Abstract
Background
Endometrial cancer is the most common genital tract carcinoma among women in developed countries, with most women presenting with stage 1 disease. Adjuvant progestagen therapy has been advocated following primary surgery to reduce the risk of recurrence of disease.
Objectives
To evaluate the effectiveness and safety of adjuvant progestagen therapy for the treatment of endometrial cancer.
Search methods
We searched the Cochrane Gynaecological Cancer Group Trials Specilaised Register, Cochrane Central Register of Controlled Trials (CENTRAL) Issue 2, 2009. MEDLINE and EMBASE up to April 2009.
Selection criteria
Randomised controlled trials (RCTs) of progestagen therapy in women who have had surgery for endometrial cancer.
Data collection and analysis
Two review authors independently abstracted data and assessed risk of bias. Risk ratios (RRs) comparing survival in women who did and did not receive progestagen were pooled in random effects meta‐analyses. .
Main results
Seven trials assessing 4556 women were identified. Three trials included women with stage one disease only, whereas four included women with more advanced disease. Meta‐analysis of four trials showed that there was no significant difference in the risk of death at five years between adjuvant progestagen therapy and no further treatment (RR = 1.00, 95% CI 0.85 to 1.18). This conclusion is also robust to single trial analyses at 4 and 7 years and in one trial across all points in time using a hazard ratio (HR). There was also no significant difference between progestagen therapy and control in terms of the risk of death from endometrial cancer, cardiovascular disease and intercurrent disease. Relapse of disease appeared to be reduced by progestagen therapy in one trial (HR = 0.71, 95% CI 0.52 to 0.97 and 5 year RR = 0.74, 95% CI 0.58 to 0.96), but there was no evidence of a difference in disease recurrence in another trial at 7 years (RR = 1.34, 95% CI 0.79 to 2.27).
Authors' conclusions
There is no evidence to support the use of adjuvant progestagen therapy in the primary treatment of endometrial cancer. There have now been several RCTs which have failed to establish a role for adjuvant progestagen therapy after primary treatment for endometrial cancer and therefore, further trials in this field are probably not justified.
Plain language summary
No evidence to support use of adjuvant progestagens to prevent recurrence of endometrial cancer after surgery
Endometrial (womb) cancer is the most common genital tract cancer in developed countries. Progestagen (a hormone) therapy is sometimes used following initial surgery to reduce the risk of recurrence. However, progestagens have been found to reduce one of the protective factors against heart disease and may also make tumours more resistant to radiotherapy. This review found no evidence to support the use of progestagen as an addition to surgery for newly diagnosed endometrial cancer. Progestagen can, however, prevent or delay recurrence of cancer in some patients.
Background
Description of the condition
Endometrial (womb) cancer encompasses a group of tumours affecting the lining of the womb (or uterus), known as the endometrium. It is the most common genital tract carcinoma in women in developed countries; the age‐standardised incidence is 9.1 per 100,000 women per year in more developed countries, compared with 1.7 per 100,000 per year in less developed countries (GLOBOCAN 2008). The incidence of endometrial cancer in the EU is 16 per 100,000 with approximately 7200 British women diagnosed each year (Baekerlandt 2009; Cancer Research UK 2010). Worldwide it is the seventh most common cancer in women and occurs predominately in postmenopausal women (91% of cases in women over 45 years old) (Baekerlandt 2009).
A large proportion of women (approximately 70%) present with stage 1 disease SEER 2011. The overall unadjusted five year survival for stage 1 disease is 96% SEER 2011, but women with advanced disease can have a survival expectancy as low as 23% (SEER 2011). In Europe, just over a fifth of women with endometrial cancer have died five years after diagnosis (EUROCARE 2003).
Description of the intervention
Adjuvant progestagen therapy has been advocated following primary surgery to reduce the risk of recurrence of disease. Progestagens have been demonstrated in observational studies to have a limited role in palliation in recurrent and extensive endometrial cancer. Clinicians used to think that administering progestagens in the adjuvant setting prolonged survival.
Why it is important to do this review
We have reviewed the published RCTs evaluating the effectiveness of this therapy. In the 1970s and 1980s several small observational studies suggested a survival advantage if progestagens were administered in a palliative setting in endometrial cancer (Kauppila 1984). Many surgeons believed that progestagens would benefit women in an adjuvant setting following surgery for endometrial cancer. The aim of this review was to evaluate evidence from RCTs.
Objectives
To evaluate the effectiveness and safety of adjuvant progestagen therapy for the treatment of endometrial cancer.
Methods
Criteria for considering studies for this review
Types of studies
RCTs
Types of participants
Women with a confirmed histological diagnosis of endometrial cancer who have had a hysterectomy
Types of interventions
Intervention
Adjuvant progestagen therapy (e.g. medroxy progesterone acetate or hydroxyprogesterone caproate)
Control
No adjuvant progestagen therapy
Types of outcome measures
Primary outcomes
Overall survival (OS): survival until death from all causes. Survival was assessed from the time when women are enrolled in the study.
Secondary outcomes
Survival until death from endometrial cancer
Survival until non‐cancer related death
Survival until death from cardio‐vascular disease
Progression‐free (PFS) or recurrence‐free survival (RFS)
Quality of life (QoL), measured using a scale that has been validated through reporting of norms in a peer‐reviewed publication.
-
Adverse events, classified according to CTCAE 2006:
haematological (leucopenia, anaemia, thrombocytopenia, neutropenia, haemorrhage)
gastrointestinal (nausea, vomiting, anorexia, diarrhoea, liver, proctitis)
genitourinary
skin (stomatitis, mucositis, alopecia, allergy)
neurological (peripheral and central)
pulmonary
other side effects such as thromboembolism and stroke
Search methods for identification of studies
Papers in all languages were sought and translations carried out if necessary.
Electronic searches
See: Cochrane Gynaecological Cancer Group methods used in reviews. The following electronic databases were searched:
The Cochrane Gynaecological Cancer Collaborative Review Group's Trial Register
Cochrane Central Register of Controlled Trials (CENTRAL), Issue 2, 2009
MEDLINE up to April 2009
EMBASE up to April 2009
The MEDLINE, EMBASE and CENTRAL search strategies are presented in Appendix 1, Appendix 2 and Appendix 3 respectively.
Databases were searched from 1966 until 2000 in the original review and up to April 2009 in this updated version.
All relevant articles found were identified on PubMed and using the 'related articles' feature, a further search was carried out for newly published articles.
Searching other resources
Unpublished and Grey literature
Metaregister, Physicians Data Query, www.controlled‐trials.com/rct, www.clinicaltrials.gov and www.cancer.gov/clinicaltrials were searched for ongoing trials.
Handsearching
First version of review
The citation list of included studies were checked through hand searching and experts in the field contacted to identify further reports trials. Sixteen journals thought to be most likely to contain relevant publications were hand searched, (Acta Cytologica, Acta Obstetrica Gynecologica Scandanavia, Acta Oncologica, American Journal of Obstetrics and Gynaecology, British Joural of Cancer, British Journal of Obstetrics and Gynaecology, British Medical Journal, Cancer, Cytopathology, Diagnostic Cytopathology, Gynecologic Oncology, International Journal of Cancer, International Journal of Gynaecological Cancer, Journal of Family Practice, Lancet, Obstetrics and Gynaecology).
Second version of review
This update is based on RCTs identified by electronic literature databases. All 16 previously handsearched publications are indexed by MEDLINE. As the accuracy of indexing RCTs is now very robust, further hand searching was not performed.
Data collection and analysis
Selection of studies
First version of review
In the original review, all possible publications identified by manual and electronic searches were collated onto an Excel spreadsheet. Two review authors (PM‐H and RL) independently scrutinised the studies to see if they met the inclusion and exclusion criteria. Diasagreements were resolved after discussion.
Second version of review
All titles and abstracts retrieved by electronic searching were downloaded to the reference management database Endnote, duplicates were then removed and the remaining references examined by two review authors (SK, PMH) independently. Those studies which clearly did not meet the inclusion criteria were excluded and copies of the full text of potentially relevant references were obtained. The eligibility of retrieved papers were assessed independently by two review authors (SK, PMH). Disagreements were resolved by discussion between the two review authors and when necessary by a third review author (RL). Reasons for exclusion are documented.
Data extraction and management
For included studies, data was abstracted as recommended in chapter 7 of the Cochrane Handbook 2008. This included data on the following:
Author, year of publication and journal citation (including language)
Country
Setting
Inclusion and exclusion criteria
Study design, methodology
-
Study population
total number enrolled
patient characteristics
age
-
Endometrial cancer details at diagnosis
the International Federation of Gynaecology and Obstetrics (FIGO) (Pecorelli 2009) stage
histological cell type
-
Intervention details
type of treatment with progestagens
duration of treatment with progestagens
-
Control details
any other reported information other than no active intervention was given
Risk of bias in study (see below)
Duration of follow‐up
Outcomes – see below
Data on outcomes will be extracted as below:
For time to event (OS and RFS) data, we extracted the log of the hazard ratio [log(HR)] and its standard error from trial reports; if these were not reported, we estimated them from other reported statistics using the methods of Parmar 1998.
For dichotomous outcomes (e.g. adverse events, or deaths if it was not possible to use a HR), we extracted the number of patients in each treatment arm who experienced the outcome of interest and the number of patients assessed at endpoint, in order to estimate a RR.
Where possible, all data extracted were those relevant to an intention‐to‐treat analysis, in which participants were analysed in groups to which they were assigned (to reduce bias).
The time points at which outcomes were collected and reported was noted.
Data were abstracted independently by two review authors (SK, PMH) onto a data abstraction form specially designed for the review. Differences between review authors were resolved by discussion or by appeal to a third review author (AB) when necessary.
Assessment of risk of bias in included studies
The risk of bias in included RCTs was assessed using the following questions and criteria:
Sequence generation
Was the allocation sequence adequately generated?
Yes: e.g. a computer‐generated random sequence or a table of random numbers
No: e.g. date of birth, clinic id‐number or surname
Unclear: e.g. not reported.
Allocation concealment
Was allocation adequately concealed?
Yes: e.g. where the allocation sequence could not be foretold
No: e.g. e.g. allocation sequence could be foretold by patients, investigators or treatment providers
Unclear: e.g. not reported
Blinding
Assessment of blinding was restricted to personnel who assessed cause of death and disease progression.
Was knowledge of the allocated interventions adequately prevented during the study?
Yes
No
Unclear
Incomplete reporting of outcome data
We recorded the proportion of participants whose outcomes were not reported at the end of the study; we noted whether or not loss to follow‐up was not reported.
Were incomplete outcome data adequately addressed?
Yes, if fewer than 20% of patients were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms
No, if more than 20% of patients were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms
Unclear if loss to follow‐up was not reported
Selective reporting of outcomes
Are reports of the study free of suggestion of selective outcome reporting?
Yes e.g if review reported all outcomes specified in the protocol
No, otherwise
Unclear, if insufficient information available.
Other potential threats to validity
Was the study apparently free of other problems that could put it at a high risk of bias?
Yes
No
Unclear
The risk of bias tool was applied independently by two review authors (AB, SK) and differences were resolved by discussion. Results were presented in both a risk of bias graph and a risk of bias summary. Results of meta‐analyses were interpreted in light of the findings with respect to risk of bias.
Measures of treatment effect
We used the following measures of the effect of treatment:
For time to event data, we used the HR, where possible. The HR summarises the chances of survival in women who received one type of treatment compared to the chances of survival in women who received another type of treatment. However, the logarithm of the HR, rather than the HR itself, is generally used in meta‐analyses.
For dichotomous outcomes, we used the RR.
Dealing with missing data
We did not impute missing outcome data.
Assessment of heterogeneity
Heterogeneity between studies was assessed by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials which cannot be ascribed to sampling variation (Higgins 2003) and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). If there was evidence of substantial heterogeneity, the possible reasons for this were investigated and reported.
Data synthesis
The results of clinically similar studies were pooled in meta‐analyses.
For any dichotomous outcomes, the RR was calculated for each study and these were then pooled.
Random effects models with inverse variance weighting were used for all meta‐analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
No sub‐group analyses were planned.
Factors such as age, stage, length of follow‐up, grade, were considered in interpretation of any heterogeneity.
Sensitivity analysis
In the Urbanski 1993 trial, which met the inclusion criteria, overall survival at five years was 97% in the progestagen group compared to 69% in the control group. Analysis of patients' risk factors demonstrated an imbalance favouring the treatment group and all treated patients with stage II and III disease survived, whereas only 55% of such patients in the control group survived. Thus we carried out a sensitivity analysis excluding this trial in the death at five years outcome. For full details see Effects of interventions.
We did not plan any sensitivity analyses a priori.
Results
Description of studies
Results of the search
The original search strategy identified references which were then screened by title and abstract to identify seven references as potentially eligible for the review. The updated search strategy identified 1740 unique references. The title and abstract screening of these references identified no further references as potentially eligible for the review. When we retrieved full copies of the text, all seven references were RCTs and met our inclusion criteria and are described in the table Characteristics of included studies.
Searches of the grey literature did not identify any additional relevant studies.
Included studies
Seven trials, randomising a total of 4556 women, met the inclusion criteria. In all trials, except that of Lewis 1974, patients had surgery (total abdominal hysterectomy and bilateral salpingo‐oophorectomy) and then adjuvant radiotherapy if indicated by standard pathological criteria. In the trial by Lewis, patients with stage I disease were divided into two groups irrespective of pathological criteria; the first group had pre‐operative intracavitary radiotherapy and surgery, while the second group was treated by surgery alone.
In some trials, only patients with stage 1 disease were recruited (Lewis 1974; Malkasian 1978; DePalo 1993), while the remaining studies included patients with more severe disease (MacDonald 1988; Vergote 1989; Urbanski 1993; COSA‐NZ‐UK 1998). Tumour stage, degree of differentiation and depth of myometrial invasion of disease are important prognostic variables which should be equally distributed between treatment and control groups. This appeared to be the case in all but one trial (Urbanski 1993), where the treatment group had systematically better prognostic features. Furthermore, in 26% of patients in the control group the depth of myometrial invasion was not known, compared to 11% in the treatment group. The trial by Vergote 1989 was the only other trial to have incomplete data on the degree of myometrial invasion. However the difference in the proportion of missing data was not great; 7% and 5% in the treatment and control groups respectively.
In theory, prolonged progestagen therapy predisposes to cardiovascular disease as it potentially increases the proportion of saturated fats in the blood stream. However, Malkasian 1978 and COSA‐NZ‐UK 1998 were the only trials that gave information about pre‐trial risk factors such as diabetes, hypertension or obesity.
In three trials, (Malkasian 1978; Urbanski 1993; COSA‐NZ‐UK 1998) all the patients initially randomised completed the study protocol. Some patients in the trials by MacDonald 1988 and DePalo 1993 did not complete the study protocol but outcome data for such patients were given. The trials of Lewis 1974 and Vergote 1989 reported insufficient data for an "intention to treat analysis".
The duration of follow‐up of patients varied from 12 to 130 months. In the trial by MacDonald 1988 all patients completed one year follow‐up and over half the patients completed five year surveillance. In the trial by Vergote 1989, follow‐up ranged from 42 to 130 months (median 72 months). In all the other trials the mean and range of follow‐up were not given, but all patients had completed the minimum follow‐up period specified.
Risk of bias in included studies
See Figure 1.
1.
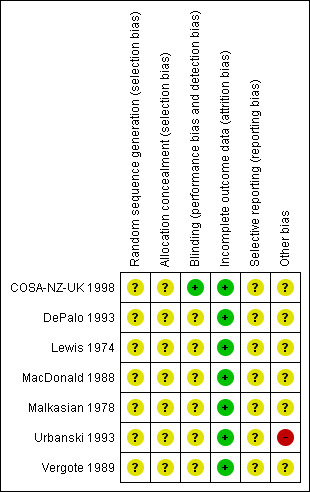
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
The method of sequence generation was not reported in any of the trials. The MacDonald 1988 trial assigned patients to treatment groups on the basis of pre‐numbered cards in sealed envelopes to allow random allocation by telephone from the regional oncology centre, but it was unclear how the cards would be randomly allocated.The trials of DePalo 1993, Lewis 1974 and COSA‐NZ‐UK 1998 were multi‐centre trials using centralised randomisation so treatment allocation was almost certainly concealed from investigators but this was not explicitly reported.The trial of MacDonald 1988 attempted to conceal treatment allocation by using sealed envelopes, but it was unclear whether these envelopes were opaque. Concealment of allocation was not reported in any of the other trials (Malkasian 1978; Vergote 1989; Urbanski 1993).
Blinding
None of the trials reported blinding of personnel assessing cause of death and disease progression.
Incomplete outcome data
Loss to follow‐up was low (<10% all assessable outcomes) in five trials (COSA‐NZ‐UK 1998; DePalo 1993; Malkasian 1978; Urbanski 1993; Vergote 1989) and of a similar proportion in both treatment arms, but was not satisfactory in two trials (Lewis 1974; MacDonald 1988).
Selective reporting
In all seven trials it was unclear as to whether outcomes had been selectively reported as there was insufficient information to permit judgement.
Other potential sources of bias
In one RCT (Urbanski 1993) there appeared to be an imbalance of poor prognostic factors in the control arm of the study. In the remaining six trials there was insufficient information to assess whether any important additional risk of bias existed.
Effects of interventions
All seven trials documented the total number of deaths in both treatment and control groups, but the time frame in which the data were analysed is incompletely reported. In the Urbanski 1993 trial, adjuvant progestagen therapy appeared to have a large and significant reduction in the rate of deaths at five years (RR = 0.10, 95% CI 0.03 to 0.30), with an overall survival rate at five years in the treatment group of 97% compared to 69% in the control group. However, analysis of patients' risk factors demonstrated an imbalance favouring the treatment group and all treated patients with stage II and III disease survived, whereas only 55% of such patients in the control group survived. These extreme results are completely out of keeping with the much more modest effects observed in the remaining six trials.
The I2 test confirmed that the trials were highly heterogeneous for overall survival at five years if Urbanski 1993 was included (I2 = 79%) but the amount of heterogeneity was substantially reduced when this trial was excluded in a sensitivity analysis (I2 = 7%). Exclusion of the trial by Urbanski 1993 from the meta‐analysis thus appears justified by the substantial heterogeneity induced by its inclusion. Since there were only three deaths in the progestagen group similar results were observed for endometrial cancer and non‐cancer related deaths.
Overall Survival
1.1. Analysis.
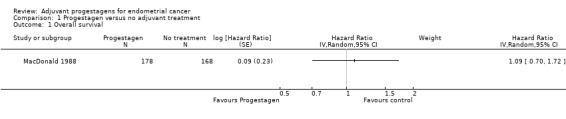
Comparison 1 Progestagen versus no adjuvant treatment, Outcome 1 Overall survival.
The trial of MacDonald 1988, found no statistically significant difference in the risk of death in women who did and did not receive progestagens (HR=1.09, 95% CI 0.70 to 1.72).
Death from any cause
1.2. Analysis.
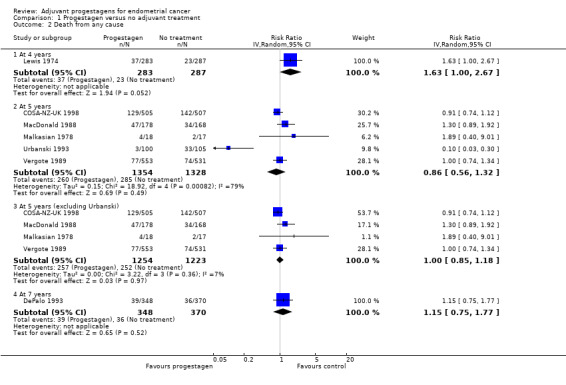
Comparison 1 Progestagen versus no adjuvant treatment, Outcome 2 Death from any cause.
At four years
The trial of Lewis 1974 (n = 563), found that women who received progestagen had an increased risk of death compared to those who did not receive progestagens, but this was only of borderline statistical significance (RR = 1.63, 95% CI 1.00 to 2.67, P = 0.05).
At five years
Meta‐analysis of five RCTs (COSA‐NZ‐UK 1998; MacDonald 1988; Malkasian 1978; Urbanski 1993: Vergote 1989) assessing 2682 participants, showed no statistically significant difference in the risk of death in women who did and did not receive progestagens (RR = 0.86, 95% CI 0.56 to 1.32). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance may represent considerable heterogeneity (I2 = 79%).
Sensitivity analysis of death from any cause at five years
A sensitivity analysis, which omitted the trial of Urbanski 1993, (four RCTs, assessing 2477 participants) showed little difference in the risk of death in women who did and did not receive progestagens (RR = 1.00, 95% CI 0.85 to 1.18). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance does not appear to be important (I2 = 7%).
At seven years
The trial of DePalo 1993 (n = 718), found no statistically significant difference in the risk of death in women who did and did not receive progestagens (RR = 1.15, 95% CI 0.75 to 1.77).
Death from endometrial cancer
1.3. Analysis.
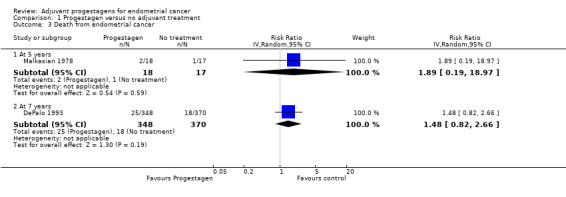
Comparison 1 Progestagen versus no adjuvant treatment, Outcome 3 Death from endometrial cancer.
At five years
The trial of Malkasian 1978 (n = 35), found no statistically significant difference in the risk of death from endometrial cancer in women who did and did not receive progestagens (RR = 1.89, 95% CI 0.19 to 18.97).
At seven years
The trial of DePalo 1993 (n = 718), found no statistically significant difference in the risk of death from endometrial cancer in women who did and did not receive progestagens (RR = 1.48, 95% CI 0.82 to 2.66).
Non‐cancer related deaths at 7 years
1.4. Analysis.
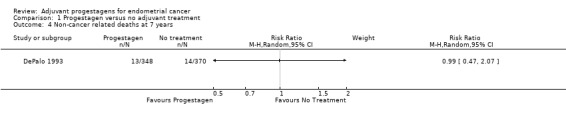
Comparison 1 Progestagen versus no adjuvant treatment, Outcome 4 Non‐cancer related deaths at 7 years.
The trial of DePalo 1993 (n = 718), found little difference in the risk of a non‐cancer related death in women who did and did not receive progestagens (RR = 0.99, 95% CI 0.47 to 2.07) .
Death due to cardiovascular disease
1.5. Analysis.
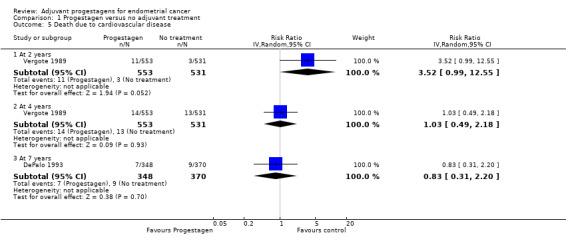
Comparison 1 Progestagen versus no adjuvant treatment, Outcome 5 Death due to cardiovascular disease.
At two years
The trial of Vergote 1989 (n = 1084), suggested that there may have been an increased risk of death due to cardiovascular disease in women who received progestagens compared to control, although this was only of borderline statistical significance in this large trial (RR = 3.52, 95% CI 0.99 to 12.55, P = 0.05).
At four years
The trial of Vergote 1989 (n = 1084), found little difference in the risk of death due to cardiovascular disease in women who did and did not receive progestagens (RR = 1.03, 95% CI 0.49 to 2.18).
At seven years
The trial of DePalo 1993 (n = 718), found no statistically significant difference in the risk of death due to cardiovascular disease in women who did and did not receive progestagens (RR = 0.83, 95% CI 0.31 to 2.20).
Recurrence‐free survival
1.6. Analysis.
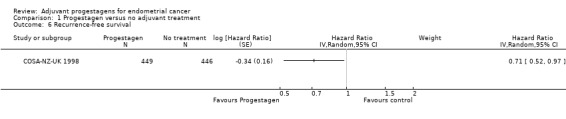
Comparison 1 Progestagen versus no adjuvant treatment, Outcome 6 Recurrence‐free survival.
The trial of COSA‐NZ‐UK 1998 (n = 895), found that women who received progestagen were associated with decreased risk of disease recurrence compared to those who received no further treatment (HR = 0.71, 95% CI 0.52 to 0.97).
Disease recurrence
1.7. Analysis.
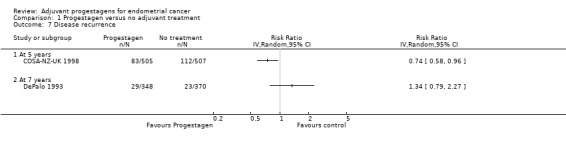
Comparison 1 Progestagen versus no adjuvant treatment, Outcome 7 Disease recurrence.
At five years
The trial of COSA‐NZ‐UK 1998 (n = 1012, which included 117 ineligible patients), found that women who received progestagen were associated with decreased risk of disease recurrence compared to those who received no further treatment (RR = 0.74, 95% CI 0.58 to 0.96). This was consistent with Analysis 1.6, which used a hazard ratio for eligible patients only.
At seven years
The trial of DePalo 1993 (n = 718), found no statistically significant difference in the risk of disease recurrence in women who did and did not receive progestagens (RR = 1.34, 95% CI 0.79 to 2.27).
Quality of Life
No trials reported QoL as an outcome.
Adverse Events
The trial of Vergote 1989 reported hypertension rates in the two arms of the study and this demonstrated no increased risk with progestagen treatment compared to no further treatment (RR = 1.12, 95% CI 0.88 to 1.42).
Discussion
Summary of main results
A review of the currently available published trials suggested that the OS of patients with endometrial cancer is not influenced by adjuvant progestagen therapy. Adjuvant progestagen therapy may be expected to have an adverse effect on deaths from intercurrent disease, particularly cardiovascular related deaths as blood bourne saturated fats increase with progestagens therefore potentially putting patients at risk. However in the two trials by MacDonald 1988 and DePalo 1993 in which long term therapy was used, there was similar risk of such deaths in the treatment and control groups. Furthermore, the trial of Vergote 1989 demonstrated no increased risk of hypertension.
Within FIGO stage I disease, subgroups of patients can be identified by surgico‐pathological staging who have a much worse five year survival. It is among these high risk patients that progestagen treatment (or any other adjuvant treatment) has the most scope to act. Thus, inclusion of all endometrial cancers could dilute the effect of progestagens in more severe cases. It might also dilute potential harm if, for example, progestagens make those tumours more resistant to radiotherapy. It is therefore a pity that only the DePalo 1993 and COSA‐NZ‐UK 1998 trials gave sufficient data for analysis of results in a subgroup with adverse prognostic factors. However, only the COSA‐NZ‐UK 1998 trial suggests a non‐significant benefit for high risk cases. While inclusion of patients with predominantly good prognostic factors might obscure the effects of progestagens on endometrial cancer related deaths, the effect of progestagens on intercurrent deaths is much less likely to be influenced by the severity of the original disease.
Overall completeness and applicability of evidence
All of these trials with the exception of Urbanski 1993 demonstrated no evidence of a difference between adjuvant progestagen therapy after surgery and no further treatment for endometrial cancer. All RCTs of progestagen therapy included in the review were conducted prior to the year 2000 and demonstrated limited benefit of adjuvant radiotherapy. Therefore many of the patients included in the six RCTS would have had adjuvant radiotherapy in contrast to current 2011 consensus guidelines. It is unlikely that the current limited use of radiotherapy would influence the efficacy of progestagens.
Quality of the evidence
Seven trials were included in this review. In total 4351 women participated. We have used a pragmatic approach to RCTs included in the comparisons. The trials used variations in the dose and route of administration of progestagens. The individual trials used different time points to measure survival and used different endpoints regarding intercurrent deaths. The RCTs were conducted during a time period when the reporting of RCTs was not standardised and many of the reports did not declare certain details regarding methodological quality and risk of bias components. As these RCTs were large multi‐centre RCTs, it is likely they employed optimal methodology but this can not be assumed.
Many of the outcomes analysed included meta analyses of only a small number of RCTs or the results of single trials due to the incomplete reporting of outcome measures and heterogeneous time points chosen which limits the conclusions which may be drawn.
Potential biases in the review process
This review used extensive electronic literature databases searches and hand searching of journals. Two review authors extracted the data with a third person settling any disagreements. We restricted the included studies to RCTs as they provide the strongest level of evidence available. Hence we have attempted to reduce bias in the review process.
The greatest threat to the validity of the review is likely to be the possibility of publication bias i.e. studies that did not find the treatment to have been effective may not have been published. We were unable to assess this possibility as the analyses were restricted to meta‐analyses of a small number of trials or single trials.
Agreements and disagreements with other studies or reviews
There are no other systematic reviews in this field. All the individual RCTs except Urbanski 1993 demonstrated the lack of effect of adjuvant progestagens. The outcomes of this update are consistent with previous versions of this review.
Authors' conclusions
Implications for practice.
Progestagens have an established place in the palliative treatment of women with advanced endometrial cancer. However, a review of the currently available trials failed to demonstrate that adjuvant progestagen therapy has a significant beneficial effect on survival. The trials included in this review are predominantly based on low risk patients where adequate statistical precision is hard to attain as the survival of early disease is extremely good. However, the available evidence points towards the conclusion that progestagens may have no role in the primary treatment of endometrial cancer.
Implications for research.
There have now been several RCTs which have failed to establish a role for adjuvant progestagen therapy after primary treatment for endometrial cancer. Therefore, further trials in this field are probably not justified.
What's new
| Date | Event | Description |
|---|---|---|
| 11 February 2015 | Amended | Contact details updated. |
| 20 November 2013 | Review declared as stable | No new trials expected on this topic area. |
History
Review first published: Issue 1, 1998
| Date | Event | Description |
|---|---|---|
| 27 March 2014 | Amended | Contact details updated. |
| 20 November 2013 | Amended | Review is being marked as stable. |
| 10 May 2011 | New search has been performed | Review updated |
| 10 May 2011 | New citation required but conclusions have not changed | Searches re‐run but no new studies included. New author added. |
| 29 June 1999 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank Chris Williams for clinical and editorial advice, Jane Hayes for designing the search strategy and running the searches, Gail Quinn and Clare Jess for their contribution to the editorial process and the review authors for their helpful comments. We would also like to thank Heather Dickinson for many helpful suggestions on updating the review.
Appendices
Appendix 1. Medline Search Strategy
Medline Ovid 1997‐ April week 2 2009
exp Endometrial Neoplasms/
(endometr* adj5 (neoplas* or carcinom* or malignan* or cancer* or tumor* or tumour*)).mp.
1 or 2
exp Progestins/
exp Medroxyprogesterone 17‐Acetate/
exp Hydroxyprogesterones/
progest*.mp.
medroxyprogesterone*.mp.
hydroxyprogesterone*.mp.
or/4‐7
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/11‐18
3 and 19 and 10
limit 20 to yr="1997 ‐ 2009"
key: mp=title, original title, abstract, name of substance word, subject heading word
ab=abstract
pt=publication type
fs=floating subheading
Appendix 2. Embase search strategy
Embase Ovid 1997 to 2009 week 16
exp Endometrium Tumor/
(endometr* adj5 (neoplas* or carcinom* or malignan* or cancer* or tumor* or tumour*)).mp.
1 or 2
exp hydroxyprogesterone/ or exp hydroxyprogesterone caproate/ or exp medroxyprogesterone/ or exp medroxyprogesterone acetate/
progest*.mp.
medroxyprogesterone*.mp.
hydroxyprogesterone*.mp.
or/4‐7
exp Controlled Clinical Trial/
randomized.ab.
placebo.ab.
dt.fs.
randomly.ab.
trial.ab.
groups.ab.
or/9‐15
8 and 3 and 16
limit 17 to yr="1997 ‐ 2009"
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name
ab=abstract
fs=floating subheading
Appendix 3. Central search strategy
CENTRAL Issue 2 2009
MeSH descriptor Endometrial Neoplasms explode all trees endometr* near/5 (neoplas* or carcinom* or malignan* or cancer* or tumor* or tumour*) (#1 OR #2) MeSH descriptor Progestins explode all trees MeSH descriptor Medroxyprogesterone 17‐Acetate explode all trees MeSH descriptor Hydroxyprogesterones explode all trees progest* medroxyprogesterone* hydroxyprogesterone* (#4 OR #5 OR #6 OR #7 OR #8 OR #9) (#3 AND #10) (#11), from 1997 to 2009
Data and analyses
Comparison 1. Progestagen versus no adjuvant treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2 Death from any cause | 7 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 At 4 years | 1 | 570 | Risk Ratio (IV, Random, 95% CI) | 1.63 [1.00, 2.67] |
| 2.2 At 5 years | 5 | 2682 | Risk Ratio (IV, Random, 95% CI) | 0.86 [0.56, 1.32] |
| 2.3 At 5 years (excluding Urbanski) | 4 | 2477 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.85, 1.18] |
| 2.4 At 7 years | 1 | 718 | Risk Ratio (IV, Random, 95% CI) | 1.15 [0.75, 1.77] |
| 3 Death from endometrial cancer | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 At 5 years | 1 | 35 | Risk Ratio (IV, Random, 95% CI) | 1.89 [0.19, 18.97] |
| 3.2 At 7 years | 1 | 718 | Risk Ratio (IV, Random, 95% CI) | 1.48 [0.82, 2.66] |
| 4 Non‐cancer related deaths at 7 years | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5 Death due to cardiovascular disease | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.1 At 2 years | 1 | 1084 | Risk Ratio (IV, Random, 95% CI) | 3.52 [0.99, 12.55] |
| 5.2 At 4 years | 1 | 1084 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.49, 2.18] |
| 5.3 At 7 years | 1 | 718 | Risk Ratio (IV, Random, 95% CI) | 0.83 [0.31, 2.20] |
| 6 Recurrence‐free survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 7 Disease recurrence | 2 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 7.1 At 5 years | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 At 7 years | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Adverse Events: Hypertension | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
1.8. Analysis.
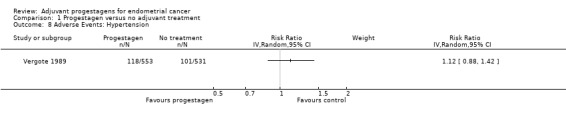
Comparison 1 Progestagen versus no adjuvant treatment, Outcome 8 Adverse Events: Hypertension.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
COSA‐NZ‐UK 1998.
| Methods | Multi‐centre RCT | |
| Participants | 1012 women with poorly differentiated endometroid, adenosquamous, clear cell or papillary serous tumours, greater than a third myometrial involvement, involvement into the cervix or spread to the adnexae and surgical complete resection was achieved. Mean age in the trial was 64.5 years (Range: 22 and 60 years). There were 486 women with FIGO Stage Ia, 321 had stage Ib, 148 stage II and 57 had stage III. 215 women had well differentiated tumour grade, 468 had moderate differentiated tumour and 329 had poorly differentiated tumours. Histology was classified as adenocarcinoma in 779 women, adenosquamous in 125, Papillary in 64, Clear cell in 28, Squamous in 2 and histology was unclassified in 6 women and was another form of cancer in a further 8 women. There was no myometrial invasion in 37 women. Myometrial invasion was <33% in 218 women, 33‐66% in 369, 67 to 100% in 350 and Into serosa in 38 women. |
|
| Interventions |
Intervention: Oral Medroxy progesterone 200 mg twice daily for at least 3 years Control: No active treatment |
|
| Outcomes | Overall death rates
Endometrial cancer deaths
Intercurrent deaths (death from non‐cancer related disease)
Relapse from disease MI and CVA related deaths |
|
| Notes | Length of follow‐up was between three to 10 years, with a mean of 5.4 years. Performance status: Group 0: 901, Group 1: 81, Group 2: 20, Group 3: 8, Confined to bed: 2 Median time from disease recurrence to death was 7 months. Statistically significant predictors of recurrence were greater myometrial invasion (P < 0.001), later stage (P < 0.001), high grade (P < 0.001), positive pelvic nodes (P < 0.001), increasing age (P < 0.05), and papillary serous or clear cell histology (P < 0.05). By the end of the trial there had been 149/505 deaths in the MPA group and 165/507 in the control group: Endometrial Cancer: 98 deaths in control group, 73 in MPA group. MI: 12 deaths in control, 17 in MPA. CVA: 9 deaths in control, 8 in MPA. Other cancers: 3 deaths in control, 9 in MPA. Other disease: 26 deaths in control 22 in MPA. Unspecified cause: 5 deaths in control, 12 in MPA. The individual causes of death shown in table 3 did not add up to the total number of deaths. The five year disease‐free (recurrence‐free) rates of 83.5% and 78% in the MPA and control groups respectively gave the number of women with disease recurrence as being larger than the reported figures given at the end of the trial. "There were 81 relapses in the MPA group and 107 in the control group (p<0.05)". The number who relapsed using the 5 year percentages were 83 in the MPA group and 112 in the control group and these figures were used in the review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation is most likely adequate because there was central randomisation, but it is uncertain. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All cases were reviewed by an ‘‘independent’’ committee after randomization and treatment to ensure eligibility and to examine for protocol violation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed:1012/1012 (100%) and 895/895 in analyses of eligible patients only. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
DePalo 1993.
| Methods | Multi‐centre RCT | |
| Participants | 771 women randomised
Women with stage 1 with no myometrial involvement did not receive any adjuvant treatment 138 (17.9%) Median age ranged between 58 and 61 across all MPA and no MPA comparisons (women with R1‐R3). 601 (83%) women had adenocarcinoma histological type, 80 (11%) had adenoacanthoma, 4 (0.5%) had clear cell, 29 (4%) had adenosquamous and 4 (0.5%) women had undifferentiated histology. 399 (55.5%) had myometrial invasion classified as M1, 192 (26.75%) as M2 and 127 (17.75%) classified as M3. |
|
| Interventions | Oral Medroxy progesterone 100mg twice daily for 1 year | |
| Outcomes | Overall death rates
Endometrial cancer deaths
Intercurrent deaths
Relapse from disease Adverse events (only reported in the treatment arm) |
|
| Notes | Patient withdrawals; disease more than stage 1 on review, patient refusal, intercurrent disease Data only available for intention to treat analysis Progestagen group 327 women Control group 370 women Duration of follow‐up 84 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Concealment of allocation is most likely adequate because there was central randomisation, but it is uncertain, "Quality control of data was performed at a centralised data centre". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 718/718 (100%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Lewis 1974.
| Methods | Multi‐centre RCT | |
| Participants | 956 women randomised All stage 1, differentiation not stated Progestagen group . < 1/3 myometrial involvement 79 (27.9%), >1/3 myometrial involvement 86 (30.3%) Control group < 1/3 myometrial involvement 80 (28.5%), > 1/3 myometrial involvement 80 (28.5%) | |
| Interventions | Pre‐operative radiotherapy group: intra‐muscular 500mg Medroxy progesterone acetate once weekly for 14 weeks Hysterectomy only group: intra‐muscular 1000mg Medroxy progesterone acetate for 14 weeks | |
| Outcomes | Overall death rates | |
| Notes | Completed protocol progestagen group 285 Completed protocol control group 287 No data for intention to treat Duration of follow‐up; 48 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 563/574 (98%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
MacDonald 1988.
| Methods | RCT | |
| Participants | 429 women randomised
Progestagen group; Stage 1a 96 (22.4%),1b 45 (10.5%), 2; 24 (5.6%), 3; 13 (3.0%)
Progestagen group; differentiation well 61 (14.2%), moderate 68 (15.8%), poor 48 (11.25)
Progestagen group; depth of penetration < 1/3 myometrial involvement 79 (18.4%), > 1/3 myometrial involvement 86 (20.0%) Control group; Stage 1a; 101 (23.54%), 1b; 43 (10.2%), 2; 11 (2.6%), 3; 13 (3.0%) Control group; differentiation well 64 (14.9%), moderate 65 ( 15.1%), poor 39 (9.0%) Control group; depth of penetration <1/3 myometrial involvement 80 (18.6%), >1/3 myometrial involvement 80 ( 18.6%) |
|
| Interventions | Intra‐Muscular Hydroxyprogesterone on diagnosis Following hysterectomy oral medroxy progesterone acetate 100mg t.d.s for 1 year and then oral medroxy progesterone acetate 100mg daily for at least 5 years | |
| Outcomes | Overall death rates Endometrial cancer deaths Intercurrent disease deaths | |
| Notes | Patient withdrawals due to inoperable disease, declined radiotherapy if indicated, inaccurate diagnosis, protocol deviations Initially enrolled Progestagen group 214 Initially enrolled Control group 215 Ineligible patients Progestagen group 36 Ineligible patients Control Group 47 Completed study Progestagen group 126 (70.8%) Completed study control group 155 (92.2%) Duration of follow‐up; 12 ‐ 60 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Dr Ian Sutherland kindly supplied pre‐numbered cards in sealed envelopes to allow random allocation by telephone from the regional oncology centre". |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether these envelopes were opaque |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 346/346 (100%) eligible patients were analysed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Malkasian 1978.
| Methods | RCT | |
| Participants | 35 women randomised
Progestagen group; Stage1a 14(40%), 1b 4 (11.4%)
Progestagen group; differentiation well 5 (14.2%), moderate 11 (31.4%), poor 2(5.7%)
Progestagen group; depth of penetration <1/3 myometrial involvement 11 (31.4%), 1/3 myometrial involvement 2 (5.71%). >1/3 myometrial involvement 5 (14.2%) Control group stage 1a 11 (31.4%), 1b 6 (17.1%) Control group; differentiation well 9 (25.7%), moderate 5 (14.2%), poor 3 (2.2%) Control group depth of penetration <1/3 myometrial involvement 11 (31.4%), 1/3 myometrial involvement 3 (2.2%), > 1/3 myometrial involvement 3 (8.6%) |
|
| Interventions | Intra‐muscular medroxy progesterone acetate 1000mg post‐op Intra‐muscular medroxy progesterone acetate 500mg once weekly for 14 weeks | |
| Outcomes | Overall death rates Endometrial cancer deaths Intercurrent deaths | |
| Notes | No withdrawals progestagen group 18 Control group 17 Duration of follow‐up; 60 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 35/35 (100%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Urbanski 1993.
| Methods | RCT | |
| Participants | 205 women with histologically confirmed FIGO stage I, II or III endometrial carcinoma who had primary treatment with hysterectomy and bilateral salpingo‐ophorectomy and, had no history of previous cancer. Median age in the trial was 56 years (Range: 41‐80 years). in the control group 65 (31.7%) had Stage 1 disease, 40 (19.5%) had stage II or greater , in the progestagen group 78 (38.0%) had stage 1 disease, 22 (10.7%) had stage II or greater in the control group 38 (18.5%) had disease < 50% myometrium, 39 (19.0%) had disease > than 50% myometrium, 28 (13.6%) not stated, in the progestagen group 52 (25.3%) had disease < 50% myometrium, 37 (18.0%) had disease > than 50% myometrium, 11 (5.36%) in the control group 9 (4.4%) had low grade disease , 36 (17.6%) had high grade disease and 60 (29.2%) without grading, in the progestagen group 18 (8.8%) had low grade disease , 31 (15.2%) had high grade disease and 51 (24.8%) without grading, |
|
| Interventions |
Intervention: Adjuvant Progestagen therapy: Started 4 to 6 weeks after surgery and consisted of biweekly intra‐muscular injections of 500mg of hydroxy‐progesterone caproate. For subjective reasons part of the patients received Progestagens intraorally. The treatment was continued for 1 year. Control: No adjuvant Progestagen therapy |
|
| Outcomes | Overall survival Endometrial cancer related deaths Cancer related deaths Disease relapse | |
| Notes | Extreme heterogeneity of results compared to all other trials, data suggest overwhelming benefit from Progestagen therapy. This in complete contrast to other RCTS included in this review. Imbalance of prognostic factors in favour of Progestagen group, i.e. stage and not stated depth of penetration; (N.B unequal distribution of risk factors between two groups). No patient was lost to follow up Duration of follow‐up; 60 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported, "Patients were randomly assigned to a control group or to a group receiving adjuvant Progestagen therapy". |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 205/205 (100%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | Inexplicable baseline imbalance of Figo stage and infiltration depth between two groups which is probable reason for extreme exaggeration of effect estimates. |
Vergote 1989.
| Methods | RCT | |
| Participants | 1048 women with histologically confirmed FIGO stage I or stage II endometrial carcinoma who underwent primary treatment with hysterectomy and bilateral salpingo‐oophorectomy and had no history of previous cancer. The median age at the time of randomisation was 61 years for both groups. FIGO stage: Stage 1: 967(92.7%), 2: 110 (10.5%), 3: 7 (0.66%) Histologic grade: well‐differentiated: 384 ( 36.64%), moderate: 397 (37.9%), poor: 155 ( 14.8%), not stated: 1 ( 0.1%) Depth of penetration: mucosa only: 123 ( 10.5%), < 1/2 myometrial involvement: 575 ( 54.8%), > 1/2 myometrial involvement: 163 (15.5%), Serosa: 8 (0.76%), not stated: 65 ( 6.2%) |
|
| Interventions |
Intervention: Adjuvant Progestagen therapy: Started 1 to 6 weeks after surgery and consisted of biweekly intramuscular injections of 1000 mg of hydroxyprogesterone caproate after a loading dose of 5000 mg given in the course of 5 days. This treatment was continued for 1 year. Control: No adjuvant Progestagen therapy |
|
| Outcomes | Overall survival Endometrial cancer deaths Intercurrent deaths Relapse | |
| Notes | All patients included in results: Progestagen group: 553, Control group: 531 The follow‐up time ranged from 42 months to 132 months (median follow‐up, 72 months). Overall survival at 5 years was calculated by enlarging plot showing percent survival in the 2 groups up to 5 years (Fig 1, P.1012) and estimated 5 year survival. it was reported that there was no loss to follow up censoring was likely to be low. By the end of the trial there had been 112/553 deaths and 67 recurrences in the progestagen group and 93/531 deaths and 75 recurrences in the control group: Endometrial Cancer: 62 deaths in control group, 61 in progestagen group. CV disease: 16 deaths in control, 25 in progestagen group. Second primary cancers: 1 death in control, 7 in progestagen group. Pulmonary disease: 1 death in control, 4 in progestagen group. Gastrointestinal disease: 3 deaths in control, 3 in progestagen group. Other: 10 deaths in control, 12 in progestagen group. "No difference in relapse rates was found between the Progestagen and control group (67 and 75 patients, respectively; P = 0.62)". "Death due to cardiovascular disease during the first 2 years after randomisation tended to be higher in the Progestagen group than in the control group (11 and three patients, respectively; P = 0.07), but was similar for the following years (14 and 13 patients, respectively)". This information was used to compute 2 and 4 year death due to cardiovascular disease in the review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed:1084/1084 (64/1148 excluded: 5.9%) 33 excluded from Progestagen group and 31 from control group due to protocol violations. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Differences between protocol and review
We did not identify any ongoing trials so the following text was removed from the unpublished and grey literature section:
"Searching other resources
Unpublished and grey literature
The main investigators of the relevant ongoing trials will be contacted for further information, as will be the major co‐operative trials groups active in this area".
Quality of life
QoL was not reported in any of the included trials, so the following sections in the protocol which discussed the handling of data for continuous outcomes were removed as they were unnecessary:
"Data extraction and management
For continuous outcomes (e.g. quality of life measures), we will extract the final value and standard deviation of the outcome of interest and the number of patients assessed at endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference between treatment arms and its standard error.
Measures of treatment effect
For continuous outcomes, we will use the mean difference between treatment arms if all trials measured the outcome on the same scale, otherwise standardised mean differences will be used.
Data synthesis
For continuous outcomes, the mean differences between the treatment arms at the end of follow‐up will be pooled if all trials measured the outcome on the same scale, otherwise standardised mean differences will be pooled".
There was an insufficient number of trials in each of the meta analyses to assess reporting biases so the following section was removed:
"Assessment of reporting biases
Funnel plots corresponding to meta‐analysis of the primary outcome will be examined to assess the potential for small study effects such as publication bias. If these plots suggest that treatment effects may not be sampled from a symmetric distribution, as assumed by the random effects model, further meta‐analyses will be performed using fixed effects models".
None of the trials imputed missing data. Although some of the outcomes that we specified were not reported in included trials, we did not contact trial authors as all trials reported over ten years ago and most significantly longer. We removed the following text from the 'dealing with missing data' section:
"Dealing with missing data
If data were missing or only imputed data were reported we contacted trial authors to request data on the outcomes only among participants who were assessed".
It was not possible to pool time to event data so the following bullet point in the data synthesis section was removed:
"Data synthesis
For time‐to‐event data (overall survival and recurrence‐free survival), HRs will be pooled using the generic inverse variance facility of RevMan 5".
Concealment of allocation and blinding of the outcome assessor was not reported in any of the trials so the following text was removed:
"Sensivity analysis
We will perform sensitivity analyses, excluding trials which do not report adequate (i) concealment of allocation, (ii) blinding of the outcome assessor".
We did however, perform a sensitivity analysis for overall survival which removed a trial that appeared to be an outlier. The removal of the Urbanski 1993 trial considerably reduced the I2 statistic meaning that there was less heterogeneity in the pooled trials. This was flagged up in the sensitivity analysis and effects of the interventions sections.
Contributions of authors
A Bryant and S Keep updated the review. P Martin‐Hirsch reviewed the updated version. P Martin‐Hirsch wrote the original systematic review with advice from the other investigators.
Sources of support
Internal sources
University of Manchester, UK.
External sources
-
Department of Health, UK.
NHS Cochrane Collaboration programme Grant Scheme CPG‐506
Royal College of Obstetricians and Gynaecologists, UK.
WellBeing Charity, UK.
Declarations of interest
None known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
COSA‐NZ‐UK 1998 {published data only}
- COSA‐NZ‐UK Endometrial Cancer Groups. Adjuvant medroxyprogesterone acetate in high‐risk endometrial cancer. International Journal of Gynecological Cancer 1998;8:387‐91. [Google Scholar]
DePalo 1993 {published data only}
- DePalo G, Mangioni C, Periti P, Vecchio M, Marubini E. Treatment of FIGO (1971) stage 1 endometrial carcinoma with intensive surgery, radiotherapy and hormonotherapy according to pathological prognostic groups. Long term results of a randomised multicentre trial. European Journal of Cancer 1993;29a:1133‐40. [DOI] [PubMed] [Google Scholar]
Lewis 1974 {published data only}
- Lewis GC, Slack NH, Mortel R, Bross I. Adjuvant progestagen therapy in the definitive treatment of endometrial cancer. Gynecologic Oncology 1974;2:368‐76. [DOI] [PubMed] [Google Scholar]
MacDonald 1988 {published data only}
- MacDonald RR, Thorogood J, Mason MK. A randomised trial of progestagens in the primary treatment of endometrial carcinoma. British Journal of Obstetrics and Gynaecology 1988;95:166‐74. [PubMed] [Google Scholar]
Malkasian 1978 {published data only}
- Malkasian G, Decker D. Aduvant progesterone therapy for stage 1 endometrial cancer. International Journal of Gynaecology and Obstetrics 1978;16:48‐9. [DOI] [PubMed] [Google Scholar]
Urbanski 1993 {published data only}
- Urbanski K, Karolewski K, Kojs Z, Klimek M, Dyba T. Adjuvant progestagen therapy improves survival in patients with endometrial cancer after hysterectomy. results of one‐institutional prospective clinical trial. European Journal of Gynaecologic Oncology 1993;14 suppl:98‐104. [PubMed] [Google Scholar]
Vergote 1989 {published data only}
- Vergote I, Kjorstad K, Abeler V, Kolstad P. A randomised trial of adjuvant progestagens in early endometrial cancer. Cancer 1989;64:1011‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Baekerlandt 2009
- Baekelandt MM, Castiglione M, on behalf of the ESMO Guidelines Working Group. Endometrial carcinoma: ESMO clinical recommendations for diagnosis, treatment and follow‐up [Endometrial carcinoma: ESMO clinical recommendations for diagnosis, treatment and follow‐up]. Annals of Oncology. 2009;20(supplement 4):29‐31. [DOI] [PubMed] [Google Scholar]
Cancer Research UK 2010
- Uterus (womb) cancer ‐ UK incidence statistics. http://info.cancerresearchuk.org/cancerstats/types/uterus/incidence/index.htm. 2010.
CTCAE 2006
- CTCAE. Common Terminology Criteria for Adverse Events. (http://ctep.cancer.gov/forms/CTCAEv3.pdf) 9th August 2006; Vol. v3.0 (CTCAE).
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG (eds). Systematic Reviews in Health Care: Meta‐Analysis in Context (2nd edition). London: BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
EUROCARE 2003
- Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, Grosclaude P, Hédelin G, Matsuda T, Møller H, Möller T, Verdecchia A, Capocaccia R, Gatta G, Micheli A, Santaquilani M, Roazzi P, Lisi D, and the EUROCARE Working Group. EUROCARE‐3: survival of cancer patients diagnosed 1990‐94 ‐ results and commentary. Annals of Oncology 2003;14 (Supplement 5):v61‐v118. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2008
- Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002. Cancer Incidence, Mortality and Prevalence Worldwide. IARC CancerBase No. 5, version 2.0. IARCPress, Lyon 2004.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kauppila 1984
- Kauppila A. Progestin therapy of endometrial, breast and ovarian carcinoma. A review of clinical observations.. Acta Obstet Gynecol Scand. 1984;63(5):441‐50. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Pecorelli 2009
- Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. International Journal of Gynecology and Obstetrics 2009;105(2):103‐4. [DOI] [PubMed] [Google Scholar]
SEER 2011
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. Surveillance Epidemiology and End Results Cancer Statistics Review, 1975‐2008. National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011.


