Abstract
Purpose
We aim to characterize infarct volume evolution within the first month post-ischemic stroke and to determine the effect of recanalization status on early infarct volume estimation.
Methods
Ischemic stroke patients recruited for the MONITOR and VISION studies were retrospectively screened and patients who had infarcts on diffusion-weighted imaging (DWI) at baseline and had at least two follow-up MR scans (n = 56) were included. Pre-defined target imaging time points, obtained on a 3-T MR scanner, were 12 hours (h), 24 h, 7 days, and ≥30 days post-stroke. Infarct tissue was manually traced blinded to the images at the other time points. Infarct expansion index was calculated by dividing infarct volume at each follow-up time point by the baseline DWI infarct volume. Recanalization was assessed within 24 h post-stroke. Correlation and statistical comparison analysis were done using the Spearman, Mann–Whitney, and Kruskal–Wallis tests.
Results
Follow-up infarct volumes were positively correlated with the baseline infarct volume (ρ > 0.81; p < 0.001) where the strongest correlation existed between baseline and 7-day post-stroke infarct volumes (ρ = 0.92; p < 0.001). The strongest correlation among the follow-up imaging was found between infarct volumes 7-day post-stroke and ≥30-day time points (ρ = 0.93; p < 0.001). Linear regression showed a close-to unity slope between 7-day and final infarct volumes (slope = 1.043; p < 0.001). Infarct expansion was higher in the non-recanalized group than the recanalized group at the 7-day (p = 0.001) and ≥30-day (p = 0.038) time points.
Conclusions
Final infarct volume can be approximated as early as 7 days post-stroke. Final infarct volume approximation is significantly associated with recanalization status.
Keywords: MRI, Infarct volume, Ischemic stroke, Sub-acute, Recanalization
Highlights
-
•
We characterize infarct volume evolution within the first month post-stroke.
-
•
We show that final infarct volume can be approximated 7 days post-stroke.
-
•
Nonrecanalized patients had a stronger correlation in 7-day vs final infarct volume.
1. Introduction
Final infarct volume is a clinically useful measure associated with the stroke functional outcome (Lev et al., 2001). It is also linked to multiple other baseline radiological scores such as the Alberta Stroke Program Early Computed Tomography Score (ASPECTS) (Coutts et al., 2004), clot burden score (Tan et al., 2009), and clinical parameters, e.g., National Institute of Health Stroke Scale (NIHSS) (Saver et al., 1999). Final infarct volume depicts the extent of infarct growth and provides a gold standard criterion to study the efficacy of treatment/management strategies (Olivot et al., 2008; Eilaghi et al., 2014). While infarct progression is widely accepted to be a dynamic process, final infarct volume has been measured at different time points in these various studies, such as after 90 days (Warach et al., 2000; Warach et al., 2006; Davis et al., 2008), 30 days, (Olivot et al., 2008; Hacke et al., 2005; Furlan et al., 2006) and 5 days (Wheeler et al., 2013; Lemmens et al., 2013). There is a tendency to use earlier time points in more recent Phase 2 clinical trials and proof-of concept studies to minimize patient drop out, minimize stroke-related complications, and reduce the overall study costs. Gaudinski et al. (2008) for example, showed that the infarct volume stabilized 30 days post-stroke compared to 3 months in 19 patients with acute stroke.
Recanalization is among the strongest predictors of clinical outcome and final infarct volume in patients with acute ischemic stroke (Rha, and Saver, 2007). Early recanalization is believed to improve the chance of survival in affected ischemic tissues. Therefore, it is reasonable to assume that recanalization status would change the infarct volume evolution. However, the exact association of infarct volume evolution with recanalization has not been quantified, particularly at the sub-acute stage. For example, it is unclear whether recanalization status affects the estimation of infarct volume size at the sub-acute stage. Recently, a linear association was reported between infarct volumes at the sub-acute (3–6 days) and chronic (>30 days) stages with both time points showing equivalent predictive value of the clinical outcome in a highly recanalized (68%) cohort (Tourdias et al., 2011). However, prediction of the final infarct volume at the early time points may be more useful in patients who do not recanalize. In such patients, prolonged follow-up is difficult to attain, as these patients are prone to clinical complications.
This study aimed to characterize infarct volume expansion in a population of patients with an intracranial occlusion who are at risk for infarct expansion after the baseline scans. We hypothesized that scanning at 7 days would be a good surrogate of final infarct volume.
2. Methods
2.1. Patient selection
This is a retrospective analysis of patients who enrolled in the MONITOR (Simon et al., 2005) or VISION (Coutts et al., 2008; Coutts et al., 2011) studies. The institutional research ethics board approved both studies and patients or their surrogates provided written informed consent. The VISION and MONITOR studies were imaging studies completed in acute ischemic stroke and TIA patients. A subset of patients with evidence of a middle cerebral artery (MCA) occlusion had serial imaging completed as part of these studies. Patients were eligible for the current analysis if they had baseline imaging including diffusion weighted (DWI) imaging (<6 h), follow-up fluid attenuated inversion recovery (FLAIR) imaging (>30 days), and at least one further FLAIR imaging at a pre-defined time point between these 2 scans. All patients had evidence of an MCA occlusion on MRA.
2.2. Image acquisition
Pre-defined target imaging time points included: baseline (<6 h), 12 h, 24 h, 7 days, and >30 days after stroke onset. Images were obtained using a 3 T MRI (Signa VH/i; GE Healthcare) with high performance gradients (40 mT/m; 184 µs rise time) using a standard quadrature head coil and established stroke imaging protocols (Lauzon et al., 2006). Single-shot echo-planar imaging was used for diffusion-weighted images (b = 0 s mm−2 and isotropic b = 1000 s mm−2; repetition time = 9000 ms; echo time = min [80–90 ms]; 240 mm field-of-view; 5.0 mm slice thickness with a 0 or 2 mm gap). ADC maps were derived from the b = 0 s mm−2 and b = 1000 s mm−2 images (DeVetten et al., 2010). FLAIR images were acquired with repetition time = 9002 ms; echo time = 140 ms; inversion time = 2250 ms; 240 mm field-of-view; 3.0 mm slice thickness; and 2.0 mm gap. MR angiography (MRA) was performed using 3D time-of-flight imaging using repetition time = 22 ms; echo time = 3.3 ms; flip angle = 15°; acquisition bandwidth of ±12.5 kHz; and a 320 × 256 × 44 mm acquired volume.
2.3. Image analysis
Images were assessed blinded to patient identifiers and time points by tracing lesions on DWI and FLAIR images in CEREBRA medical imaging software, (Gobbi et al., 2012) (M.K., A.E.) and edited using MIPAV (Medical Image Processing, Analysis, and Visualization, BIRSS; NIH, Bethesda, Maryland, USA). Acute lesions were identified on baseline DWI while follow-up lesions were identified on FLAIR images. The infarct regions were confirmed by a stroke neurologist (M.A.). Infarct expansion index was calculated by finding the ratio of the infarct volume on FLAIR imaging at each time point to the baseline DWI infarct volume. To assess the effect of initial infarct volume, the cohort was then split into patients with large (≥10 ml; top 20th percentile by volume) and small (<10 ml) infarct baseline infarct volumes. Recanalization was assessed on MRA within 24 h post-stroke using the Thrombolysis In Myocardial Infarction (TIMI) (Anon, 1985) recanalization score (M.A.). TIMI scores of 2 and 3 (partial and complete recanalization) were considered evidence of positive recanalization.
2.4. Statistical analysis
Values are reported as mean and standard deviation (SD) unless otherwise noted. Normality was tested using the Kolmogorov–Smirnov tests. The difference between lesion volumes at different time points was investigated using the Mann–Whitney and Kruskal–Wallis rank tests. The relationship between lesion volumes at different time points was investigated using a Spearman rank correlation test. p < 0.05 was considered statistically significant.
3. Results
Overall 964 patients were screened from both MONITOR (Simon et al., 2005) and VISION (Coutts et al., 2008; Coutts et al., 2011) studies, and 59 patients were eligible for inclusion in this sub-study. One patient with evidence of acute hemorrhage and two patients with significant motion artifact were excluded, leaving a total of 56 patients included in this analysis. The numbers of scans at each imaging time point were 56 (baseline), 17 (12-hour), 29 (1-day), 56 (7-day), and 56 (>30 day). Patient demographics are shown in Table 1. Post-onset interval for the DWI was 2 ± 2 h (range: 0.5–6 h), and for the follow-up FLAIR imaging at the pre-defined nominal study time points was: 12 h (13 ± 8 h, range: 4–26 h), 1 day (1.1 ± 0.3 day, range: 0.7–1.9 day), 7 days (6.5 ± 2.7 days, range: 3–11 days), and >30 days (57 ± 11 days, range: 22–111 days).
Table 1.
Patient baseline characteristics.
| Characteristics | Value |
|---|---|
| Mean age years (SD) | 68 (12) |
| Female sex, number (%) | 24 (43) |
| Hypertension, number (%) | 30 (54) |
| Mean glucose level (mmol/l) (SD) | 7.2 (2.6) |
| Diabetes mellitus, number (%) | 9 (16) |
| Right hemisphere (%) | 27 (48) |
| Mean systolic pressure (mm Hg) (SD) | 156.2 (28.9) |
| Mean diastolic pressure (mm Hg) (SD) | 82.5 (18.9) |
| Mean baseline NIHSS (SD) | 5 (5) |
| Thrombolytic treatment, number (%) | 12 (21) |
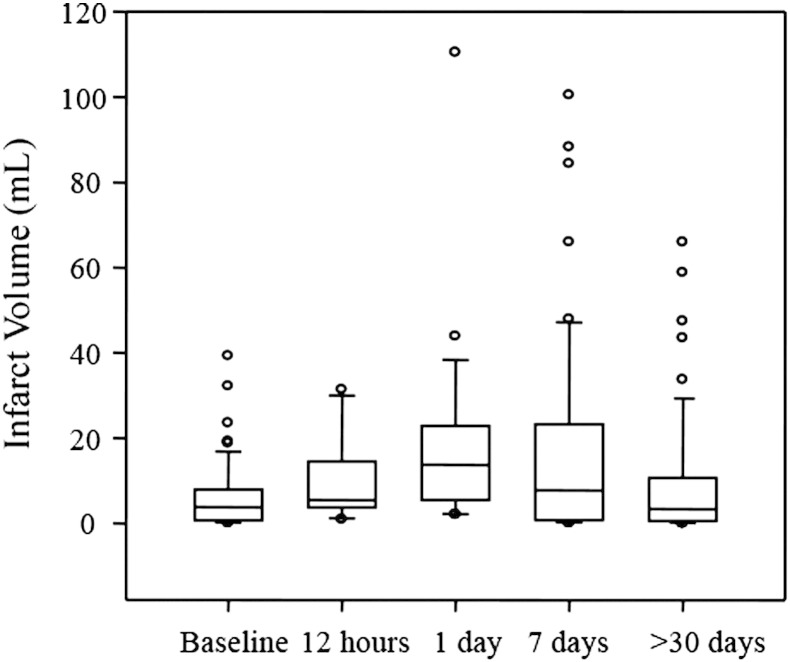
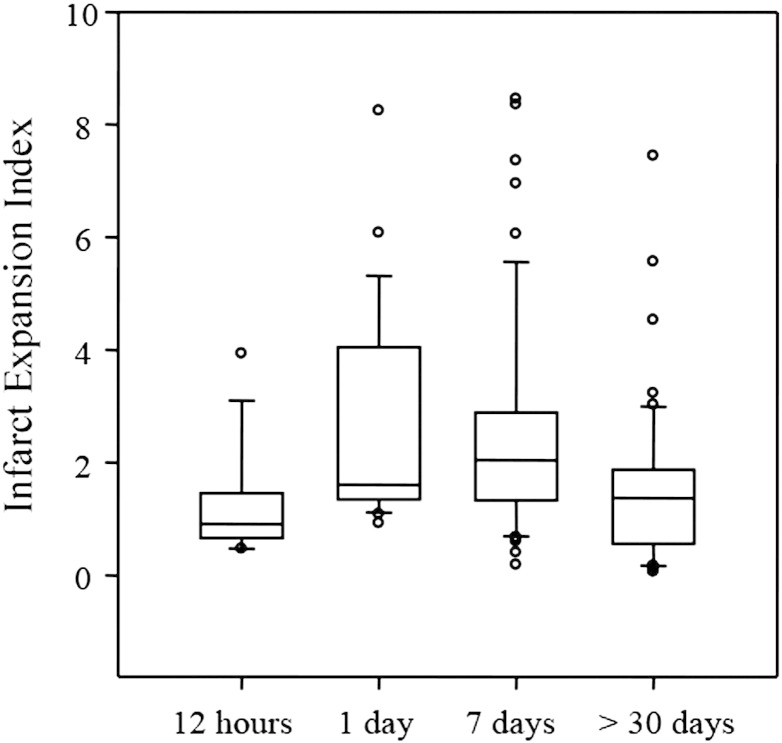
An example of infarct volume evolution at different time points is shown in Fig. 1. Infarct volume at each time point were: 6.45 ± 8.03 ml (baseline), 9.32 ± 9.33 ml (12-hour), 18.81 ± 21.40 ml (1-day), 16.86 ± 23.07 ml (7-day) and 9.51 ± 14.84 ml (>30 day). The measured infarct volumes across all time points for all patients were not normally distributed (Kolmogorov–Smirnov normality tests, p ≤ 0.003). Fig. 2 shows the median and quartile ranges for infarct volume. The differences in the median values of infarct volumes were significantly different (Kruskal–Wallis test, p ≤ 0.001). The mean ± SD values of the infarct expansion index by follow-up time were: 1.27 ± 0.97 (12-hour), 2.59 ± 1.80 (1-day), 2.44 ± 1.88 (7-day) and 1.52 ± 1.36(>30 day). Similar to the infarct volumes, the infarct expansion indices were significantly different (Kruskal–Wallis test, p ≤ 0.001). The distribution of infarct expansion indices including median and quartile ranges is presented in Fig. 3.
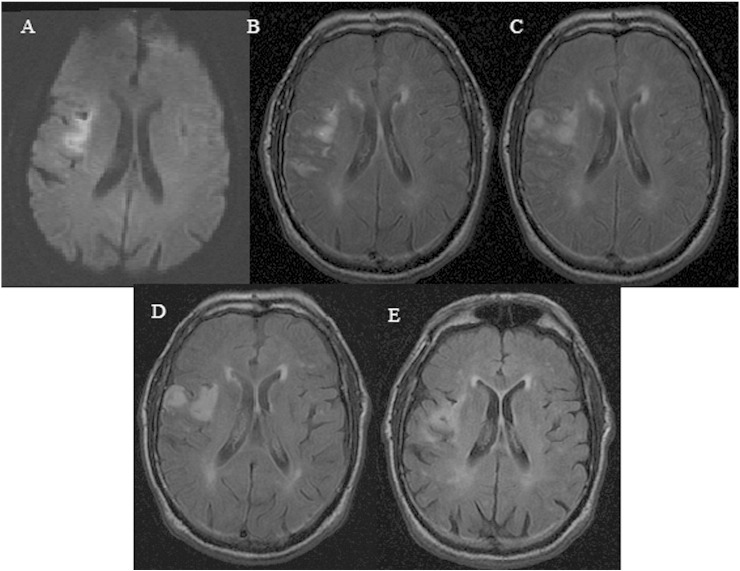
Fig. 1.
Infarct volume changes in a 79-year-old male patient presented with left-sided weakness and occlusion of the superior division of the right MCA. This figure represents diffusion-weighted imaging (DWI) at 5 hours after onset (A) and consecutive fluid attenuated inversion recovery (FLAIR) imaging sessions obtained 12 hours (B), 28 hours (C), 5 days (D) and 40 days (E) post stroke.
Fig. 2.
Infarct volume distribution measured at consecutive imaging time points. In this box plot, the ends of the boxes define the 25th and 75th percentiles, with a line at the median and error bars extending to the 10th and 90th percentiles.
Fig. 3.
Infarct expansion index, calculated as the infarct volume divided by lesion size on the baseline DWI, declines after the sub-acute phase within the first week post-stroke. Ends of the boxes define the 25th and 75th percentiles, with a line representing the median value and error bars extending to the 10th and 90th percentiles.
Spearman rank correlation tests showed high level of correlation between infarct volumes measured at multiple time points. Correlation coefficients (ρ) ranged from 0.80 to 0.93 (p < 0.001 across all 10 (4 + 3 + 2 + 1) pairwise Spearman rank correlation combinations). The largest correlation coefficient (ρ = 0.93) was found between 7-day and final time points, whereas the smallest correlation coefficient (ρ = 0.80) was found between 24-h and final infarct volumes.
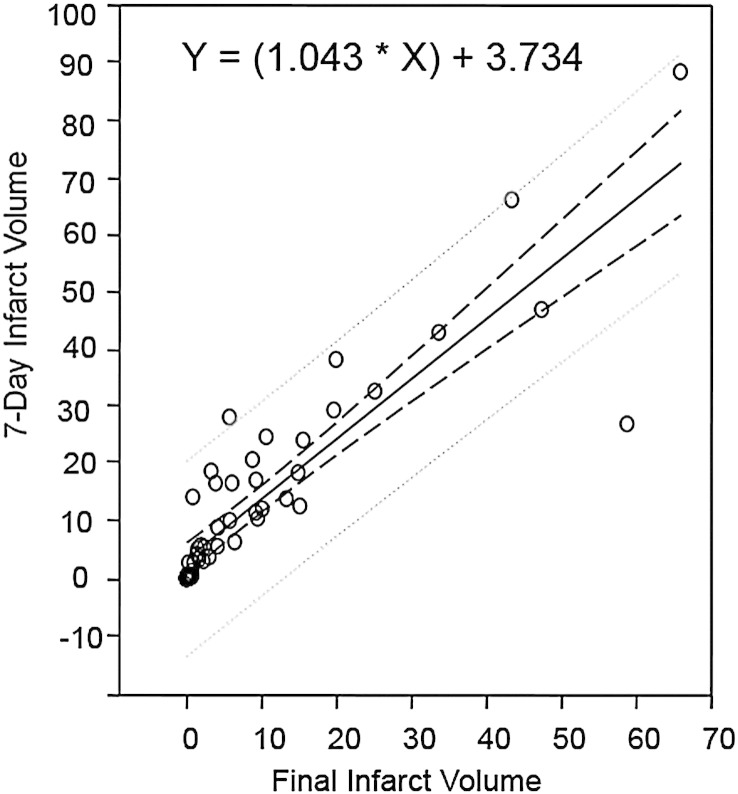
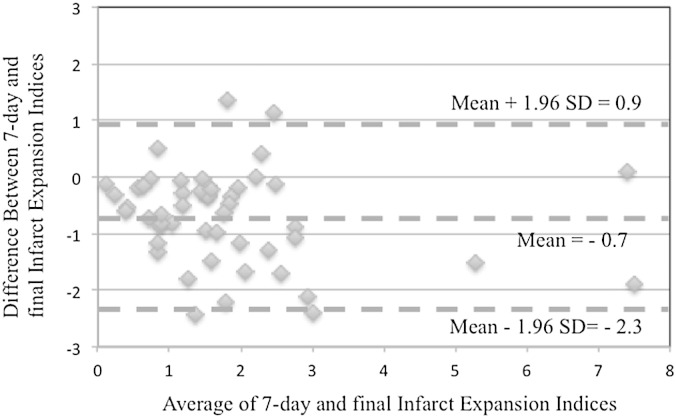
The correlation coefficient and associated p value between 7-day and final infarct volumes in the smaller baseline infarct volume group were ρ = 0.919 and p < 0.001, and in the larger baseline infarct volume group were ρ = 0.833 and p = 0.002. A linear regression analysis of 7-day and final infarct volumes showed a slope of 1.04 (p < 0.001) and intercept of 3.734 (p = 0.009) with R = 0.89 (Fig. 4). Bland-Altman analysis found a mean = −0.7 and standard deviation of 0.8 in difference between infarct expansion indices at 7-day and final assessment time points (Fig. 5).
Fig. 4.
Linear regression analysis of 7-day and final infarct volumes. The solid line represents the regression line and dashed lines represent the 95% prediction and confidence intervals. Calculated p values for slope (1.043) and intercept (3.734) were <0.001 and 0.009, respectively.
Fig. 5.
Bland-Altman analysis of infarct expansion at 7-day and final assessment time points.
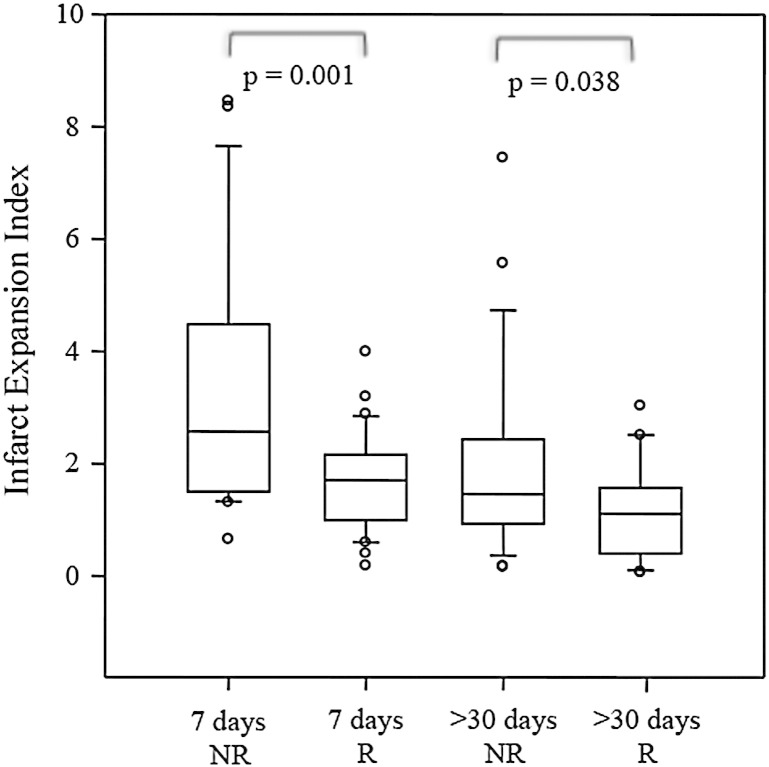
Patients with successful recanalization (n = 30) had a lower infarct expansion index than the non-recanalized group (n = 15) at the 7-day (Mann–Whitney test, p = 0.001) and final (Mann–Whitney test, p = 0.038) assessment time points (Fig. 6). Patients in the non-recanalized group showed a stronger Spearman correlation between 7-day and final infarct volumes (ρ = 0.727; p = 0.009) than those in the recanalized group (ρ = 0.566, p = 0.002). No significant differences were found in infarct expansion of patients treated (n = 9) and not-treated (n = 45) with intra-venous tPA at the 7-day (Mann–Whitney test, p = 0.712) and final (Mann–Whitney test, p = 0.623) assessment time points.
Fig. 6.
Infarct expansion was significantly different in recanalized (R) and non-recanalized (NR) groups at both 7-day and final (>30-day) time points. Ends of the boxes define the 25th and 75th percentiles, with a line at the median and error bars extending to the 10th and 90th percentiles.
4. Discussion
This study demonstrates the feasibility of approximating final infarct volume based on 1-week post-stroke volumes. While infarct volumes were significantly different at all time-points, the strongest correlation was found between infarct volume assessments at 7-day and >30 day time points. Further, patients with negative recanalization status showed even stronger correlations between 7-day and >30 day final infarct volumes.
Early estimation of the final infarct volume can substantially minimize dropout rates from clinical trials, decrease costs, and reduce the need for repeat imaging. Our data are consistent with the recent literature showing some level of overestimation in the infarct volume 7 days post-stroke, compared to volumes after a month (Figs. 2 and 3) (Gaudinski et al., 2008). However, a strong correlation between later infarct volumes is to be expected. A Bland-Altman analysis of the infarct expansion at these two time points suggests that approximation of the final volume is feasible 7 days post-onset. This finding is also consistent with a previous study (Tourdias et al., 2011), which showed that the 3–6 day assessments of infarct volume provide the same predictive value of the stroke clinical outcome as chronic volume assessments. High correlation between the 7-day and >30 day infarct volumes supports a similar correlation pattern between NIHSS and infarct volume assessed after sub-acute, and chronic time points (Ebinger et al., 2009).
We found stronger correlations between early (7-day) and final (>30 day) assessments in patients who failed to achieve recanalization. This observation indicates that in these patients the infarct core tends to expand into the penumbra in the absence of recanalization. Therefore, the FLAIR imaging abnormality seen on early assessments without recanalization represents an accurate approximation of the final infarct region. In addition, early assessment of the final infarct volume in these patient cohorts is important because these patients are typically impaired clinically and are more likely to be excluded from clinical trials due to loss of long-term follow-up. This exclusion can lead to biased interpretation of the study results since the focus would be on patients who recanalized and experienced a less severe stroke. For example, it has been shown that replacing the 90-day infarct volume assessment with the one week assessment resulted in five times lower patient exclusion due to lack of follow-up (Tourdias et al., 2011). Infarct volume assessment after the first week appears to be sufficient to demonstrate treatment efficacy (Ebinger et al., 2009) and supports the desirability for earlier imaging endpoint particularly in Phase 2 clinical trials and proof-of-concept studies (MR Stroke Collaborative Group et al., 2006). Early follow-up imaging has been already adopted in a multicenter prospective cohort study to assess response to endovascular reperfusion (Lansberg et al., 2012).
Early estimation of the final infarct volume may also have important implications for rehabilitation planning. The correlation between clinical parameters and imaging estimation of the final infarct core can be used to guide the selection of candidates who will likely benefit from rehabilitation. The significant association between limited baseline infarct volume and improved functional recovery after stroke is established (Heiss et al., 2000). Within the first few weeks post-onset, the presence of pre-infarct edema can result in functional impairment and blurs the estimation of the final infarct core (Rosenberg, 1999). Therefore, unmasking areas of edema through early approximation of the final infarct volume may help predict functional recovery and thus intensify the rehabilitation of such patients (Hu et al., 2010) for improved functional outcome.
This study is subject to several limitations. The data set used does not include imaging for all patients at all time-points. Fifty-six patients entered the study with baseline DWI within 6 h of onset. The smallest group was the early follow-up assessment after 12 h (n = 17). However, the majority of patients had imaging at all other time points. In our study, the average infarct volume and the median baseline NIHSS are lower than the typical ischemic stroke cohort (Eilaghi et al., 2014). Patients with smaller stroke volumes were more likely to have imaging at multiple time points and particularly after 30 days post-stroke. Therefore, results may not necessarily apply to patients with larger or proximal infarcts. This perhaps explains why only the top 20th percentile of the cohort had baseline infarct volume >10 ml. Of note, the presented 56 patients were screened from 964 patients that participated in two single-center clinical trials (Simon et al., 2005; Coutts et al., 2008; Coutts et al., 2011). Despite the reduced sample size, we were able to identify a significantly higher correlation between 7-day and infarct volumes in those with negative recanalization status. Recanalization status was obtained during the first 24 h, but only provides a single time measure of vessel state. In this study it was not possible to accurately determine time of recanalization. Finally, our protocol did not obtain perfusion maps at follow-up imaging; reperfusion (occurring spontaneously or achieved due to intervention) is known to impact on the dynamic of infarct evolution post-stroke (Eilaghi et al., 2013).
In summary, final infarct volume approximation is significantly associated with recanalization status of the patient. Final infarct volumes can be estimated as early as 7 days post-stroke potentially increasing inclusion rate for clinical trials, and reducing the need for repeated imaging, and decreasing healthcare clinical costs.
Conflicts of interest
None.
Sources of funding
The Vascular Imaging of acute Stroke for Identifying predictors of clinical Outcome and recurrent ischemic events (VISION) study was supported by grant funding from the Canadian Institutes for Health Research (CIHR MOP-118096) and Heart and Stroke Foundation of Alberta, NWT, and Nunavut (HSFA). The Modeling the evolution of Infarcting Tissue in the setting of Occlusion and Recanalization (MONITOR) study was funded by HSFA. The 3.0-T MR scanner in the Seaman Family MR Research Centre used in this study was partially funded by Canada Foundation for Innovation. AMD is supported by a salary award from Alberta Innovates-Health Solutions (AI-HS) and the HSFA Chair in Stroke Research. SBC received salary support from the AI-HS and the Heart and Stroke Foundation of Canada (HSFC) Distinguished Clinician Scientist award, supported in partnership with the Canadian Institute of Health Research (CIHR), Institute of Circulatory and Respiratory Health and AstraZeneca Canada, Inc. RF was supported by the Canada Research Chair Program and is the Hopewell Professor of Brain Imaging. AE was supported with University of Calgary Eyes' High and Natural Sciences and Engineering Research Council (NSERC) of Canada CREATE Program fellowships.
Contributor Information
Mark Krongold, Email: markkrongold@gmail.com.
Mohammed A. Almekhlafi, Email: malmekhl@ucalgary.ca.
Andrew M. Demchuk, Email: ademchuk@ucalgary.ca.
Shelagh B. Coutts, Email: scoutts@ucalgary.ca.
Richard Frayne, Email: rfrayne@ucalgary.ca.
Armin Eilaghi, Email: aeilaghi@ucalgary.ca.
References
- MR Stroke Collaborative Group. Phan T.G., Donnan G.A., Davis S.M., Byrnes G. Proof-of-principle phase II MRI studies in stroke: sample size estimates from dichotomous and continuous data. Stroke. 2006;37:2521–2525. doi: 10.1161/01.STR.0000239696.61545.4b. 16931782 [DOI] [PubMed] [Google Scholar]
- Anon The thrombolysis in myocardial infarction (TIMI) trial. Phase I findings. TIMI study group. N. Engl. J. Med. 1985;312(14):932–936. doi: 10.1056/NEJM198504043121437. 4038784 [DOI] [PubMed] [Google Scholar]
- Coutts S.B., Eliasziw M., Hill M.D., Scott J.N., Subramaniam S., Buchan A.M. An improved scoring system for identifying patients at high early risk of stroke and functional impairment after an acute transient ischemic attack or minor stroke. Int J Stroke. 2008;3(1):3–10. doi: 10.1111/j.1747-4949.2008.00182.x. 18705908 [DOI] [PubMed] [Google Scholar]
- Coutts S.B., Hill M.D., Eliasziw M., Fischer K., Demchuk A.M., VISION study group Final 2 year results of the vascular imaging of acute stroke for identifying predictors of clinical outcome and recurrent ischemic eveNts (VISION) study. BMC Cardiovasc. Disord. 2011;11:18. doi: 10.1186/1471-2261-11-18. 21513559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts S.B., Lev M.H., Eliasziw M., Roccatagliata L., Hill M.D., Schwamm L.H. ASPECTS on CTA source images versus unenhanced CT: added value in predicting final infarct extent and clinical outcome. Stroke. 2004;35(11):2472–2476. doi: 10.1161/01.STR.0000145330.14928.2a. 15486327 [DOI] [PubMed] [Google Scholar]
- Davis S.M., Donnan G.A., Parsons M.W., Levi C., Butcher K.S., Peeters A. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7(4):299–309. doi: 10.1016/S1474-4422(08)70044-9. 18296121 [DOI] [PubMed] [Google Scholar]
- DeVetten G., Coutts S.B., Hill M.D., Goyal M., Eesa M., O’Brien B. Acute corticospinal tract Wallerian degeneration is associated with stroke outcome. Stroke. 2010;41(4):751–756. doi: 10.1161/STROKEAHA.109.573287. 20203322 [DOI] [PubMed] [Google Scholar]
- Ebinger M., Christensen S., De Silva D.A., Parsons M.W., Levi C.R., Butcher K.S. Expediting MRI-based proof-of-concept stroke trials using an earlier imaging end point. Stroke. 2009;40(4):1353–1358. doi: 10.1161/STROKEAHA.108.532622. 19246703 [DOI] [PubMed] [Google Scholar]
- Eilaghi A., Brooks J., d’Esterre C., Zhang L., Swartz R.H., Lee T.Y. Reperfusion is a stronger predictor of good clinical outcome than recanalization in ischemic stroke. Radiology. 2013;269(1):240–248. doi: 10.1148/radiol.13122327. 23716707 [DOI] [PubMed] [Google Scholar]
- Eilaghi A., d’Esterre C.D., Lee T.Y., Jakubovic R., Brooks J., Liu R.T. Toward patient-tailored perfusion thresholds for prediction of stroke outcome. AJNR Am. J. Neuroradiol. 2014;35(3):472–477. doi: 10.3174/ajnr.A3740. 24113471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan A.J., Eyding D., Albers G.W., Al-Rawi Y., Lees K.R., Rowley H.A. Dose escalation of desmoteplase for acute ischemic stroke (dedas): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke. 2006;37(5):1227–1231. doi: 10.1161/01.STR.0000217403.66996.6d. 16574922 [DOI] [PubMed] [Google Scholar]
- Gaudinski M.R., Henning E.C., Miracle A., Luby M., Warach S., Latour L.L. Establishing final infarct volume: stroke lesion evolution past 30 days is insignificant. Stroke. 2008;39(10):2765–2768. doi: 10.1161/STROKEAHA.107.512269. 18635854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi D.G., Lu Q., Frayne R., Salluzi M. Cerebra-WML: a rapid workflow for quantification of white matter hyperintensities. Canadian Stroke Congress. 2012 [Google Scholar]
- Hacke W., Albers G., Al-Rawi Y., Bogousslavsky J., Davalos A., Eliasziw M. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke. 2005;36(1):66–73. doi: 10.1161/01.STR.0000149938.08731.2c. 15569863 [DOI] [PubMed] [Google Scholar]
- Heiss W.D., Kracht L., Grond M., Rudolf J., Bauer B., Wienhard K. Early [(11)c]Flumazenil/h(2)o positron emission tomography predicts irreversible ischemic cortical damage in stroke patients receiving acute thrombolytic therapy. Stroke. 2000;31(2):366–369. doi: 10.1161/01.str.31.2.366. 10657407 [DOI] [PubMed] [Google Scholar]
- Hu M.H., Hsu S.S., Yip P.K., Jeng J.S., Wang Y.H. Early and intensive rehabilitation predicts good functional outcomes in patients admitted to the stroke intensive care unit. Disabil. Rehabil. 2010;32(15):1251–1259. doi: 10.3109/09638280903464448. 20131942 [DOI] [PubMed] [Google Scholar]
- Lansberg M.G., Straka M., Kemp S., Mlynash M., Wechsler L.R., Jovin T.G. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11(10):860–867. doi: 10.1016/S1474-4422(12)70203-X. 22954705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzon M.L., Sevick R.J., Demchuk A.M., Frayne R. Stroke imaging at 3.0 T. Neuroimaging Clin. N. Am. 2006;16(2):343–366. doi: 10.1016/j.nic.2006.02.004. 16731371 [DOI] [PubMed] [Google Scholar]
- Lemmens R., Mlynash M., Straka M., Kemp S., Bammer R., Marks M.P. Comparison of the response to endovascular reperfusion in relation to site of arterial occlusion. Neurology. 2013;81(7):614–618. doi: 10.1212/WNL.0b013e3182a08f07. 23851962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev M.H., Segal A.Z., Farkas J., Hossain S.T., Putman C., Hunter G.J. Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: prediction of final infarct volume and clinical outcome. Stroke. 2001;32(9):2021–2028. doi: 10.1161/hs0901.095680. 11546891 [DOI] [PubMed] [Google Scholar]
- Olivot J.M., Mlynash M., Thijs V.N., Kemp S., Lansberg M.G., Wechsler L. Relationships between infarct growth, clinical outcome, and early recanalization in diffusion and perfusion imaging for understanding stroke evolution (DEFUSE) Stroke. 2008;39(8):2257–2263. doi: 10.1161/STROKEAHA.107.511535. 18566302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rha J.H., Saver J.L. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38(3):967–973. doi: 10.1161/01.STR.0000258112.14918.24. 17272772 [DOI] [PubMed] [Google Scholar]
- Rosenberg G.A. Ischemic brain edema. Prog Cardiovasc Dis. 1999;42(3):209–216. doi: 10.1016/s0033-0620(99)70003-4. 10598921 [DOI] [PubMed] [Google Scholar]
- Saver J.L., Johnston K.C., Homer D., Wityk R., Koroshetz W., Truskowski L.L. Infarct volume as a surrogate or auxiliary outcome measure in ischemic stroke clinical trials. The RANTTAS investigators. Stroke. 1999;30(2):293–298. doi: 10.1161/01.str.30.2.293. 9933262 [DOI] [PubMed] [Google Scholar]
- Simon J.E., Bristow M.S., Lu H., Lauzon M.L., Brown R.A., Manjón J.V. A novel method to derive separate gray and white matter cerebral blood flow measures from MR imaging of acute ischemic stroke patients. J. Cereb. Blood Flow Metab. 2005;25(9):1236–1243. doi: 10.1038/sj.jcbfm.9600130. 15889045 [DOI] [PubMed] [Google Scholar]
- Tan I.Y., Demchuk A.M., Hopyan J., Zhang L., Gladstone D., Wong K. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am. J. Neuroradiol. 2009;30(3):525–531. doi: 10.3174/ajnr.A1408. 19147716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourdias T., Renou P., Sibon I., Asselineau J., Bracoud L., Dumoulin M. Final cerebral infarct volume is predictable by MR imaging at 1 week. AJNR Am. J. Neuroradiol. 2011;32(2):352–358. doi: 10.3174/ajnr.A2271. 20966063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warach S., Kaufman D., Chiu D., Devlin T., Luby M., Rashid A. Effect of the Glycine Antagonist Gavestinel on cerebral infarcts in acute stroke patients, a randomized placebo-controlled trial: the GAIN MRI substudy. Cerebrovasc. Dis. 2006;21(1-2):106–111. doi: 10.1159/000090208. 16340185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warach S., Pettigrew L.C., Dashe J.F., Pullicino P., Lefkowitz D.M., Sabounjian L. Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 investigators. Ann. Neurol. 2000;48(5):713–722. 11079534 [PubMed] [Google Scholar]
- Wheeler H.M., Mlynash M., Inoue M., Tipirneni A., Liggins J., Zaharchuk G. Early diffusion-weighted imaging and perfusion-weighted imaging lesion volumes forecast final infarct size in DEFUSE 2. Stroke. 2013;44(3):681–685. doi: 10.1161/STROKEAHA.111.000135. 23390119 [DOI] [PMC free article] [PubMed] [Google Scholar]