Abstract
OBJECTIVE
We aim to characterize the effects on total body fat and distribution of a 1-year intensive lifestyle intervention (ILI) for weight loss in overweight and obese adults with type 2 diabetes and to examine whether changes in adipose tissue (AT) depots were associated with changes in metabolic biomarkers.
RESEARCH DESIGN AND METHODS
Participants were 54 females and 38 males (age 57.8 ± 6.7 years [mean ± SD]; BMI 31.7 ± 3.5 kg/m2) enrolled in the Look AHEAD (Action for Health in Diabetes) trial randomized to ILI or diabetes support and education (DSE) from whom baseline and 1-year MRI measures of total AT (TAT) and regional (arm, trunk, leg) AT, including subcutaneous AT (SAT), visceral AT (VAT), and intermuscular AT (IMAT), were acquired. We tested whether mean changes in ILI and DSE were equal and, within groups, whether changes were different from zero. Regression models tested whether changes in AT compartments were associated with metabolic variable changes.
RESULTS
Body weight changed −0.52 ± 3.62 kg (P = 0.31) in DSE and −7.24 ± 5.40 kg (P < 0.0001) in ILI. Mean ILI changes were different from DSE (P < 0.001 for TAT, SAT, and IMAT and P < 0.01 for VAT in females). Within ILI, SAT and VAT decreased in males and females (P < 0.0001), but IMAT was unchanged (0.00 ± 0.54 kg; P = 0.99). In DSE, SAT and VAT did not change, but IMAT increased by 0.46 ± 0.55 kg (P < 0.001). Controlling for weight loss, reduction of specific AT depots was associated with improvement in metabolic biomarkers.
CONCLUSIONS
Weight loss of 7–10% from an ILI over 1 year reduced SAT and VAT and prevented an increase in IMAT. Reductions in AT depots were associated with improvements in biomarkers.
Introduction
Most individuals with type 2 diabetes are overweight or obese. Using cross-sectional data, we have shown that adipose tissue (AT) distribution is significantly altered in type 2 diabetes compared with nondiabetic controls, with larger amounts of visceral AT (VAT) and intermuscular AT (IMAT) and with less subcutaneous AT (SAT) than controls (1). The changes in these depots have been shown to be associated with exacerbation of insulin resistance (IR) (2,3). The seeming inability of SAT to absorb additional excess energy in obese patients results in accretions in other depots and a resultant increase in metabolic risk (4).
While diet and exercise interventions are the initial step in treating obesity, the precise effects of such interventions on specific AT depots are unknown. Insofar as AT may play a role in IR and different depots may contribute differently to IR or cardiovascular disease (CVD) risk, it is important to establish how various AT depots respond to weight-loss interventions. Most published studies have included a single abdominal slice as a surrogate measure of total VAT or abdominal SAT, but whole-body SAT and whole-body IMAT have rarely been measured (e.g., [4,5]).
Only a small number of clinical trials of the effect of lifestyle change (diet and/or exercise interventions) in which whole-body MRI measures of AT were acquired have been published (6–8). In nondiabetic postmenopausal women who lost 10% body weight over 16 weeks on a moderate hypocaloric diet, 78% of weight lost was AT, of which 93% was SAT and 7% was VAT (6). Relative to baseline values, reductions in SAT and VAT were 16.6 and 25%, respectively. In another study of both lean and obese men with and without type 2 diabetes participating in 13 weeks of supervised aerobic exercise, significant reductions in TAT, abdominal SAT, and VAT were observed in both groups (7). Although the reductions in TAT and abdominal SAT were not different between groups, the reduction in VAT was greater in the obese (−16%) and type 2 diabetes groups (−22%) by comparison with the lean group (−13%). In abdominally obese women and men with and without type 2 diabetes randomized to exercise or nonexercise control for 24 weeks, greater reductions of TAT and VAT (after adjustment for baseline weight, age, and sex) were observed in men than women in the aerobic exercise group only (8). Men and women within the same treatment group (resistance exercise, aerobic exercise, combined exercise, nonexercise) did not differ significantly in their response to exercise. It thus remains uncertain how specific AT depots respond to weight-loss interventions in adults with type 2 diabetes.
There is also the question of whether reductions in the size of specific AT depots are associated with improvements in biological indicators of metabolic health such as fasting glucose, insulin sensitivity, and serum lipids. Evidence of improvements in CVD risk factors after 1 year of intentional weight loss in the Look AHEAD (Action for Health in Diabetes) trial has been published (9), but studies have not investigated whether improvements are related to an overall reduction of body weight or to changes in specific body compartments or to something else entirely. In cross-sectional studies, larger amounts of VAT and IMAT have been associated with elevations in lipids, glucose, insulin, and impaired glucose tolerance (10–12), whereas larger amounts of subcutaneous lower-body AT deposits have been associated with beneficial/protective effects (13,14). In the current study, the detailed MRI in vivo quantification of AT depots enabled us to investigate in a longitudinal, noninvasive fashion associations of metabolic markers with changes in specific regional AT depots. To date, there have been no systematic studies of AT depot associations with biomarkers.
The goals of this prospective study were 1) to compare the effect of a 1-year randomized intensive lifestyle intervention (ILI) for weight loss versus usual diabetes support and education (DSE) on total AT (TAT) and its subdepots (SAT, VAT, and IMAT) and regional segments (arm, trunk, and leg) of SAT and IMAT and 2) to examine whether changes in the size of specific AT depots are associated with changes in biological indicators of metabolic health in a cohort of overweight and obese adults with type 2 diabetes.
Research Design and Methods
Study Design and Participants
Participants with type 2 diabetes, BMI ≥25 and ≤41, and enrolled in the Look AHEAD trial at the New York and Pittsburgh sites were invited to enroll in this ancillary study after randomization but before initiation of any intervention. Participants who were claustrophobic or who did not fit within the field of view for MRI (BMI >41 kg/m2) were excluded. Recruitment began in January 2002. The Look AHEAD clinical trial of the effect of weight loss on the prevention of CVD in men and women, ages 45–76 years, with a BMI ≥25 kg/m2 and type 2 diabetes, has been described elsewhere (15). Participants in the ILI group received weekly diet, exercise, and behavior modification counseling in groups or individually for the first 24 weeks, followed by 18 additional treatment sessions during the next 6 months. The intervention included a moderately intense physical activity goal of ≥175 min a week, such as brisk walking or similar unsupervised at-home aerobic exercise. The DSE group received usual medical care provided by their own primary care physicians, plus three group educational sessions per year (16). Preintervention (baseline) and 1-year follow-up data were used in this analysis. Selected 1-year results from the Look AHEAD trial (17,18) and baseline results of this MRI ancillary study have been previously published (1).
Biological markers of metabolic health (fasting serum glucose, glycosylated hemoglobin [HbA1c], total cholesterol, HDL cholesterol [HDL-C], LDL cholesterol [LDL-C], triglycerides, and systolic and diastolic blood pressure [BP]) were collected by the parent Look AHEAD trial and were obtained for this analysis from the Look AHEAD Data Coordinating Center.
This study was conducted in accordance with Good Clinical Research Practice guidelines and the Declaration of Helsinki. All studies were approved by the Institutional Review Board of St. Luke’s-Roosevelt Hospital or the University of Pittsburgh, and all subjects gave written consent to participate.
Procedures
Body Composition Measures
Body weight was measured to the nearest 0.1 kg (Avery Weigh-Tronix, New York, NY; Scale-Tronix, Wheaton, IL) and height to the nearest 0.5 cm using a stadiometer (Holtain, Crosswell, Wales, U.K.).
MRI
TAT, including SAT, VAT, and IMAT, was measured using whole-body multislice MRI as previously described (1,19). Subjects at both sites (New York and Pittsburgh) were placed on a 1.5T scanner (General Electric, 6× Horizon, Milwaukee, WI) platform with arms extended above head. The protocol involved the acquisition of ∼40 axial images, 10 mm thickness, and at 40-mm intervals across the whole body. SliceOmatic 4.2 image analysis software (TomoVision, Montreal, Canada) was used to analyze images on a PC workstation (Gateway, Madison, WI). MRI-volume estimates were converted to mass using an assumed density of 0.92 kg/L for AT (20). All scans were read by the same technician in the Image Analysis Laboratory of the New York Obesity Nutrition Research Center. The technical error for three repeated readings of the same scan by the same observer for SAT, VAT, and IMAT volumes in our laboratory are 0.96, 1.97, and 0.65%, respectively.
Statistical Analysis
Descriptive statistics (number, mean, and SD) for continuous variables and percentages for categorical variables were calculated for subject baseline characteristics and for changes from baseline to 1 year. The t test was used to test the null hypothesis that the mean baseline characteristics of the two groups, DSE and ILI, were equal and to test the hypothesis that the 1-year changes for ILI and DSE were equal. The paired t test was used to test the hypothesis that the mean 1-year change from baseline within each group was equal to zero. Separate analyses were performed for males and females.
Multiple linear regression was used to model the relationship between changes in AT depots and changes in metabolic variables. First, models were run to determine whether the association of AT depot changes with metabolic changes, if any, depended on sex and treatment group. If results differed by sex or group, they are reported only for the sex or group where associations were significant. If the model detected no interactions, then all subjects were pooled. Subsequent models adjusted for baseline value of the metabolic variable, change in body weight, and baseline size and change in the AT depot. Between 1 and 6 cases were excluded from the model to reduce residual variability or for excessive leverage. Subjects who started or stopped medication intended to affect a metabolic variable during the study period were excluded from those analyses. Subjects taking insulin at any time were excluded from analyses of fasting glucose and HbA1c. Only subjects who had both baseline and 1-year measures of all AT depots were included in these analyses. N varied between 62 and 70 depending on how many cases were excluded because of medications or missing values. Tests for preferential VAT loss were carried out using an allometric model as described by Hallgreen and Hall (21) and de Souza et al. (22). The statistical calculations were performed using SAS (version 9.4, SAS Institute Inc., Cary, NC) and STATA (version 11.0, College Station, TX). The level of significance for all statistical tests was two-tailed P ≤ 0.05.
Results
Demographic and Body Composition Characteristics
Subject demographic characteristics and mean values for AT compartments, by sex, for ILI and DSE groups are shown in Table 1. The ethnic distribution was 11 African Americans, 2 Asians, 26 Caucasians, and 3 Hispanics in the ILI group and 14 African Americans, 2 Asian, 30 Caucasians, and 3 Hispanics in the DSE group. Mean duration of diabetes in the sample was 7.0 ± 6.4 years. With the exception of arm IMAT in women, ILI and DSE groups did not differ statistically at baseline for any of the variables in Table 1.
Table 1.
Subject characteristics and AT depots at baseline
| Female ILI n = 28 | Female DSE n = 26 | Male ILI n = 14 | Male DSE n = 24 | |
|---|---|---|---|---|
| Age (years) | 57.8 ± 6.7 | 56.8 ± 6.3 | 57.7 ± 7.7 | 58.8 ± 6.3 |
| Weight (kg) | 82.13 ± 12.33 | 84.57 ± 12.19 | 93.16 ± 7.61 | 99.48 ± 12.33 |
| Height (m) | 1.62 ± 0.06 | 1.61 ± 0.07 | 1.75 ± 0.06 | 1.76 ± 0.08 |
| BMI (kg/m2) | 31.38 ± 4.38 | 32.70 ± 3.40 | 30.39 ± 2.64 | 31.84 ± 2.64 |
| Duration of diabetes (years) | 8.4 ± 7.7 | 6.3 ± 4.7 | 6.8 ± 9.0 | 6.5 ± 4.5 |
| TAT (kg) | 36.99 ± 9.60 | 39.86 ± 8.53 | 31.12 ± 4.71 | 33.35 ± 6.93 |
| SAT (kg) | 31.55 ± 8.12 | 34.08 ± 7.49 | 23.13 ± 3.57 | 25.42 ± 5.95 |
| Arms (kg) | 3.19 ± 1.02 | 3.36 ± 0.82 | 2.34 ± 0.55 | 2.48 ± 0.56 |
| Trunk (kg) | 18.82 ± 5.04 | 20.09 ± 4.82 | 14.84 ± 2.52 | 16.49 ± 4.29 |
| Legs (kg) | 9.54 ± 3.55 | 10.63 ± 3.10 | 5.94 ± 1.18 | 6.44 ± 1.59 |
| VAT (kg) | 3.83 ± 1.71 | 3.85 ± 1.82 | 5.92 ± 1.60 | 6.01 ± 1.78 |
| IMAT (kg) | 1.61 ± 0.69 | 1.92 ± 0.70 | 2.08 ± 0.48 | 1.92 ± 0.70 |
| Arms (kg) | 0.07 ± 0.03 | 0.10 ± 0.04* | 0.13 ± 0.05 | 0.12 ± 0.05 |
| Trunk (kg) | 0.86 ± 0.46 | 1.01 ± 0.43 | 1.10 ± 0.28 | 1.03 ± 0.42 |
| Legs (kg) | 0.68 ± 0.29 | 0.81 ± 0.32 | 0.84 ± 0.25 | 0.76 ± 0.29 |
Values are mean ± SD.
*Within sex, there were no significant differences between the two study groups at baseline using the t test except for female arm IMAT, which was larger in the DSE group (P < 0.05).
Changes in Body Weight
The mean ± SD of the weight changes was −0.52 ± 3.62 kg (P = 0.31) in the DSE group and −7.24 ± 5.40 kg (P < 0.0001) in the ILI group. Weight loss in the ILI group was ∼7% in females and ∼10% in males. For both men and women, AT constituted 81% of the weight lost.
Changes in AT Depots
Table 2 presents mean changes from baseline to 1 year for both groups, by sex. Within the ILI group, TAT, SAT, and VAT decreased in males and females (P < 0.0001), whereas IMAT was unchanged. Within the DSE group, there were no significant changes in TAT, SAT, or VAT, but IMAT increased in both sexes by similar amounts, ∼0.46 kg (P < 0.002).
Table 2.
Mean changes in body weight and AT depots at 1 year
| Female ILI
n = 28 |
Female DSE
n = 26 |
Male ILI
n = 14 |
Male DSE
n = 24 |
|||||
|---|---|---|---|---|---|---|---|---|
| kg | % | kg | % | kg | % | kg | % | |
| Weight | −5.95 ± 5.56a,b | −7.04 | −0.30 ± 3.81 | — | −9.83 ± 4.12a,b | −10.5 | −0.76 ± 3.48 | — |
| TAT | −4.80 ± 5.57a,b | −12.5 | 0.22 ± 3.41 | — | −8.00 ± 4.77a,b | −25.6 | 0.09 ± 3.26 | — |
| SAT | −4.11 ± 4.56a,b | −12.5 | −0.21 ± 2.98 | — | −5.57 ± 3.22a,b | −23.6 | −0.07 ± 2.59 | — |
| Arms | −0.31 ± 0.63a | −8.8 | −0.05 ± 0.46 | — | −0.40 ± 0.48a,b | −14.7 | −0.04 ± 0.53 | — |
| Trunk | −2.81 ± 2.79a,b | −14.7 | −0.16 ± 2.04 | — | −3.90 ± 2.44a,b | −25.9 | 0.27 ± 2.09 | — |
| Legs | −0.99 ± 1.57a,b | −9.0 | 0.09 ± 1.40 | — | −1.28 ± 0.83a,b | −20.7 | −0.30 ± 0.96 | — |
| VAT | −0.83 ± 0.83a,b | −22.9 | −0.03 ± 0.62 | — | −2.15 ± 1.48a,b | −37.5 | −0.30 ± 0.98 | — |
| IMAT | 0.14 ± 0.38b | — | 0.46 ± 0.66a | 26.8 | −0.28 ± 0.72b | — | 0.47 ± 0.44a | 28.1 |
| Arms | 0.03 ± 0.04a | 45.9 | 0.05 ± 0.06a | 58.5 | 0.00 ± 0.08 | — | 0.01 ± 0.07 | — |
| Trunk | 0.01 ± 0.19b | — | 0.19 ± 0.37a | 19.7 | −0.18 ± 0.39b | — | 0.14 ± 0.27a | 13.5 |
| Legs | 0.11 ± 0.20a | 20.8 | 0.23 ± 0.37a | 38.1 | −0.10 ± 0.33b | — | 0.31 ± 0.32a | 49.7 |
Values are mean ± SD. Percent values are mean changes at 1 year as a percentage of baseline. Percentages are shown only for compartments where change was statistically different from zero (P < 0.05).
aMean change from baseline is significantly different from zero using a paired t test (P < 0.05).
bMean changes from baseline are significantly different for ILI vs. DSE groups using a t test (P < 0.05).
With respect to regional SAT changes, all ILI regional SAT depots decreased in both sexes, and all ILI changes were significantly different (except arm SAT, women; P = 0.07) from those in DSE where there were no significant changes from baseline for either sex.
With respect to changes in regional IMAT depots, in the DSE group, women increased in all regions while men increased in trunk and leg but not arm. In the ILI group, IMAT increased in arms and legs for women but did not change in men. Changes were significantly different between DSE and ILI groups in the trunk region for women and in trunk and leg for men, with the intervention seeming to prevent or attenuate IMAT increase.
Table 2 also shows mean changes at 1 year expressed as a percentage of the baseline value. Males in ILI show near twofold percentage losses for TAT, SAT, and IMAT (VAT 1.6 times) compared with females even though females had larger initial TAT and SAT deposits.
Changes in Specific Depots as a Percentage of Total Depot Change in ILI
If TAT loss is partitioned into its component parts, for females, 86% of the reduction was from SAT and 17% from VAT, while IMAT increased 3%. For males, the corresponding values were 70% from SAT, 27% from VAT, and 3% from IMAT. Thus, as a proportion of TAT loss, females have a greater reduction in SAT than males and males have a greater reduction in VAT than females (both P < 0.001). The IMAT difference is not statistically reliable.
Reductions in regional SAT as a proportion of total SAT loss were similar for females and males, respectively: arms (7.5 vs. 7.2%), trunk (68.4 vs. 70.0%), and legs (24.1 vs. 23.0%). Thus, for a given reduction in SAT, males and females did not differ in regional decreases.
Changes in regional IMAT as a proportion of total IMAT change appeared to be different for females and males. Females gained a small amount of IMAT (0.14 kg) with the gain distributed as arms 21.4%, trunk 7.1%, and legs 78.6%; males lost a small amount of IMAT (−0.28 kg), with the loss occurring in trunk 64.3% and legs 35.7%. Statistical tests of sex differences in regional IMAT changes adjusted for total IMAT change did not reach significance because of large interindividual variability in percentages.
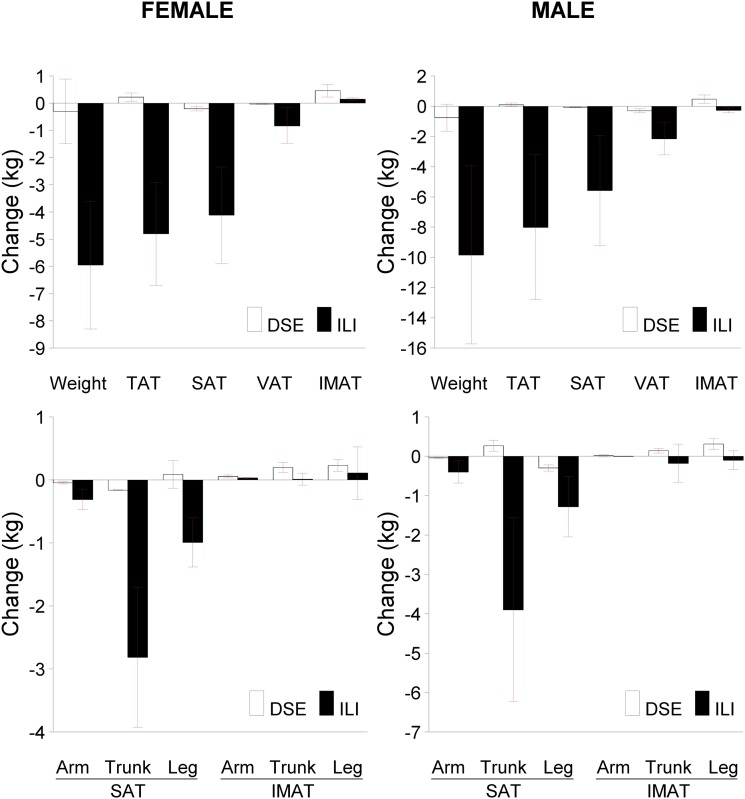
Figure 1 shows the mean values and 95% CIs for changes in body weight and AT compartments, including arm, trunk, and leg regions, in the ILI and DSE groups.
Figure 1.
Top panels: Changes in ILI and DSE groups for body weight, TAT, SAT, VAT, and IMAT. Bottom panels: Changes in regional SAT and IMAT deposits. Bars show group mean and 95% CI. All AT depots are by MRI. Baseline values are given in Table 1.
Associations of Changes in Weight and AT Depots With Metabolic Variables
We first tested whether changes in body weight were reliably associated with changes in metabolic variables as reported in the parent Look AHEAD trial (9). This was found to be the case for eight of the nine metabolic markers available; for six of these eight, the association was qualified by group or sex (Table 3). No associations were found with LDL-C, and it was not included in the table.
Table 3.
Regression coefficients for changes in metabolic variables with changes in weight and AT depots
| Glucose (mg/dL) | HbA1c % (mmol/mol) | Cholesterol (mg/dL) | HDL-C (mg/dL) | Cholesterol/HDL | Triglycerides (mg/dL) | Systolic BP (mmHg) | Diastolic BP (mmHg) | |
|---|---|---|---|---|---|---|---|---|
| Weight (kg) | ILI 2.1† | ILI 0.04 (0.4)‡ | M 1.7† | −0.35* | ILI 0.07‡ | 2.2† | ILI 0.65* | ILI 0.60‡ |
| TAT (kg) | ILI 2.0† | ILI 0.03 (0.3)* | M 1.8† | F −0.56† | ILI 0.07‡ | 2.1† | 0.59* | ILI 0.58‡ |
| SAT | ILI 2.5† | ILI 0.04 (0.4)* | M 2.7† | F −0.75† | ILI 0.09‡ | 2.5* | 0.85* | ILI 0.82‡ |
| Arm | ILI 0.51* | 26.6† | ILI 6.1‡ | |||||
| Trunk | ILI 4.2† | ILI 0.06 (0.7)* | ILI 3.1† | F −1.18† | ILI 0.12‡ | 3.8* | 1.5† | ILI 1.2‡ |
| Leg | ILI 7.0* | ILI 0.15 (1.6)* | ILI 7.5† | F −1.40* | ILI 0.30‡ | ILI 2.5‡ | ||
| VAT | ILI 8.8† | ILI 0.13 (1.4)* | ILI 5.6* | −1.60* | 0.26‡ | 12.2‡ | 2.9* | 2.2‡ |
| IMAT | ILI 17.7* | 0.27* | M 25.3† | |||||
| Arm | M 3.8* | |||||||
| Trunk | ILI 41.5* | M 0.75* | M 41.7* | |||||
| Leg | 17.2* | F 7.1* |
The regression coefficient estimates the change in the metabolic variable associated with a 1-kg change in the AT compartment. Changes in compartments and metabolic variables were expressed as differences, year 1 minus baseline. When associations were different by group or sex, the qualifier is shown. F, females; M, males.
*P < 0.05.
†P < 0.01.
‡P < 0.001.
A second set of models tested whether changes in AT compartments were related to changes in metabolic variables without controlling for concomitant changes in body weight. Associations were found for every metabolic variable except, as noted above, for LDL-C (Table 3). Notably, TAT, VAT, SAT, and trunk SAT were associated with every variable except LDL-C, while IMAT was associated with three of the metabolic variables. Regression coefficients were all positive, indicating that the values of variables (AT compartment size and metabolic marker) increased or decreased together, except for HDL-C, where negative coefficients indicated that decreases in AT depots were associated with increases in HDL-C.
A third set of models examined whether any association remained between changes in a specific AT depot and a metabolic marker after taking effects of total weight change into account. The following associations approached or exceeded statistical significance: VAT with fasting glucose (ILI group, regression coefficient β = 9.6; P = 0.06), cholesterol/HDL ratio (β = 0.16; P = 0.058), triglycerides (β = 9.8; P = 0.04), and diastolic BP (β = 1.7; P = 0.03); arm SAT with triglycerides (β = 21.2; P = 0.014), systolic BP (β = 1.7; P = 0.03), and diastolic BP (β = 4.5; P = 0.02); trunk SAT with systolic BP (β = 2.1; P = 0.04) and diastolic BP (β = 0.9; P = 0.07); and leg SAT with diastolic BP (β = 2.2; P = 0.02).
Conclusions
In overweight and obese adults with type 2 diabetes, weight loss of ∼7% in females and ∼10% in males from a 1-year ILI resulted in significant reductions in TAT, SAT, and VAT and all regional depots of SAT. IMAT did not decrease; however, the intervention prevented or reduced the IMAT gain which otherwise occurred in trunk and leg compartments. Increased leg AT in the DSE group was made up of a disproportionate increase in IMAT (women 0.25 kg, men 0.33 kg) relative to SAT (women 0.15 kg, men −0.25 kg). Also, the magnitude of IMAT increase in the DSE group is remarkable, with an increase near 27% in the whole body and near 50% in some regions in a single year. This rate of increase in the DSE group and the resistance of IMAT to an ILI may be a clinically significant observation, as IMAT is known to be independently associated with fasting glucose and IR (23,24).
Sex Differences in AT Changes
Numerous studies have investigated whether changes in fat depots differ between sex when fat is lost, reaching a variety of conclusions depending on whether changes are expressed as mass or as a percentage of baseline or percentage of fat lost and whether adjusted for initial compartment size, amount of TAT lost, and/or other covariates. Of particular interest in this study are the IMAT and VAT depots because of their potential metabolic roles. While IMAT increased similarly in males and females in the DSE groups, in females in the ILI group, it continued to increase despite the intervention. Additional follow-up is needed to confirm this sex difference and to determine whether it persists.
With respect to VAT, when expressed as mass or percentage change or percentage of TAT lost, men appear to lose more VAT than women, e.g., in this study 27 vs. 17% of TAT or 36 vs. 22% of baseline VAT in men and women, respectively (see also [25,26]). However, when adjusted for initial compartment size, sex differences often disappeared (26,27). A systematic review of weight-loss studies by Chaston et al. (28,29) investigating change in VAT compared with abdominal SAT found no sex differences but noted a preferential loss of VAT that was greatest with modest weight loss and was attenuated or absent when large amounts of weight were lost. Following up on these observations and those of Smith and Zachwieja (30), Hallgreen and Hall (21) proposed that changes in VAT are described by an allometric relationship where the change of VAT to the change of fat mass (FM) is proportional to the initial ratio of VAT to FM, i.e., dVAT/dFM = k ×VAT/FM, where k is a constant (21). With a constant of 1.3, the model appeared to fit a variety of weight-loss interventions, including bariatric surgery, caloric restriction with or without exercise, and both sexes. In a recent report, de Souza et al. (22) applied this allometric model to data from the POUNDS LOST trial and found the constant to be significantly different for men and women: adjusted for baseline ratios of visceral to total FM, women lost more visceral fat than did men (k = 1.32 vs. 1.15, respectively, at 6 months). Using the same inclusion criteria as de Souza et al. (22) (weight loss 5 kg or more and losses in both VAT and FM compartments), we found no sex difference in preferential VAT loss (k = 1.37 ± 0.13, n = 11 for men; 1.38 ± 0.14, n = 16 for women), consistent with the original report of Hallgreen and Hall (21). The reason for these differences between studies remains, at present, unknown and the issue of preferential VAT loss unresolved.
Implications of Changes in Regional Adipose Depots for Metabolic Risk
The interest in understanding how specific AT depots respond to a weight-loss intervention derives from observations in cross-sectional studies that different AT depots are independently associated with different metabolic risk (31). Excess VAT is associated with risk factors for coronary artery disease (32) and type 2 diabetes (2) and an impairment in the response of the liver to insulin (33). Whole-body IMAT is an important independent correlate of IR (23). Femoral-gluteal SAT and femoral-gluteal IMAT have independent and opposing relationships with CVD risk factors (24). Lower amounts of upper-leg SAT (i.e., femoral-gluteal AT) have been found to play a role in IR (3), and greater amounts of upper-leg SAT (possibly through a triglyceride storage function) may exert a degree of cardio-protection having been found to relate favorably to glucose and lipid levels (24,34).
In the current study, weight loss in overweight and obese females and males with type 2 diabetes at 1-year ILI was strongly associated with reductions in fasting plasma glucose and HbA1c, confirming numerous prior observations of the positive effects of weight loss to improve glycemic control. Moreover, in this study with its longitudinal data, we confirmed some of the cross-sectional associations between AT distribution and metabolic control. VAT change was associated with changes in glycemic control, dyslipidemia, and BP. Taken together, the VAT change was significantly associated with eight biomarkers of metabolic control, and four of these approached or exceeded significance after controlling for weight: fasting glucose, cholesterol/HDL ratio, triglycerides, and diastolic BP. This is consistent with cross-sectional associations of VAT with serum glucose, lipids, and BP (35,36). The associations seen in this study of trunk SAT change with metabolic parameters followed a pattern quite similar to that observed for VAT and thus are consistent with reports of greater metabolic risk with upper-body obesity. We did not, however, observe evidence of a protective effect of lower-body fat, specifically leg SAT. Instead, positive associations of change (decreases) in leg SAT with decreases in glucose, improvements in dyslipidemia, and reductions in BP were noted in our within-subject data. These results pertaining to change in leg SAT conformed closely to the pattern of associations found for change in VAT and in trunk SAT. This close similarity of associations might be a consequence of a pervasive similarity rather than regional differences of IR across AT depots in type 2 diabetes. While the data obtained on regional volumes of AT do not enable a corresponding assessment of potential regional variations in AT IR, the congruence of associations between metabolic parameters with change in weight (which was largely attributable to loss of AT) and change in VAT, trunk SAT, and leg SAT, respectively, would seem consistent with a hypothesis of AT IR that is more similar than diverse across regional AT depots in type 2 diabetes and argues against an AT depot that affords protection against IR.
The moderating effects of sex on associations of AT change with lipid variables (e.g., in males, SAT associates with total cholesterol, in females, with HDL-C) may indicate genuine sex differences in associations with AT depot change as, for instance, reported by Therkelsen et al. (37) for trunk IMAT and dyslipidemia in men in the Framingham study.
There is a current debate as to whether VAT or other AT depots are causal or merely correlated with CVD risk, and attempts have been made to investigate the link by surgical alteration of depot size. In the case of VAT, investigators have surgically removal intraabdominal fat in animal models (38) and in humans, either alone or as an adjunct to bariatric surgery, with mixed results (39,40). The influence of abdominal SAT on metabolic markers has been investigated in studies before and after liposuction, with negative results (41). The evidence for a causal role of AT depots from surgical studies, therefore, is not conclusive.
The reason that many of the associations of AT change with metabolic markers were observed only in the ILI group may be that although there was a range of weight changes in both treatment groups, the DSE group included more persons who gained weight or lost relatively small amounts of weight, hence there was less opportunity for a clear relationship to emerge.
Study Limitations
The sample size for this study was reduced by the necessity of excluding a large number of subjects who started or stopped taking a medication related to the metabolic markers being studied. Also, the inclusion of medicated subjects who remained on medication for the duration of the study period may have muted any effect of AT compartment change on the metabolic marker. The study of regional AT involves segmenting compartments into smaller subcompartments, which increases measurement error. Furthermore, our measurements of SAT did not distinguish superficial from deep SAT, which may be metabolically different compartments. Finally, the investigation of associations of 10 AT compartments with 9 biomarkers with adjustment for weight change necessarily involves a large number of statistical tests, hence the reported significant findings need to be confirmed in subsequent studies.
Conclusion
A 1-year ILI in overweight and obese adults with type 2 diabetes resulted in a weight loss of 7–10% and reduced SAT and VAT, but not IMAT. The DSE group did not lose weight and experienced an increase in trunk and leg IMAT. Within-person changes in TAT, SAT, and VAT mass were associated with changes in CVD risk factors in a fashion similar to associations seen in cross-sectional studies.
Article Information
Acknowledgments. The authors thank Else Ruts and Qing He (project coordinators), Elli Ioannidou (dual-energy X-ray absorptiometry analyst), and Mark Punyanitya (MRI analysis quality control) of St. Luke’s-Roosevelt Hospital and Jowanda Green and Carol Kelley (project coordinators) of the Obesity/Nutrition Research Center, University of Pittsburgh.
Funding. This work was supported by National Institutes of Health grants R01-HL-070298, P30-DK-26687, P30-DK-046204, and UL-1RR-024156.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.G. was involved in study design, data collection in New York, data analysis, manuscript writing and provided administrative support and supervision. S.H. and J.T. were involved in data analysis and manuscript writing. D.E.K. was principal investigator at the Pittsburgh Look AHEAD site, was coinvestigator on this study, and was responsible for data collection in Pittsburgh and manuscript writing. L.B. was involved in MRI quality control for type 2 diabetes data collected at both sites. F.X.P.-S. is principal investigator at the New York Look AHEAD site, was coinvestigator on this study, and contributed to administrative support and manuscript writing. J.P. was the project coordinator for the New York Look AHEAD site. J.M. was a lifestyle interventionist for the Look AHEAD study in Pittsburgh and was involved in recruitment for this ancillary study. J.M.C. is a Look AHEAD coinvestigator at the Johns Hopkins site and was involved in the design of the DSE intervention as well as the safety monitoring plan for the study. S.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Appendix
The MRI Ancillary Study Working Group (Look AHEAD Trial) consists of Dympna Gallagher, EdD; David E. Kelley, MD; Stanley Heshka, PhD; John Thornton, PhD; Lawrence Boxt, MD; Isaiah Janumala, MD; Lance Davidson, PhD; F. Xavier Pi-Sunyer, MD; Jennifer Patricio, MS; and Juliet Mancino, MS.
Footnotes
A complete list of members of the MRI Ancillary Study Working Group (Look AHEAD Trial) can be found in the appendix.
References
- 1.Gallagher D, Kelley DE, Yim JE, et al. MRI Ancillary Study Group of the Look AHEAD Research Group . Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr 2009;89:807–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr 2000;71:885–892 [DOI] [PubMed] [Google Scholar]
- 3.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med 2005;165:777–783 [DOI] [PubMed] [Google Scholar]
- 4.Azuma K, Heilbronn LK, Albu JB, Smith SR, Ravussin E, Kelley DE, Look AHEAD Adipose Research Group . Adipose tissue distribution in relation to insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 2007;293:E435–E442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eastwood SV, Tillin T, Wright A, et al. Thigh fat and muscle each contribute to excess cardiometabolic risk in South Asians, independent of visceral adipose tissue. Obesity (Silver Spring) 2014;22:2071–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallagher D, Kovera AJ, Clay-Williams G, et al. Weight loss in postmenopausal obesity: no adverse alterations in body composition and protein metabolism. Am J Physiol Endocrinol Metab 2000;279:E124–E131 [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Kuk JL, Davidson LE, et al. Exercise without weight loss is an effective strategy for obesity reduction in obese individuals with and without Type 2 diabetes. J Appl Physiol (1985) 2005;99:1220–1225 [DOI] [PubMed] [Google Scholar]
- 8.Davidson LE, Hudson R, Kilpatrick K, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med 2009;169:122–131 [DOI] [PubMed] [Google Scholar]
- 9.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Look AHEAD Research Group . Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 2007;30:1374–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albu JB, Murphy L, Frager DH, Johnson JA, Pi-Sunyer FX. Visceral fat and race-dependent health risks in obese nondiabetic premenopausal women. Diabetes 1997;46:456–462 [DOI] [PubMed] [Google Scholar]
- 11.Ryan AS, Nicklas BJ. Age-related changes in fat deposition in mid-thigh muscle in women: relationships with metabolic cardiovascular disease risk factors. Int J Obes Relat Metab Disord 1999;23:126–132 [DOI] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care 2003;26:372–379 [DOI] [PubMed] [Google Scholar]
- 13.Van Pelt RE, Jankowski CM, Gozansky WS, Schwartz RS, Kohrt WM. Lower-body adiposity and metabolic protection in postmenopausal women. J Clin Endocrinol Metab 2005;90:4573–4578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snijder MB, Dekker JM, Visser M, et al. Hoorn study . Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care 2004;27:372–377 [DOI] [PubMed] [Google Scholar]
- 15.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD Research Group . Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–628 [DOI] [PubMed] [Google Scholar]
- 16.Wadden TA, West DS, Delahanty L, et al. Look AHEAD Research Group . The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it [published correction appears in Obesity (Silver Spring) 2007;15:1339]. Obesity (Silver Spring) 2006;14:737–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakicic JM, Jaramillo SA, Balasubramanyam A, et al. Look AHEAD Study Group . Effect of a lifestyle intervention on change in cardiorespiratory fitness in adults with type 2 diabetes: results from the Look AHEAD Study. Int J Obes (Lond) 2009;33:305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albu JB, Heilbronn LK, Kelley DE, et al. Look AHEAD Adipose Research Group . Metabolic changes following a 1-year diet and exercise intervention in patients with type 2 diabetes. Diabetes 2010;59:627–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr 2004;79:874–880 [DOI] [PubMed] [Google Scholar]
- 20.Snyder WS, Cooke MJ, Mnassett ES, Larhansen LT, Howells GP, Tipton IH. Report of the Task Group on Reference Man. Oxford, Pergamon, 1975 [Google Scholar]
- 21.Hallgreen CE, Hall KD. Allometric relationship between changes of visceral fat and total fat mass. Int J Obes (Lond) 2008;32:845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Souza RJ, Bray GA, Carey VJ, et al. Effects of 4 weight-loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: results from the POUNDS LOST trial. Am J Clin Nutr 2012;95:614–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr 2005;82:1210–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yim JE, Heshka S, Albu J, et al. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int J Obes (Lond) 2007;31:1400–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doucet E, St-Pierre S, Alméras N, et al. Reduction of visceral adipose tissue during weight loss. Eur J Clin Nutr 2002;56:297–304 [DOI] [PubMed] [Google Scholar]
- 26.Gasteyger C, Larsen TM, Vercruysse F, Pedersen D, Toubro S, Astrup A. Visceral fat loss induced by a low-calorie diet: a direct comparison between women and men. Diabetes Obes Metab 2009;11:596–602 [DOI] [PubMed] [Google Scholar]
- 27.Janssen I, Ross R. Effects of sex on the change in visceral, subcutaneous adipose tissue and skeletal muscle in response to weight loss. Int J Obes Relat Metab Disord 1999;23:1035–1046 [DOI] [PubMed] [Google Scholar]
- 28.Chaston TB, Dixon JB. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes (Lond) 2008;32:619–628 [DOI] [PubMed] [Google Scholar]
- 29.Chaston TB, Dixon JB, O’Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond) 2007;31:743–750 [DOI] [PubMed] [Google Scholar]
- 30.Smith SR, Zachwieja JJ. Visceral adipose tissue: a critical review of intervention strategies. Int J Obes Relat Metab Disord 1999;23:329–335 [DOI] [PubMed] [Google Scholar]
- 31.Bjørndal B, Burri L, Staalesen V, Skorve J, Berge RK. Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J Obes 2011;2011:490650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang YK, Chen M, Clements RH, Abrams GA, Aprahamian CJ, Harmon CM. Human mesenteric adipose tissue plays unique role versus subcutaneous and omental fat in obesity related diabetes. Cell Physiol Biochem 2008;22:531–538 [DOI] [PubMed] [Google Scholar]
- 33.Virtanen KA, Lönnroth P, Parkkola R, et al. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J Clin Endocrinol Metab 2002;87:3902–3910 [DOI] [PubMed] [Google Scholar]
- 34.Snijder MB, Visser M, Dekker JM, et al. Health ABC Study . Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 2005;48:301–308 [DOI] [PubMed] [Google Scholar]
- 35.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007;116:39–48 [DOI] [PubMed] [Google Scholar]
- 36.Rhéaume C, Arsenault BJ, Bélanger S, et al. Low cardiorespiratory fitness levels and elevated blood pressure: what is the contribution of visceral adiposity? Hypertension 2009;54:91–97 [DOI] [PubMed] [Google Scholar]
- 37.Therkelsen KE, Pedley A, Speliotes EK, et al. Intramuscular fat and associations with metabolic risk factors in the Framingham Heart Study. Arterioscler Thromb Vasc Biol 2013;33:863–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabriely I, Ma XH, Yang XM, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes 2002;51:2951–2958 [DOI] [PubMed] [Google Scholar]
- 39.Fabbrini E, Tamboli RA, Magkos F, et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology 2010;139:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dillard TH, Purnell JQ, Smith MD, et al. Omentectomy added to Roux-en-Y gastric bypass surgery: a randomized, controlled trial. Surg Obes Relat Dis 2013;9:269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klein S, Fontana L, Young VL, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med 2004;350:2549–2557 [DOI] [PubMed] [Google Scholar]