and anti-inflammatory and antioxidant properties (31,32). Serum HDL-C in patients with type 2 diabetes can be modified into glycated HDL-C and oxidized HDL-C, and the modifications of HDL-C could lead to an acceleration of cancer progression in diabetic patients (33). In the ILLUMINATE (Investigation of Lipid Level Management to Understand Its Impact in Atherosclerotic Events) trial, torcetrapib, an inhibitor of cholesterylester transfer protein, was associated with increased HDL-C levels, but cancer and infections were also increased (34). The adverse effect was possibly a result of the generation of dysfunctional HDL-C (35).
The major strengths of the current investigation include a large sample size with serum cholesterol prospectively measured, long follow-up time, and use of administrative databases to avoid differential recall bias. We used baseline HDL-C levels and updated mean values of HDL-C during follow-up in the analyses, which can avoid potential bias from a single baseline measurement. In addition, study subjects used the same public health-care system, which minimizes the influence of health-care accessibility. One limitation of the study is that the analysis was not performed on a representative sample of the population, which limits the generalizability of the results; however, LSUHCSD hospitals are public hospitals and cover >1.6 million patients, most of whom are low-income residents of Louisiana. The results of the current study will have wide applicability for populations with low income and without health insurance living in the U.S. Another limitation is that cancer incidence was defined using ICD-9-CM administrative coding rather than through cancer registry data. However, the use of ICD-9 codes to define eligibility has been used in other cohort studies, such as the Hong Kong Diabetes Registry (9). Finally, although we adjusted the analyses for an extensive set of confounding factors, residual confounding resulting from measurement error in the assessment of confounding factors and unmeasured factors such as physical activity, education, and dietary factors cannot be excluded.
In summary, this study found that higher HDL-C concentrations are associated with a decreased risk of cancer among men and women with type 2 diabetes. An influence of preclinical disease to lower cholesterol concentrations appears to explain part of the inverse association, but etiologic role cannot be completely ruled out. Additional studies in specific cancer populations as well as experimental investigations to elucidate potential mechanisms will be useful for a more complete understanding of the association between HDL-C and the risk of cancer.
Supplementary Material
Article Information
Funding. This work was supported in part by the Louisiana State University Improving Clinical Outcomes Network, Louisiana Clinical Data Research Network, Colon Cancer Coalition Foundation, and grant 1-U54-GM-104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. W.Z. and J.G. contributed to researching data and writing the manuscript. R.H. and G.H. contributed to researching data and reviewing and editing the manuscript. W.L. and Y.W. contributed to researching data. X.W. contributed to reviewing and editing the manuscript. G.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-0523/-/DC1.
References
- 1.Stattin P, Björ O, Ferrari P, et al. Prospective study of hyperglycemia and cancer risk. Diabetes Care 2007;30:561–567 [DOI] [PubMed] [Google Scholar]
- 2.Strickler HD, Wylie-Rosett J, Rohan T, et al. The relation of type 2 diabetes and cancer. Diabetes Technol Ther 2001;3:263–274 [DOI] [PubMed] [Google Scholar]
- 3.Czyzyk A, Szczepanik Z. Diabetes mellitus and cancer. Eur J Intern Med 2000;11:245–252 [DOI] [PubMed] [Google Scholar]
- 4.Moorman PG, Hulka BS, Hiatt RA, et al. Association between high-density lipoprotein cholesterol and breast cancer varies by menopausal status. Cancer Epidemiol Biomarkers Prev 1998;7:483–488 [PubMed] [Google Scholar]
- 5.Furberg AS, Veierød MB, Wilsgaard T, Bernstein L, Thune I. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Natl Cancer Inst 2004;96:1152–1160 [DOI] [PubMed] [Google Scholar]
- 6.Kucharska-Newton AM, Rosamond WD, Mink PJ, Alberg AJ, Shahar E, Folsom AR. HDL-cholesterol and incidence of breast cancer in the ARIC cohort study. Ann Epidemiol 2008;18:671–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kucharska-Newton AM, Rosamond WD, Schroeder JC, McNeill AM, Coresh J, Folsom AR, Members of the Atherosclerosis Risk in Communities Study . HDL-cholesterol and the incidence of lung cancer in the Atherosclerosis Risk in Communities (ARIC) study. Lung Cancer 2008;61:292–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn J, Lim U, Weinstein SJ, et al. Prediagnostic total and high-density lipoprotein cholesterol and risk of cancer. Cancer Epidemiol Biomarkers Prev 2009;18:2814–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jafri H, Alsheikh-Ali AA, Karas RH. Baseline and on-treatment high-density lipoprotein cholesterol and the risk of cancer in randomized controlled trials of lipid-altering therapy. J Am Coll Cardiol 2010;55:2846–2854 [DOI] [PubMed] [Google Scholar]
- 10.Yang X, So WY, Ma RC, et al. Predicting values of lipids and white blood cell count for all-site cancer in type 2 diabetes. Endocr Relat Cancer 2008;15:597–607 [DOI] [PubMed] [Google Scholar]
- 11.Morton J, Ng MK, Chalmers J, et al. ADVANCE Collaborative Group . The association of high-density lipoprotein cholesterol with cancer incidence in type II diabetes: a case of reverse causality? Cancer Epidemiol Biomarkers Prev 2013;22:1628–1633 [DOI] [PubMed] [Google Scholar]
- 12.Li W, Wang Y, Chen L, et al. Increasing prevalence of diabetes in middle or low income residents in Louisiana from 2000 to 2009. Diabetes Res Clin Pract 2011;94:262–268 [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Li W, Wang Y, et al. Increasing prevalence of hypertension in low income residents within Louisiana State University Health Care Services Division Hospital System. Eur J Intern Med 2012;23:e179–e184 [DOI] [PubMed] [Google Scholar]
- 14.Zhao W, Katzmarzyk PT, Horswell R, et al. Blood pressure and stroke risk among diabetic patients. J Clin Endocrinol Metab 2013;98:3653–3662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao W, Katzmarzyk PT, Horswell R, et al. HbA1c and lower-extremity amputation risk in low-income patients with diabetes. Diabetes Care 2013;36:3591–3598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Johnson J, Hu G. HbA1c and coronary heart disease risk among diabetic patients. Diabetes Care 2014;37:428–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao W, Katzmarzyk PT, Horswell R, et al. Aggressive blood pressure control increases coronary heart disease risk among diabetic patients. Diabetes Care 2013;36:3287–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Johnson J, Hu G. HbA1c and heart failure risk among diabetic patients. J Clin Endocrinol Metab 2014;99:E263–E267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao W, Katzmarzyk PT, Horswell R, Wang Y, Johnson J, Hu G. Sex differences in the risk of stroke and HbA(1c) among diabetic patients. Diabetologia 2014;57:918–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Katzmarzyk PT, Horswell R, Zhao W, Johnson J, Hu G. Kidney function and the risk of cardiovascular disease in patients with type 2 diabetes. Kidney Int 2014;85:1192–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Diabetes Association . Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 22.Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 2000;321:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon DJ, Rifkind BM. High-density lipoprotein—the clinical implications of recent studies. N Engl J Med 1989;321:1311–1316 [DOI] [PubMed] [Google Scholar]
- 24.Filippatos TD, Elisaf MS. High density lipoprotein and cardiovascular diseases. World J Cardiol 2013;5:210–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Circulating cholesterol level and risk of death from cancer in men aged 40 to 69 years. Experience of an international collaborative group. JAMA 1982;248:2853–2859 [DOI] [PubMed] [Google Scholar]
- 26.Kökoğlu E, Karaarslan I, Karaarslan HM, Baloğlu H. Alterations of serum lipids and lipoproteins in breast cancer. Cancer Lett 1994;82:175–178 [DOI] [PubMed] [Google Scholar]
- 27.Knapp ML, al-Sheibani S, Riches PG. Alterations of serum lipids in breast cancer: effects of disease activity, treatment, and hormonal factors. Clin Chem 1991;37:2093–2101 [PubMed] [Google Scholar]
- 28.Yang X, So WY, Ma RC, et al. Low HDL cholesterol, metformin use, and cancer risk in type 2 diabetes: the Hong Kong Diabetes Registry. Diabetes Care 2011;34:375–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonda TA, Tu S, Wang TC. Chronic inflammation, the tumor microenvironment and carcinogenesis. Cell Cycle 2009;8:2005–2013 [DOI] [PubMed] [Google Scholar]
- 31.Negre-Salvayre A, Dousset N, Ferretti G, Bacchetti T, Curatola G, Salvayre R. Antioxidant and cytoprotective properties of high-density lipoproteins in vascular cells. Free Radic Biol Med 2006;41:1031–1040 [DOI] [PubMed] [Google Scholar]
- 32.von Eckardstein A, Hersberger M, Rohrer L. Current understanding of the metabolism and biological actions of HDL. Curr Opin Clin Nutr Metab Care 2005;8:147–152 [DOI] [PubMed] [Google Scholar]
- 33.Pan B, Ren H, Ma Y, et al. High-density lipoprotein of patients with type 2 diabetes mellitus elevates the capability of promoting migration and invasion of breast cancer cells. Int J Cancer 2012;131:70–82 [DOI] [PubMed] [Google Scholar]
- 34.Barter PJ, Caulfield M, Eriksson M, et al. ILLUMINATE Investigators . Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109–2122 [DOI] [PubMed] [Google Scholar]
- 35.Katsiki N, Athyros VG, Karagiannis A, Mikhailidis DP. High-density lipoprotein, vascular risk, cancer and infection: a case of quantity and quality? Curr Med Chem 2014;21:2917–2926 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.