Abstract
In diabetic retinopathy, neovascularization is hypothesized to develop due to hypoxia in the retina. However, evidence for retinal hypoxia is limited, and the progressive changes in oxygenation are unknown. The objective of this study was to determine if retinal hypoxia occurs early in the development of diabetes. Intraretinal oxygen (PO2) profiles were recorded with oxygen-sensitive microelectrodes in control and diabetic Long-Evans rats at 4 and 12 weeks after induction of diabetes. Diabetes did not affect oxygen consumption in the photoreceptors in either dark or light adaptation. Oxygenation of the inner retina was not affected after 4 weeks of diabetes, although vascular endothelial growth factor levels increased. At 12 weeks, average inner retinal PO2, normalized to choriocapillaris PO2, was higher in diabetic rats than in age-matched controls, which was opposite to what was expected. Thus retinal hypoxia is not a condition of early diabetes in rat retina. Increased inner retinal PO2 may occur because oxygen consumption decreases in the inner retina.
Introduction
Diabetic retinopathy is clinically defined as damage to the retinal vasculature. The early clinical changes, which include microaneurysms and small intraretinal hemorrhages, are followed by capillary loss and, eventually, neovascularization. Increased vascular endothelial growth factor (VEGF) is an important mediator of these vascular abnormalities and a major contributor to the pathology of diabetic retinopathy (1).
Tissue hypoxia is one of the possible causes of increased VEGF. Hypoxia is implicated in the pathogenesis of diabetic retinopathy (2), but its onset, severity, and role in VEGF upregulation are still unclear. Several studies have provided evidence of hypoxia in the diabetic retina. Direct intraretinal oxygen measurements in cats with long-term diabetes (6–8 years) showed that there was inner retinal hypoxia, but this study was limited to three animals at one time point (3). Also, oxygenation across the inner retina was heterogeneous, with some areas exhibiting hypoxia and other areas showing normal oxygenation. A second study suggested that the inner retina was hypoxic in rats at 5 weeks, but few details were available (4). Finally, diabetic mice showed, after 5 months, increased inner retinal staining with pimonidazole HCl, a histochemical marker for tissue hypoxia (5). Studies in humans also provide indirect evidence of retinal hypoxia. In diabetic patients with minimal retinopathy, breathing 100% oxygen significantly improved contrast sensitivity but had no effect on the contrast sensitivity of control subjects without diabetes (6). Some animal studies do not show hypoxia in diabetes, but these studies have been confined to vitreal PO2 measurements, which may be less sensitive than intraretinal measurements, and have generally been performed at relatively early time points (7–9). At a very late time point, in humans with proliferative retinopathy, the vitreous has been shown to have lower PO2 in diabetic rats than in controls (10,11)
The purpose of these experiments was to begin to determine the timing and extent of oxygenation changes in diabetic rats with intraretinal PO2 measurements and relate oxygenation to other pathological changes in these animals (12). While rats do not exhibit all of the vascular changes seen in human patients with diabetes, they do lose pericytes and endothelial cells (13), have increased VEGF (12,14), and have substantial changes in retinal gene expression (15,16). Here we have recorded intraretinal PO2 profiles in rats at 4 and 12 weeks after the induction of diabetes, with the hypothesis that diabetic rats would have lower PO2 in the inner retina than controls.
Research Design and Methods
Induction of Diabetes
Animal experiments were performed in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by Northwestern University’s Institutional Animal Care and Use Committee. Diabetes was induced in male Long-Evans rats between 50 and 57 days old with a single intraperitoneal injection of streptozotocin (Axxora LLC, San Diego, CA; 65 mg/kg rat, 6.5 mg/mL) in 0.05 mol/L sodium citrate buffer (pH 5). Rats with blood glucose levels greater than 300 mg/dL 2 days after induction were deemed diabetic. The rats were not treated with insulin. Age-matched controls received a single intraperitoneal injection of 0.05 mol/L sodium citrate buffer only (0.01 mL/g rat). Rats were weighed weekly (Supplementary Fig. 1A), and nonfasting blood glucose levels were measured from the tail vein using a Bayer CONTOUR Meter (Bayer HealthCare LLC, Mishawaka, IN). The meter read “HI” if blood glucose exceeded 600 mg/dL. Those readings were set at 600 mg/dL for the purpose of averaging (Supplementary Fig. 1B). Diabetic animals had blood glucose levels of 500 mg/dL or higher; controls had blood glucose levels of ∼120 mg/dL.
Experimental Design
The rats were divided into four groups: control and diabetic rats at 4 or 12 weeks after induction of diabetes. Table 1 gives the number of animals in each group. PO2 data were collected during dark and light adaptation from all groups. The first priority was to make the measurements in dark adaptation (3–12 profiles/rat). PO2 profiles were collected from a smaller number of animals in light adaptation using illumination sufficient to saturate rod responses in the electroretinogram (∼80 lux). Data from 9 out of 10 control animals used in this study were also included in a study on normal rat retina (17).
Table 1.
Physiological parameters for each group as measured during intraretinal PO2 recordings
| 4 weeks |
12 weeks |
|||
|---|---|---|---|---|
| Control | Diabetic | Control | Diabetic | |
| Number of animals* | 4 (4) | 5 (4) | 6 (5) | 5 (3) |
| Arterial pH | 7.32 ± 0.06 | 7.36 ± 0.03 | 7.37 ± 0.01 | 7.28 ± 0.03 |
| PaCO2 (mmHg) | 44.6 ± 5.8 | 36.7 ± 2.3 | 39.1 ± 1.2 | 45.0 ± 5.4 |
| PaO2 (mmHg) | 111.0 ± 6.2 | 110.2 ± 5.6 | 98.4 ± 4.5 | 96.1 ± 2.1 |
| Blood glucose (mg/dL) | 136 ± 6 | 354 ± 48 | 134 ± 14 | 359 ± 44 |
| Temperature (°C) | 38.2 ± 0.6 | 37.3 ± 0.4 | 37.8 ± 0.1 | 38.5 ± 0.3 |
| Arterial pressure (mmHg) | 98 ± 6 | 99 ± 4 | 98 ± 4 | 90 ± 10 |
| Heart rate (bpm) | 395 ± 4 | 365 ± 12 | 360 ± 11 | 348 ± 15 |
Values are given as n or mean ± SEM.
*The number of rats from which light-adapted profiles were also collected appears in parentheses.
At ∼4 and 12 weeks after induction of diabetes, the rats were anesthetized with isoflurane for surgery and urethane during recordings, and PO2 depth profiles were recorded from the retina in vivo using double-barreled oxygen/voltage microelectrodes. Electrode preparation, animal preparation, and data collection and analysis were performed as previously described (17). After completing surgical preparations, the animal was paralyzed with pancuronium bromide and artificially ventilated. An arterial sample was taken shortly after paralysis for measurement of blood gases and glucose. Arterial values were adjusted if necessary by changes in the tidal volume provided by the respirator and the fraction of inspired oxygen. Thereafter, arterial samples were taken at ∼1 h intervals and adjusted if necessary. The blood gas and glucose values during the experiments are given in Table 1. Arterial blood pressure was measured via the arterial cannula, and if average pressure fell below ∼75 mmHg, the animal was given 6% hydroxyethyl starch (Hespan or Vetastarch) intravenously.
The eccentricity of penetrations could not be obtained, but the entry point of the electrode in the eye and the electrode angle were calculated to place the recordings in central retina, usually at one anterior-posterior “latitude,” and over an ∼30° arc of nasal-temporal locations.
Statistics
All values in the text and figures are reported as mean ± SEM unless stated otherwise. Statistical significance was determined using a two-factorial ANOVA with two levels in each factor (2 × 2 ANOVA) and was defined as P < 0.05. The factors for the ANOVA were time point (levels 4 and 12 weeks) and treatment (levels control and diabetic). ANOVA results are shown in Supplementary Data. Fisher protected least significant difference was used for post hoc analysis.
Results
Intraretinal PO2 Profiles
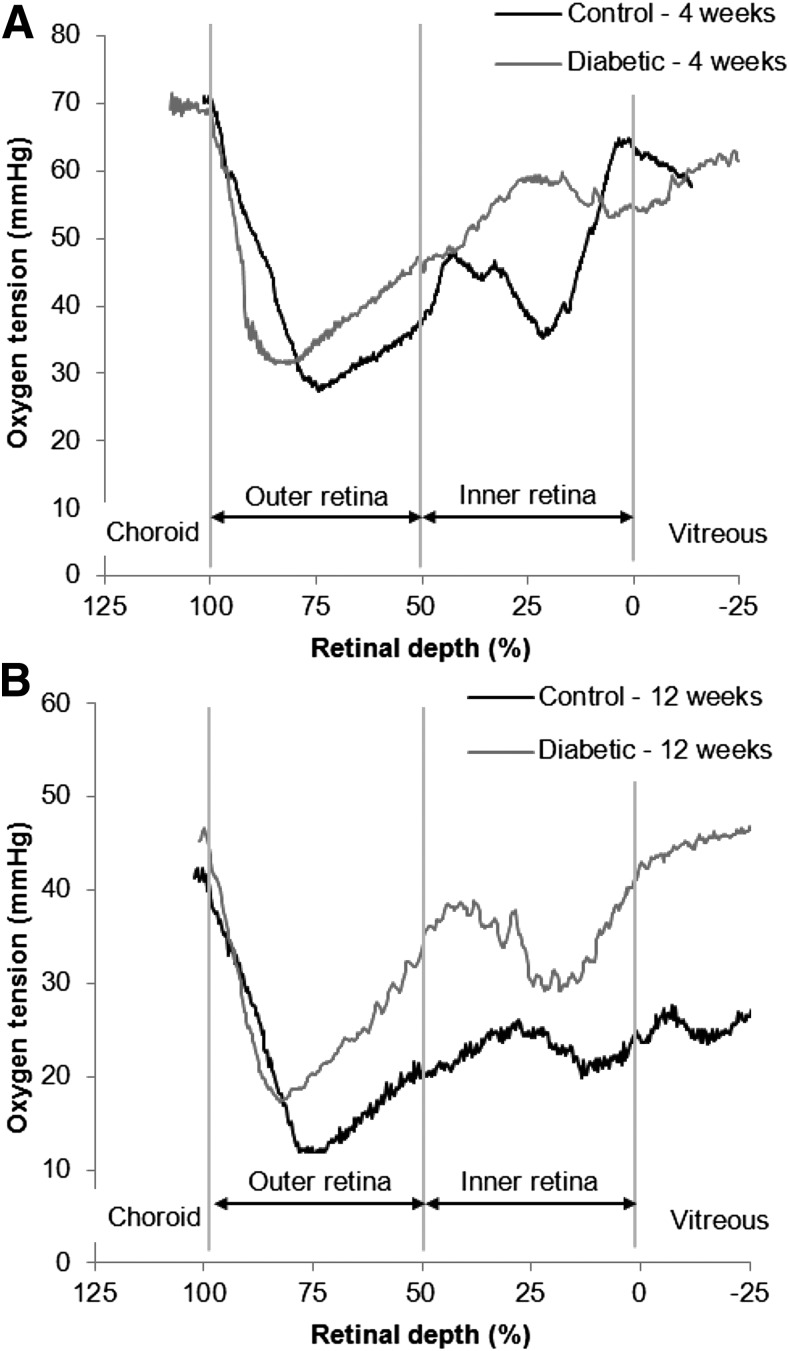
The PO2 profiles were similar for control and diabetic rats at both time points, as shown by representative intraretinal PO2 profiles obtained during dark adaptation (Fig. 1A, 4 weeks; Fig. 1B, 12 weeks). The major features of intraretinal oxygen profiles in rats were described previously (17,18). The outer retina, where the photoreceptors lie, is avascular and is located at 50–100% retinal depth. The minimum PO2 in the outer retina (Pmin) occurs at ∼75–85% retinal depth and corresponds to the photoreceptor inner segments. The inner retina is located at 0–50% retinal depth and is characterized by peaks corresponding to the location of the retinal vasculature.
Figure 1.
Example intraretinal PO2 profiles from control and diabetic rats at 4 weeks (A) and 12 weeks (B). The choroid is located beyond 100% retinal depth. The outer retina, where the photoreceptors lie, is avascular and is located at 50–100% retinal depth. The minimum PO2 in the outer retina (Pmin) occurs in the inner segment layer. The location of Pmin in profiles can be affected by whether the electrode pulls on the retina and was not consistently different between diabetic rats and controls. The inner retina is located at 0–50% retinal depth and the vitreous is located at <0% retinal depth.
Characteristics of Outer Retinal Oxygenation in Diabetic Rat Retina
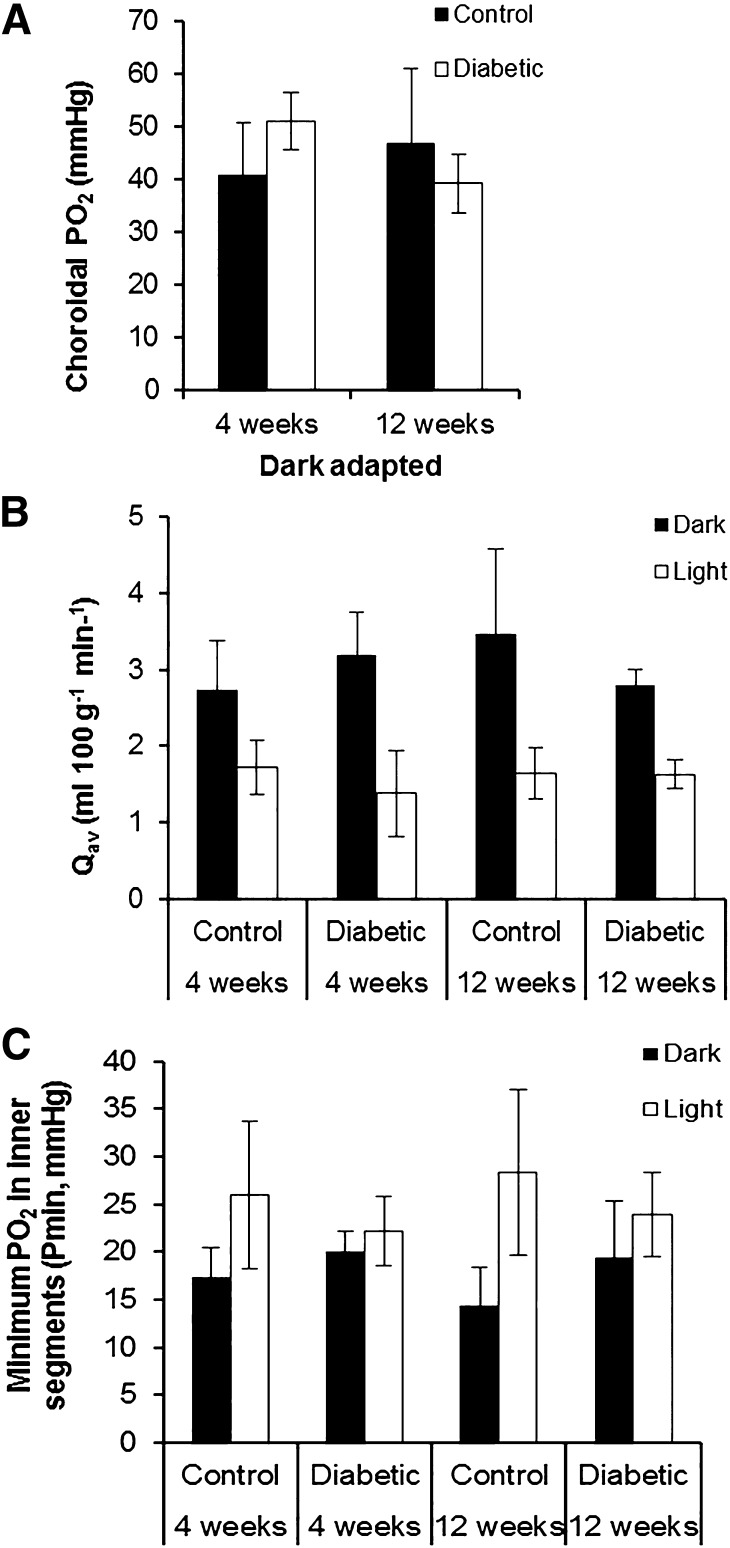
Choroidal PO2 (PC) was not different between control and diabetic rats at either time point (Fig. 2A, Supplementary Table 1). Photoreceptor oxygen consumption (Qav), obtained by fitting a model to the data (17), was also not significantly different between control and diabetic rats in dark or light adaptation (Fig. 2B, Supplementary Table 2). Light adaptation decreased Qav to 51–65% of the dark-adapted value in each group, and diabetes had no effect on the light-induced change in Qav.
Figure 2.
Characteristics of outer retinal oxygenation were comparable for control and diabetic rats after 4 and 12 weeks of diabetes. A: The dark-adapted choroidal PO2 was not statistically different between the four groups. Black bars represent controls; white bars represent diabetic rats. B: Photoreceptor oxygen consumption (Qav) in both dark and light adaptation was comparable in the control and diabetic animals at 4 and 12 weeks after induction of diabetes. Light adaptation decreased Qav compared with dark adaptation by a similar magnitude in all four groups. Black bars represent dark adaptation; white bars represent light adaptation. C: Minimum PO2 in inner segments of photoreceptors (Pmin) was comparable for control and diabetic rats at 4 and 12 weeks. Light adaptation increased Pmin in all four groups. Black bars represent dark adaptation; white bars represent light adaptation. All error bars are SEM.
Diabetes also had no effect on the minimum PO2 in the outer retina (Pmin) in dark adaptation (Fig. 2C, Supplementary Table 3). As expected, light adaptation increased Pmin for all groups, and diabetes had no effect on light-adapted Pmin.
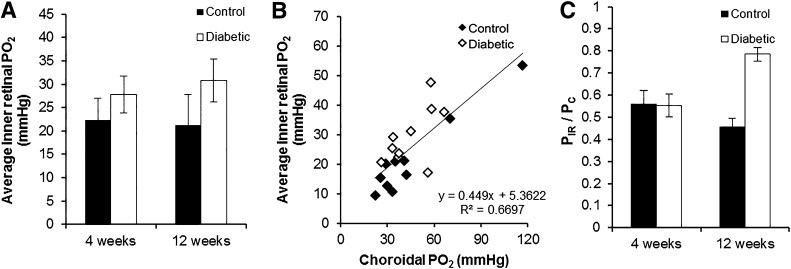
Average Inner Retinal PO2
The average inner retinal PO2 was computed for each profile over the inner 50% of the retina (PIR), and an average was then obtained across profiles for each animal. PIR during dark adaptation appeared to be higher in the diabetic animals than in the controls, but the differences were not significant (Fig. 3A, Supplementary Table 4). However, in analyzing these data, we found that PIR was significantly correlated with PC in the same animal (Fig. 3B). Since there was variability within and between groups, and because part of this correlation could be caused by errors applying the in vitro calibration to the in vivo data, PIR was normalized to choroidal PO2 (PIR/PC) for each rat (Fig. 3C). When analyzed this way, ANOVA showed a significant main effect for the treatment factor (diabetes; P = 0.005) and a significant interaction between time point and treatment (P = 0.004) (Supplementary Table 5). Post hoc Fisher protected least significant difference showed that diabetic rats at 12 weeks had significantly higher PIR/PC than the other three groups (P < 0.005). All other comparisons were not significant.
Figure 3.
A: The average inner retinal PO2 (PIR) in control and diabetic rats was not significantly different after 4 and 12 weeks. B: Correlation between PIR and choroidal PO2 (PC) for each rat. C: PIR normalized to PC, which showed that PIR/PC was comparable between control rats at 4 and 12 weeks and diabetic rats at 4 weeks. However, PIR/PC was significantly higher in diabetic rats at 12 weeks than the three other groups (P < 0.005). In A and C, black bars represent control; white bars represent diabetic; and error bars are SEM.
Discussion
This study is only the third set of intraretinal PO2 measurements in any diabetic animal (3,4). As expected, diabetes did not affect outer retinal oxygenation or oxygen consumption. The choroidal PO2, Pmin, and Qav were comparable for the control and diabetic rats at both time points. Also, the response to light adaptation was similar for all four groups. All the physiological parameters indicated that the outer retina is unaffected in the early stages of diabetes.
Diabetes ultimately damages the vasculature of the inner retina. Therefore, the inner retinal oxygenation was hypothesized to decrease in diabetes, but the result was opposite to what was expected. Instead of hypoxia, diabetes caused PIR/PC at 12 weeks to increase by ∼45% compared with age-matched controls. The confidence in this result is reinforced by a study using a very different technique, pimonidazole and immunofluorescence labeling, which showed that 12-week Long-Evans diabetic rats had significantly less staining in all layers of the retina than controls (19). This also strongly suggests a relative increase in inner retinal PO2 in diabetic rats at this time point and tends to rule out the possibility that the present result arose from recording selectively from different parts of the retina in control versus diabetic animals. The increase in PIR could result from two possible causes. First, diabetes could cause retinal blood flow to increase, thereby bringing more oxygen to the inner retina and imbalancing the relation between supply and demand. No information is available about blood flow in pigmented rats. In albino (Sprague-Dawley and Wistar) rats, retinal blood flow decreased at 1, 2, 4, and 6 weeks after induction of diabetes compared with controls (20,21). There is evidence of molecular differences among rat strains with respect to diabetes (16), so blood flow may also be affected differently in different strains. In the only previous intraretinal PO2 measurements in rats, the inner retina was reported to be hypoxic at 5 or 6 weeks (4). This was reported very briefly, but based on previous work from the same group, it is likely that these were Sprague-Dawley rats.
The second possible cause for increased PIR/PC could be decreased oxygen consumption in the inner retina due to hyperglycemia-induced dysfunction or loss of neurons, assuming that blood flow does not concomitantly increase. We currently do not have methods to quantify inner retinal oxygen consumption in vivo. The oxygen profiles were collected in one dimension, and modeling oxygen consumption in the inner retina is a three-dimensional problem because of the presence of the retinal circulation. Nevertheless, other studies show neural cell loss in diabetes, which could lead to decreased oxygen consumption. Streptozotocin-induced diabetes significantly increased apoptosis and ganglion cell loss in Sprague-Dawley (22) and Brown Norway (23) rat retinas beginning as early as 1 month. Molecular data in Long-Evans rats also indicates neural dysfunction (12). In humans, retinal venous oxygen saturation is elevated even in background retinopathy (24,25), which may correspond to the present results in rats and could also be caused by decreased metabolism.
The time points used in this study, 4 and 12 weeks after induction of diabetes, still represent very early stages in diabetes. We and others have shown that VEGF protein levels are higher in diabetic rats at 4 weeks (12,14,16,23) and now can be certain that this is not caused by hypoxia. At 12 weeks of diabetes in rats, however, VEGF is not elevated relative to control (12,14), which is consistent with the lack of hypoxia at this time. VEGF levels are elevated again in rats at 6 months (26). Retinal oxygenation may continue to change as the disease progresses, so retinal hypoxia should not yet be ruled out as a characteristic of long-standing diabetes in rats.
Supplementary Material
Article Information
Funding. This study was funded in part by National Eye Institute training grant T32 EY007128 and National Eye Institute grant R01 EY021165.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.C.M.L. performed all experiments and was primarily responsible for data analysis and a draft of the manuscript. R.A.L. performed all experiments and edited and revised the manuscript. R.A.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the Annual Meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, FL, 2–6 May 2010.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db14-0101/-/DC1.
References
- 1.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med 2012;366:1227–1239 [DOI] [PubMed] [Google Scholar]
- 2.Frank RN. Diabetic retinopathy. N Engl J Med 2004;350:48–58 [DOI] [PubMed] [Google Scholar]
- 3.Linsenmeier RA, Braun RD, McRipley MA, et al. Retinal hypoxia in long-term diabetic cats. Invest Ophthalmol Vis Sci 1998;39:1647–1657 [PubMed] [Google Scholar]
- 4.Yu DY, Cringle S, Yu PK, et al. Retinal cellular metabolism and its regulation and control. In Neurovascular Medicine: Pursuing Cellular Longevity for Healthy Aging. Maiese K, Ed. New York, Oxford University Press, 2009, p. 69–100 [Google Scholar]
- 5.de Gooyer TE, Stevenson KA, Humphries P, Simpson DA, Gardiner TA, Stitt AW. Retinopathy is reduced during experimental diabetes in a mouse model of outer retinal degeneration. Invest Ophthalmol Vis Sci 2006;47:5561–5568 [DOI] [PubMed] [Google Scholar]
- 6.Harris A, Arend O, Danis RP, Evans D, Wolf S, Martin BJ. Hyperoxia improves contrast sensitivity in early diabetic retinopathy. Br J Ophthalmol 1996;80:209–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alder VA, Yu DY, Cringle SJ, Su EN. Changes in vitreal oxygen tension distribution in the streptozotocin diabetic rat. Diabetologia 1991;34:469–476 [DOI] [PubMed] [Google Scholar]
- 8.Ernest JT, Goldstick TK, Engerman RL. Hyperglycemia impairs retinal oxygen autoregulation in normal and diabetic dogs. Invest Ophthalmol Vis Sci 1983;24:985–989 [PubMed] [Google Scholar]
- 9.Stefansson E, Peterson JI, Wang YH. Intraocular oxygen tension measured with a fiber-optic sensor in normal and diabetic dogs. Am J Physiol 1989;256:H1127–H1133 [DOI] [PubMed] [Google Scholar]
- 10.Holekamp NM, Shui YB, Beebe D. Lower intraocular oxygen tension in diabetic patients: possible contribution to decreased incidence of nuclear sclerotic cataract. Am J Ophthalmol 2006;141:1027–1032 [DOI] [PubMed] [Google Scholar]
- 11.Lange CA, Stavrakas P, Luhmann UF, et al. Intraocular oxygen distribution in advanced proliferative diabetic retinopathy. Am J Ophthalmol 2011;152:406–412 [DOI] [PubMed]
- 12.Lau JC, Kroes RA, Moskal JR, Linsenmeier RA. Diabetes changes expression of genes related to glutamate neurotransmission and transport in the Long-Evans rat retina. Mol Vis 2013;19:1538–1553 [PMC free article] [PubMed] [Google Scholar]
- 13.Engerman RL, Kern TS. Retinopathy in animal models of diabetes. Diabetes Metab Rev 1995;11:109–120 [DOI] [PubMed] [Google Scholar]
- 14.Schrufer TL, Antonetti DA, Sonenberg N, Kimball SR, Gardner TW, Jefferson LS. Ablation of 4E-BP1/2 prevents hyperglycemia-mediated induction of VEGF expression in the rodent retina and in Muller cells in culture. Diabetes 2010;59:2107–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brucklacher RM, Patel KM, VanGuilder HD, et al. Whole genome assessment of the retinal response to diabetes reveals a progressive neurovascular inflammatory response. BMC Med Genomics 2008;1:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirwin SJ, Kanaly ST, Linke NA, Edelman JL. Strain-dependent increases in retinal inflammatory proteins and photoreceptor FGF-2 expression in streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci 2009;50:5396–5404 [DOI] [PubMed] [Google Scholar]
- 17.Lau JCM, Linsenmeier RA. Oxygen consumption and distribution in the Long-Evans rat retina. Exp Eye Res 2012;102:50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cringle SJ, Yu DY, Yu PK, Su EN. Intraretinal oxygen consumption in the rat in vivo. Invest Ophthalmol Vis Sci 2002;43:1922–1927 [PubMed] [Google Scholar]
- 19.Wright WS, McElhatten RM, Messina JE, Harris NR. Hypoxia and the expression of HIF-1alpha and HIF-2alpha in the retina of streptozotocin-injected mice and rats. Exp Eye Res 2010;90:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higashi S, Clermont AC, Dhir V, Bursell SE. Reversibility of retinal flow abnormalities is disease-duration dependent in diabetic rats. Diabetes 1998;47:653–659 [DOI] [PubMed] [Google Scholar]
- 21.Pouliot M, Hétu S, Lahjouji K, Couture R, Vaucher E. Modulation of retinal blood flow by kinin B₁ receptor in Streptozotocin-diabetic rats. Exp Eye Res 2011;92:482–489 [DOI] [PubMed] [Google Scholar]
- 22.Barber AJ, Lieth E, Khin SA, Antonetti DA, Buchanan AG, Gardner TW. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. J Clin Invest 1998;102:783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kusari J, Zhou S, Padillo E, Clarke KG, Gil DW. Effect of memantine on neuroretinal function and retinal vascular changes of streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci 2007;48:5152–5159 [DOI] [PubMed] [Google Scholar]
- 24.Hammer M, Vilser W, Riemer T, et al. Diabetic patients with retinopathy show increased retinal venous oxygen saturation. Graefes Arch Clin Exp Ophthalmol 2009;247:1025–1030 [DOI] [PubMed] [Google Scholar]
- 25.Hardarson SH, Stefánsson E. Retinal oxygen saturation is altered in diabetic retinopathy. Br J Ophthalmol 2012;96:560–563 [DOI] [PubMed] [Google Scholar]
- 26.Hammes HP, Lin J, Bretzel RG, Brownlee M, Breier G. Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes 1998;47:401–406 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.