Abstract
Introduction
Morquio A syndrome (mucopolysaccharidosis type IVA, MPS IVA) is one of the lysosomal storage diseases and is caused by the deficiency of N-acetylgalactosamine-6-sulfate sulfatase (GALNS). Deficiency of this enzyme leads to accumulation of glycosaminoglycans (GAGs), keratan sulfate (KS) and chondroitin-6-sulfate (C6S). The majority of KS is produced by chondrocytes, and therefore, the undegraded substrates accumulate mainly in cells and extracelluar matrix (ECM) of cartilage. This has a direct impact on cartilage and bone development, leading to systemic skeletal dysplasia. In patients with Morquio A, cartilage cells are vacuolated, and this results in abnormal chondrogenesis and/or endochondral ossification.
Areas covered
This article describes the advanced therapies of Morquio A, focused on enzyme replacement therapy (ERT) and gene therapy to deliver the drug to avascular bone lesions. ERT and gene therapies for other types of MPS are also discussed, which provide therapeutic efficacy to bone lesions.
Expert opinion
ERT, gene therapy and hematopietic stem therapy are clinically and/or experimentally conducted. However, there is no effective curative therapy for bone lesion to date. One of the limitations for Morquio A therapy is that targeting avascular cartilage tissues remains an unmet challenge. ERT or gene therapy with bone-targeting system will improve the bone pathology and skeletal manifestations more efficiently.
Keywords: enzyme replacement therapy, GALNS, gene therapy, keratan sulfate, mucopolysaccharidosis IVA, tandem mass spectrometry
1. Introduction
Morquio A syndrome (mucopolysaccharidosis type IVA, MPS IVA) is an autosomal recessive lysosomal storage disorder (LSD) caused by deficiency of N-acetylgalactosamine-6-sulfate sulfatase (GALNS). This enzyme deficiency leads to progressive accumulation of the glycosaminoglycans (GAGs), keratan sulfate (KS) and chondroitin-6-sulfate (C6S). KS is produced mainly in cartilage tissue, and consequently, the GAGs accumulate primarily in the lysosomes of cartilage cells, associated ligaments, and extracellular matrix (ECM) produced by these cells [1-4]. Thus, excessive storage of KS causes systemic skeletal dysplasia such as striking short trunk stature, cervical spinal cord compression, pectus carinatum, kyphoscoliosis, knock-knee, hypermobile joints and an abnormal gait with an increased tendency to fall [5-8].
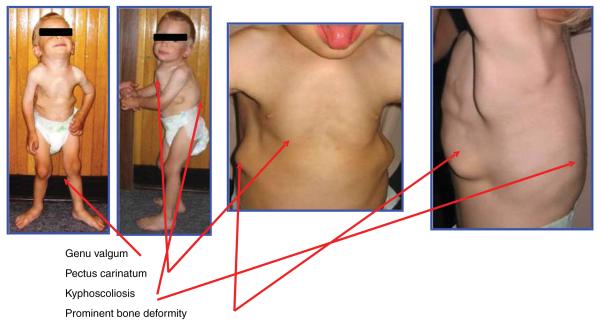
Patients with Morquio A appear healthy at birth. Major signs and symptoms in most Morquio A patients are usually observed before their first birthday, including kyphosis, protrusion of the chest and prominent forehead. Milestones for walking are often delayed. Patients with Morquio A are usually evaluated during the second year of life for unique skeletal features including knock-knee, growth retardation, laxity of joints and abnormal gait with a tendency to fall in addition to kyphosis, protrusion of the chest and prominent forehead (Figures 1–3). Patients with Morquio A can usually be distinguished clinically from patients with other MPS by preservation of intelligence and characteristic skeletal changes manifesting as a spondyloepiphyseal dysplasia with unique laxity of joints (knee, hand cervical spine, hip) and cervical instability. Odontoid hypoplasia is the most critical skeletal feature to be found in most Morquio A patients. Odontoid hypoplasia in combination with ligamentous laxity and extradural GAGs deposition can result in atlantoaxial subluxation and/or cervical stenosis with or without cord compression, cervical myelopathy or even death [5-7].
Figure 1.
Clinical features of Morquio A patient (copyright permission from International Morquio Organization). A 3-year-old patient had bone deformities (short stature, pectus carinatum, kyphoscoliosis, genu valgum, prominent forehead and abnormal gait); height 85 cm, 25 percentile of male Morquio A growth chart; body weight 10 kg.
Figure 3.
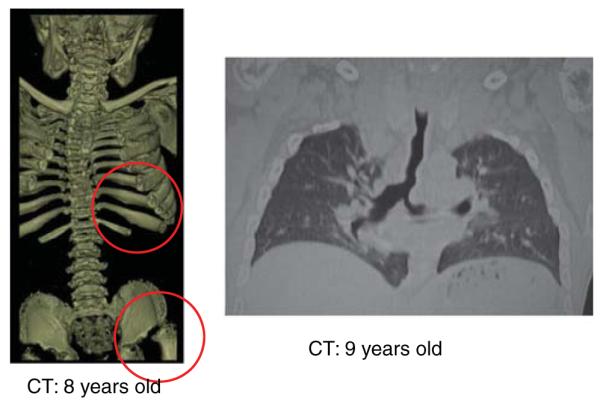
Computed tomography pictures in Morquio A patients. Left panel shows 3D reconstructed CT indicating scoliosis, platyspondyly, oar-like ribs and drastic hip joint with a flattered femoral cap. Right panel shows the twisted and abnormally shaped lumen of trachea and the left bronchus. The redundancy and buckling of the main trachea are observed.
Other potential complications include airway and pulmonary compromise (Figure 4), muscle weakness, valvular heart disease, hearing loss, fine corneal clouding and widely spaced teeth with abnormally thin enamel [5-7]. Patients with severe phenotype often do not survive beyond the second or third decade of life, primarily related to cervical instability and pulmonary compromise. Most Morquio A patients have difficulty with anesthesia because of narrow airway and a small, restrictive lung. Difficulty with both upper and lower airways of Morquio A patients increases as the disease progresses and greatly increases the risk of anesthesia and sedation [9]. Patients with mild manifestations of Morquio A have been reported to survive into the seventh decade of life [5-7].
Figure 4.
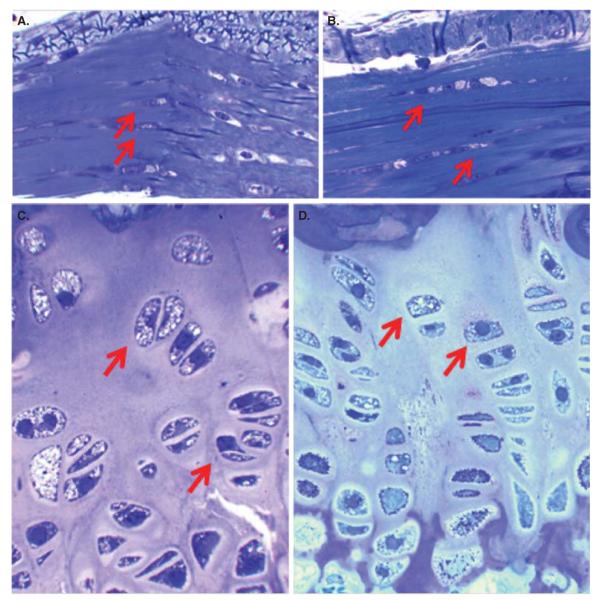
Histopathology after 3 months of ERT. Connective tissues surrounding the article (A, B) and growth plate (C, D) are shown. Sections are stained with toluidine blue. Magnifications: ×100. Connective tissue: The cells in connective tissues surrounding the articular cartilage in an untreated MPS IVA mouse contain the vacuoles (A), and ERT with SUMF1–GALNS shows still storage material (B). Growth plate: An untreated MPS IVA mouse shows storage material in chondrocytes and the disorganized cartilage layer (C). Most chondrocytes were vacuolated with lysosomal distension. The storage materials in the chondrocytes were reduced to some extent, and the column structure was organized more after ERT with SUMF1–GALNS (D). Magnification: ×100 in A–D. Scale bar (A–D) = 2 μM.
In spite of the devastating cartilage disease, there have been almost no data reporting histological and molecular evaluations of bone and cartilage pathology in Morquio A patients. Biopsies from two affected brothers have shown a poorly organized morphological structure in both the superficial and deep zones of articular cartilage [10]. Abnormal collagen modification was also found in articular cartilage [11]. Since in Morquio A patients the degeneration of cartilage is observed even at birth, chondrogenesis and endochondral ossification during early growth development may be already affected by accumulation of undegraded KS in cartilage.
A recent autopsied case revealed the accumulation of foam cells and macrophages in multiples tissues including bone, cartilage, ligaments, heart valves, aorta, lung, liver and kidney. These findings indicate that chronic inflammation may worsen clinical features in MPS IVA patients [12]. It was of interest that C6S was accumulated in aorta and heart valves.
Therapies for MPS have been developed experimentally and clinically. These include enzyme replacement therapy (ERT), gene therapy, hematopoietic stem cell transplantation (HSCT) and substrate reduction therapy (SRT), all of which lead to the partial improvement of clinical phenotypes. ERT is approved for use in patients with mucopolysaccharidosis I (MPS I) [13], MPS II [14,15] and MPS VI [16-19]. A clinical trial for Morquio A has been conducted. Patients treated with ERT showed clinical improvement of somatic manifestations and improved quality of life. However, there are several limitations with current ERT: i) limited effect on neurological and skeletal symptoms [20,21], ii) rapid clearance from the circulation and iii) immunological issues (antibody production leads to reduced efficacy) [13,22-24]. To resolve the above issues, a long circulating bone-targeting enzyme has been created [25,26].
Experimental gene therapies have been tested in animal models and human subjects [27,28]. However, viral vectors for gene therapy have not been delivered to bone efficiently, and targeting the viral vector to bone remains a major challenge. Gene therapy with bone-targeting adeno-associated virus (AAV) vectors has been experimentally evaluated in Morquio A mouse models [6,7,29]. HSCT shows some benefits in physical activity and bone mineral density of treated mice, but longer term observations are needed. HSCT of MPS patients improves quality of life, but therapeutic effect remains limited in bone lesions [30].
Surgical procedures for MPS IVA are often required and these increase with age; procedures include adenoidectomy, tonsillectomy, ear tube, cervical decompression/fusion, osteotomy, knee correction surgery and hip correction surgery. Most patients with Morquio A have subdural depositions of GAGs causing stenosis and odontoid hypoplasia resulting in subluxation and instability of cervical C1–C2 region. Therefore, physicians prescribe cervical spine fusion/decompression surgery for the most affected patients even without signs and symptoms.
Procedures to fix bone lesions remain an unmet challenge. Specific delivery of drugs to avascular cartilage tissue has not yet been attained, although several promising results targeting drugs to bone to treat a range of bone diseases have been demonstrated to be effective both experimentally and clinically [26,31-38,9].
In this article, advanced therapies, ERT and gene therapy for Morquio A syndrome are described along with their expectations and limitations. These therapies are also reviewed for other types of MPS to investigate therapeutic effect in bone lesions.
2. Advanced therapies for Morquio A syndrome
2.1 Enzyme replacement therapy
ERT provides limited impact on bones and joints. The success of ERT largely depends on biodistribution and concentration of the infused enzyme, which can easily reach visceral organs such as liver and spleen, but cannot readily access cartilage and ligaments due to their avascularity. Clinical trials with ERT in MPS I, II and VI showed limited improvement in joint pain, stiffness or joint range of motion. Skeletal dysplasia cannot be cured by the current ERT [39-41], since there is little evidence that conventional ERT directly delivers enzyme to avascular cartilage lesions. Most lysosomal enzymes have a short half-life in the circulation due to rapid clearance in liver, kidney and spleen by carbohydrate-recognizing receptors, further limiting the availability of enzyme for delivery to bone and cartilage lesions.
ERT markedly reduces serum levels of tumor necrosis factor (TNF) and receptor activator of nuclear factor (NF)-κB (RANKL) in MPS VI rats, and therefore, ERT may have positive secondary anti-inflammatory effects on the organs that are not easily accessible to the enzyme, providing additional clinical benefits [42]. Another study on animals with MPS VI and VII indicated that ERT can prevent vertebral and facial bone abnormalities, but cannot reverse progressive deformities [43]. Degenerative joint changes are not delayed in animals treated since birth, although skeletal pathology is reduced, with more normalized bone dimensions and with more uniform bone density and a trabecular pattern [43].
An MPS IVA clinical trial has been conducted and has shown a longer 6-min walk distance in a weekly infusion group compared with a placebo group [44]. The positive clinical effects of ERT are not derived from direct delivery of the enzyme to the cartilage. A surgical remnant from a 17-year-old Morquio A patient who had been in an extension clinical trial for 3 months did not show any evidence of reduction of vacuoles in cells of cartilage tissues. The underlying problems associated with progressive skeletal deformity and laxity of joints will remain unsolved by the current ERT that uses native enzyme coexpressed with sulfatase-modifying factor (SUMF 1) [45,46]. Since bone pathology is observed at birth [47] or even earlier [48] in patients and newborn MPS IVA mice, a new approach such as bone-targeting system is needed to achieve significant clinical efficacy for the skeleton. Six months of ERT for adult MPS IVA mice was effective at reducing the development of pathology in visceral organs, but vacuolated chondrocytes in articular and epiphyseal cartilage still remained [25].
Pathological analysis of MPS IVA mice treated continuously from birth to 3 months showed that although storage materials were still found in chondrocytes of growth plate and surrounding connective tissue, the column structure of the growth plate was more normally organized than that in untreated mice (Figure 4). Although this is not a complete remission in bone pathology, this finding demonstrates the importance of early treatment. Thus, the therapeutic efficacy of the current ERT could be improved if patients are treated at a younger age [49].
Careful long-term evaluation of ERT will be required to determine whether the infused enzyme can eventually improve skeletal pathology in Morquio A patients. Shortly after the start of ERT, most patients show decreased urine KS level, but a decrease in blood KS level has not yet been demonstrated. Urine KS could be derived from KS stored or filtered in kidney, and consequently reduced urine KS seen in treated Morquio A patients may not correspond to any improvement in bone pathology or any other clinical signs and symptoms. The majority of blood KS originates from the chondrocytes and their ECM, and therefore, its reduction would be a more direct indicator of bone improvement. It would be of great interest to know whether blood KS levels decrease over time, the extent to which blood and urine KS levels are correlated, how clinical improvements are correlated with reduction of blood and urine KS, and how blood and urine KS composition and characteristics differ.
To improve bone pathology, two alternative approaches of ERT have been developed using modified enzymes. One is to chemically modify an enzyme to prolong its half-life to treat MPS VII mice and the other uses a bone-targeting strategy to treat MPS IVA and hypophosphatasia mice.
β-Glucuronidase (GUS) was chemically modified to destroy mannose-6 phosphate moieties (PerT-GUS) so that the enzyme escapes clearance by mannose 6-phosphate and mannose receptors. This modified enzyme showed a markedly prolonged circulation half-life (over 100 times), compared with native GUS enzyme. The enzyme reduced central nervous system (CNS) storage more effectively than native GUS in an MPS VII mouse model [50]. To evaluate the effectiveness of long-circulating PerT-GUS in reducing the skeletal pathology, MPS VII mice were treated for 12 weeks with PerT-GUS or native GUS. Reduced storage material and a more organized growth plate were observed in PerT-GUS-treated mice compared with native GUS-treated mice. Long-circulating PerT-GUS provides a significant impact in rescuing bone lesions as well. This chemically modified GALNS enzyme into MPS IVA mice is now under investigation.
Hydroxyapatite (HA) is a major inorganic matrix in bone, but is not found in soft tissues. Drugs attached to HA will be released in the process of the bone resorption. Thus, targeting a drug to HA is a potential method for a selective drug delivery to bone. Recently, a novel bone-targeting peptide has been attached to an enzyme (alkaline phosphatase) and shown that the tagged enzyme is delivered more specifically to bone than unmodified native enzyme. This tagged enzyme improves the clinical and pathological consequence of systemic bone disease, hypophosphatasia and osteoarthritis [31,32,38]. A clinical trial for hypophosphatasia using this bone-targeting system showed substantial improvement of bone pathology and was clinically effective [33].
Human GALNS has been bioengineered to tag a hexaglutamate sequence (E6) to its N-terminus (E6-GALNS). This tagged enzyme has a markedly prolonged retention in the circulation, increasing the blood levels to 20 times higher than that of the untagged enzyme since the glycosylation site has presumably been changed. The tagged enzyme was also retained longer in bone. Pathological analysis of MPS IVA mice treated with the targeting enzyme showed clearance of the storage materials from chondrocytes, especially after 24 weekly injections. These findings suggest that the use of the tagged enzyme enhances delivery and improves bone pathology in MPS IVA mice [26].
2.2 Gene therapy
The recent approval of the first gene therapy products in Russia [51] and Europe [52] has brought new hopes into the gene therapy field. Although several studies have shown promising results for MPS [53,54], only three clinical trials have been approved as of July 8, 2013 [55]. The results of clinical trials have not been published yet. In this section, a review of research of potential gene therapies for MPS over the past 5 years is presented (Table 1). For MPS IVA, preclinical trials have been performed to demonstrate the feasibility of correction of the disease using adeno-associated virus (AAV)-derived vectors. Unique aspects of studies of MPS IVA include the first study of the impact of systemic coadministration of replacement enzyme with the sulfatase-modifying factor 1 (SUMF1) and the development of a modified AAV vector carrying a bone-tag peptide to improve its delivery to bone. Here the current trend for gene therapies in MPS and prospects for novel gene therapies for MPS IVA are summarized.
Table 1.
Gene therapy studies for mucopolysaccharidosis (2008 - 2013).
| Disease | Vector | Animal and duration | Conclusions | Refs. |
|---|---|---|---|---|
| MPS I | Plasmid | Adult mice (3 weeks) |
Production of the fusion protein transferrin-alpha-l-iduronidase (IDUA) allowed higher enzymatic activity in the brain than an unmodified IDUA enzyme, after hydrodynamic tail vein injection |
[56] |
| Transposon sleeping beauty |
Adult mice (3 months) |
IDUA activity levels maintained 100-fold wild-type levels with immunomodulation; reduction of GAG storage in most tissues, including the brain; correction of several clinical manifestations |
[58,59] | |
| Plasmid minicircle DNA | Adult mice (up to 80 days) |
Immunomodulation was necessary to achieved long-term IDUA activity levels for up to 80 days |
[57] | |
| γ-Retroviral | Neonatal and adult mice | Gamma-retroviral vectors showed better results in enzymatic activity, GAG storage clearance and clinical corrections than self-inactivating retroviral vector. Long-term expression was observed in 90% of treated animals, which prevented biochemical abnormalities in several organs, including complete correction of brain, hearing, and vision abnormalities |
[60,63,64,109] | |
| γ-Retroviral | Newborn dogs (1 year) |
Enzymatic activity was up to 26-fold wild-type levels. Normalization of GAG was observed in most tissues, as well as an improvement of several clinical manifestations. Cervical spine disease was severe and difficult to correct. |
[62,61] | |
| AAV8 | Newborn mice (10 months) |
Intracerebroventricular administration achieved IDUA increase in the hippocampus and motor and limbic neurons. Treatment prevented neurocognitive dysfunction. |
[67] | |
| AAV5 | Adult dog (up to 8 months) |
Improvements in brain markers such as storage of GAGs, GM2 and GM3 were achieved but values remain higher than those observed in nonaffected dogs |
[66] | |
| Lentiviral | Adult mice (6 months) |
Hematopoietic stem cells transduced with lentiviral vector showed IDUA activity levels in brain 4.5-fold wild type; therapeutic benefit provided by the use of transduced hematopoietic stem cells gene therapy on critical MPS I manifestations was higher than that observed only after HSCT. |
[65] | |
| MPS II | Plasmid | Adult mice | Electro gene transfer achieved iduronate-2-sulfatase (IDS) activity levels in muscle, and therapeutic benefits were limited to this tissue. |
[68] |
| AAV2/8 | Adult mice | Supraphysiological activity levels were observed in plasma and several tissues. GAG storage were completely corrected in all analyzed organs. |
[69] | |
| MPS IIIA | Lentiviral | Adult mice (6 - 7 months) |
Normalization of enzymatic activity, reduction of GAG in the brain, improvement in behavioral performance; no obvious effect of mannitol pretreatment |
[77,78] |
| Canine AdV | Adult and newborn mice (up to 20 weeks) |
Transient gene expression; partial reduction of GAG in the brain; production of neutralizing antibodies against the vector |
[79,80] | |
| AAV9 | Adult mice | Transgene expression in the brain and peripheral tissues after intravenous infusion; normalization of GAG; reduction of brain inflammation; improvement of animal survival |
[74] | |
| MPS IIIB | AAV2 and AAV9 | Adult mice (until the last stage of clinical manifestations) |
Prolonged life span, improved behavioral performance, reduction of GAG, delay of late- stage clinical manifestation. Mannitol pretreatment enhanced transduction of brain cells; long-term vector maintenance and expression. AAV9 vector showed better results than AAV2 vector. |
[71,72,75] |
| AAV2/5 | Newborn mice (1 year) |
Improvement of life span, motor function, hearing impairment, activity onset. GAG reduction was not observed. Combination with BMT had a synergistic effect on hearing impairment and GAG storage but antagonist on life span. |
[73] | |
| AAV5 | Adult dogs (4 months) |
Immunosuppression is necessary for enzyme production and partial reduction of GAG storage. |
[66] | |
| AAV2/5 and lentiviral | Newborn mice (1 year) |
Combination of intracranial AAV and intravenous lentivirus vector administration produced better results in recovery of motor function and hearing impairment than each vector separately. |
[70] | |
| Lentiviral | Adult mice (6 months) |
Reduction of storage lesions in the brain, behavioral performance recovery | [76] | |
| MPS IVA | γ-retroviral | In vitro | Enzymatic activity was increased in several cell types transduced with the vector, with levels higher than those observed in normal cells. |
[93] |
| AAV2 |
In vitro and adult mice (12 weeks) |
Eukaryotic promoters induced similar expression levels than those observed with viral promoter. Coexpression with SUMF1 produced a significant increase in enzymatic activity and favored therapeutic levels in bone. |
[94,95,98] | |
| Bone-tagged AAV2 | Adult mice (4 weeks) |
Modified vector showed significantly increase in bone affinity, which led to a significant increment in gene expression in bone cells and enzymatic activity of 41.9% of wild-type levels |
[104,105] | |
| AAV2 and lentivirus | In vitro | AAV vectors carried both GALNS and SUMF1 genes showed better results than when both genes were transduced separately. Lentiviral vectors allowed higher enzymatic activities than those observed with AAV vectors. |
[108] | |
| MPS VI | Lentivirus |
In vitro and direct knee joint injection in rats |
Expression for up to 41 days in culture cells and up to 8 weeks in transduced synovial cells after direct knee joint injection |
[85] |
| AAV2/8 | Newborn and juvenile cats (up to 2 years) |
Wild-type enzymatic activities in animals treated reduction of GAGs in urine and tissues. Improvement in heart abnormalities, femur length, growth plate architecture, facial dysmorphism and mobility test. No improvement in spine abnormalities Preexisting neutralizing anti-AAV8 antibodies negatively impact serum arylsulfatase B (ARSB) activity, GAG levels and femur length. |
[81,82] | |
| -Retroviral | Newborn cats (up to 8 years) |
Serum ARSB activity up to 18-fold normal. Normalization of GAG in urine and tissues, facial dysmorphism, body weight, femur length and heart abnormalities. Most of the spine abnormalities remained unaltered. High enzymatic activities can be maintained for up to 8 years without side effects |
[84] | |
| MPS VII | Plasmid | Adult mice | Liver GUSB production and GAGs clearance in the peripheral tissues and brain | [86] |
| Sleeping beauty transposon |
Adult mice (14 weeks) |
GUSB activity levels were 100-fold wild type. Lysosomal pathology in the liver and other organs were partially corrected. |
[58] | |
| γ-Retroviral | Neonatal dogs (up to 12 years) |
GUSB activity present in serum and tissues (including brain) for up to 12 years. GAGs were reduced in most tissues and femur length and mobility was improved but spine abnormalities were not completely corrected. |
[62,87,88] | |
| AAV | Adult mice (6 months) |
The hGBp promoter showed GUSB expression levels in the brain at similar levels as neuron-specific promoter. |
[91] | |
| AAV9 and modified capsid AAV |
Adult mice | Modified capsid AAV corrected cognitive deficits and GAGs storage in the brain. AAV9 was inhibited by sialic acid present in the brain. |
[90] | |
| Lentiviral | Newborn and adult mice | Somatic correction was observed in neonatal and 7-week-old gene therapy mice. Lumbar and femoral lengths were significantly improved. |
[89] | |
| Lentiviral | Adult mice | With intravenous administration, brain pathology was partially corrected, while intracerebroventricular administration produced better correction in the brain and eye. |
[92] |
GUSB: beta-glucuronidase; HSCT: Hematopoietic stem cell therapy; hGBp: GUSB minimal promoter
2.2.1 MPS I (Hurler syndrome)
During the past 5 years, nonviral (plasmids or transposon) and viral (gamma-retrovirus, lentiviral or AAV) vectors have been used for gene therapy of MPS I in animal models (Table 1). Nonviral vector-based therapy was conducted using plasmid [56], minicircle DNA plasmid [57] and sleeping beauty transposon [58,59]. In all cases, immunomodulation was necessary to achieve long-term gene expression, increased therapeutic enzymatic activities and reductions of accumulated GAGs. The potential for nonviral vector-based therapy was highlighted by the use of sleeping beauty transposon to obtain supraphysiological enzymatic activity levels [58,59] and by the use of a fusion of α-iduronidase to transferrin to enable the delivery of enzyme across the blood–brain barrier after an intravenous administration [56]. Nonviral vectors could avoid some of the side effects associated with the use of viral vectors, but low and short-term expression still limit the impact of these vectors on gene therapies for MPS. In contrast to nonviral vectors, γ-retroviral vectors allowed long-term expression and normal or even supraphysiological levels of enzymatic activity in serum and tissues in MPS I mice and dogs [60,61]. GAG and secondarily elevated enzymes (β-hexosaminidase and β-glucuronidase) were reduced in most tissues, leading to the correction of hernias, chest deformities, joint disease, facial dysmorphia, corneal clouding, valvular heart disease and aortic dilatation. However, lesions in aorta and cervical spine were more difficult to correct, even when animals were treated in the neonatal period and with supraphysiological enzyme levels that were maintained for long periods (up to 7 years) [62,63]. Self-inactivating (SIN) retroviral vectors have also been evaluated to reduce the insertional mutagenesis associated with γ-retroviral vectors. Although SIN vectors allowed significant increases in enzymatic activity, correction of some biomarkers (e.g., GAG and secondary elevated enzymes) and improvements of clinical manifestations, they were not as effective as therapies with γ-retroviral vectors [60,64]. As an alternative approach, lentivirus has been used to transduce hematopoietic stem cells followed by transplantation of the transduced cells. This hematopoietic stem cell gene therapy provided better results than HSCT with untransduced cells and resulted in a complete normalization of bone deformities (i.e., skull width and femur and humerus length) and improvement of behavioral performance [65]. Finally, AAV5 and AAV8 vectors were evaluated in MPS I mice and dogs [66,67]. After a direct brain administration of vectors, only AAV8 showed significant improvement in accumulation of GAGs and neurocognitive dysfunction, suggesting the importance of selection of the vector.
2.2.2 MPS II (Hunter syndrome)
Two studies of MPS II gene therapy have been reported during the past 2 years (Table 1). In the first case, electro gene transfer was used to mediate the delivery of a plasmid to muscle cells [68]. Although a significant increase of enzymatic activity was observed in muscle from treated mice, activity was not detected in liver, spleen, kidney, lung and heart, and reduction in GAG storage was not observed in treated mice. Intravenous infusion of AAV2/8 vector was also used to mediate gene transfer in an adult MPS II mouse model, providing supraphysiological enzymatic activity in tissues, normalization of GAG storage and reduction of neuropatho-logical alterations [69].
2.2.3 MPS III (Sanfilippo syndrome)
Impairment of the CNS is the main characteristic of MPS III in both animal models and patients. Therefore, studies on gene therapy for MPS III have mainly focused on the correction of the neurodegeneration. As shown in Table 1, AAV [66,70-75], lentivirus [70,76-78] and canine adenovirus (AdV) vectors [79,80] have been evaluated for MPS IIIA and IIIB gene therapies, which have been delivered intravenously or by direct brain injection (i.e., intracranial, intraventricular, intracisternal or intracranial). A common approach for the treatment of MPS III was intravenous pretreatment with mannitol, which in most cases allowed an increase of the number of transduced cells in both brain and peripheral organs, as well as improvement of the therapeutic effect of the therapy [71,72,75,78]. Except for the studies carried out with canine AdV vector [79,80], gene therapy for MPS IIIA and IIIB showed long-term vector maintenance and expression, improvement in behavioral performance, reduction of GAG in urine and/or tissues, increase in life span and normalization of secondary enzymatic activities (e.g., β-glucuronidase or β-hexosaminidase). Nevertheless, enzymatic activity in serum and tissues ranged between normal and subnormal levels, showing that supraphysiological enzymatic activities are not required to achieve a therapeutic effect in MPS III. Gene therapy (AAV vector) combined with bone marrow transplantation (BMT) has a synergistic effect of the correction of motor function, hearing impairment, and GAG storage, but negatively impacts the life span of the treated animals [73]. One of the studies using MPS IIIB dogs reported that immunosuppression was required to allow the production of the enzyme and the partial reduction of the GAG storage in the brain [66]. It is noteworthy that the correction of behavioral and neurological impairments in treated adult MPSIII mice represents the promising results for the treatment of MPS III patients.
2.2.4 MPS VI (Maroteaux–Lamy syndrome)
During the past 5 years, two important studies have been carried out for MPS VI (Table 1). In the first study, AAV2/8 vectors were infused to MPS VI cats at 5 or 50 days of age [81]. Reduction of GAGs in urine and tissues, and improvement in heart abnormalities, femur length, growth plate architecture, facial dysmorphism, and mobility test (walk, run, and jump) were observed in treated animals, while improvement in spinal and joint abnormalities was limited regardless of the doses and the age of treatment [81]. Later, it was observed that the presence of neutralizing antibodies (Nabs) significantly impact the serum arylsulfatase B (ARSB) activity, limiting the effect of the vector administration in GAG reduction and femur length [82]. Since antibodies to AAV8 have been observed in 15 – 25% of healthy human populations [83], these results show that clinical applications of this gene therapy will require Nab screening of patients prior to treatment.
One of the most promising studies of MPS VI gene therapy indicates that a γ-retroviral vector carrying feline ARSB gene should be infused into newborn MPS VI cats [84]. The animals were monitored for up to 8 years postinfusion, showing maintenance of supraphysiological enzymatic activity in serum and tissues, normalization of GAG in urine and tissues, and less pronounced facial dysmorphism, reduced body weight, reduced femur length, and heart abnormalities. Spinal abnormalities remained although some improvements were observed 6 years postinfusion. This study showed that, at least in cats, the coexpression of SUMF1 is not required to produce high levels of the deficient sulfatase and that high enzymatic activities can be maintained for up to 8 years without side effects.
The potential of lentiviral vector for MPS VI treatment has been evaluated in vitro (fibroblast and chondrocytes) and in vivo (direct knee joint injection in rats), showing expression for up to 41 days in culture cells and up to 8 weeks in transduced synovial cells after direct knee joint injection [85].
2.2.5 MPS VII (Sly syndrome)
MPS VII has been one of the most studied MPSs in the development of gene therapy strategies for this group of diseases. During the past 20 years, gene therapy reports for MPS VII have used plasmid [86], sleeping beauty transposon [58] and gamma-retroviral [62,87,88], lentiviral [89] and AAV [90,91] vectors (Table 1). Nonviral vector-based therapy using a plasmid allowed pathology correction both in the peripheral tissues and in the brain [86], while the use of a sleeping beauty transposon vector allowed an improvement only in somatic tissues reaching β-glucuronidase levels 100-fold higher than in wild-type animals, although immunomodulation was required to achieve long-term expression with this vector [58].
One of the most promising results of the use of γ-retroviral vectors in gene therapy for MPS was reported by Xing et al. [88]. MPS VII dogs were treated 2 – 3 days after birth and followed for up to 11 years. Treated animals showed that the therapeutic levels of enzymatic activity were maintained throughout the studied period without side effects associated with the therapy, and GAG storage in urine and tissues was significantly reduced. Although a significant correction was observed for most bone deformities, therapy failed to normalize calcification of the ventral epiphysis of the vertebral bodies, where GAG remained elevated, and treatment did not prevent intervertebral disk degeneration [62,87]. However, treated dogs showed a significant improvement in life span and mobility. Lentiviral vectors allowed significant improvements in lumbar and femoral lengths in treated newborn and adult MPS VII mice after an intravenous administration [89], while intracerebroventricular administration allowed biochemically and histopathologically brain correction and better clearance of storage material from the eye [92]. The use of AAV vectors to treat MPS VII mice showed stable GUSB expression for up to 6 months in the brain [91], and comparison between AAV9 and capsid-modified AAV vectors showed that the modified vector reduced cognitive deficits and brain GAG storage [90].
2.2.6 MPS IVA (Morquio A syndrome)
The first study of gene therapy for Morquio A was conducted using a γ-retrovirus vector. This resulted in increased GALNS activity and reduced GAGs in transduced normal and Morquio A human lymphoblastoid B cells, human keratinocytes, murine myoblasts (C2C12) and rabbit synoviocytes (HIG-82) [93]. Subsequently, AAV vectors were selected for Morquio A gene therapy studies and divided into three phases. In the first phase, the effect of the promoter region on GALNS expression was evaluated in vitro, showing that eukaryotic promoters (elongation factor 1α or α1-antitrypsin) induced similar expression levels to those observed with cytomegalovirus (CMV) promoter [94-96]. These alternative promoters may help avoid the gene silencing associated with the CMV promoter [97]. Coexpression with SUMF1 resulted in a fourfold increase in enzymatic activity and favored the secretion of the enzyme [94]. In the second phase, 12 weeks after a Morquio A mouse model was treated with a single intravenous administration of these AAV vectors, plasma enzymatic activity levels were restored to 19 or 8.5% of wild-type levels, with or without coadministration with SUMF1. Enzymatic activity was increased in the peripheral tissues by up to 22% of wild-type levels, and coadministration of SUMF1 allowed significantly increased activity in most tissues. Of note, GALNS activity was not restored in bone when Morquio A mice were treated with AAV–GALNS vector alone, but coadministration of AAV–SUMF1 vector resulted in 33% of wild-type levels in this tissue. Thus, SUMF1 coexpression enabled achievement of therapeutic levels of enzyme in the most severely affected tissue [98]. Finally, in the third phase, the benefits of AAV retargeting through the modification of viral capsid were exploited [99-103]. The AAV vector was modified by the insertion of multiple copies of a short acidic amino acid peptide within the viral capsid to confer affinity of the virus for hydroxyapatite (HA), the major constituent of bone matrix. This increase in affinity for HA resulted in higher vector genome copies in bone, which led to a significant increase in gene expression in bone cells and enzymatic activity that was 42% of wild-type levels [104,105]. Recently, a new set of AAV vectors carrying both GALNS and SUMF1 genes was constructed using internal ribosome entry site (IRES), either viral or human. Furthermore, lentiviral vectors carrying GALNS or SUMF1 complementary DNA (cDNA) were also evaluated. In HEK293 cells, enzymatic activities produced using SUMF1–IRES–GALNS AAV vectors, carrying either viral or human IRES, were higher than those produced using AAV–GALNS: AAV–SUMF1 cotransduction [106-108]. GALNS transduction mediated by the lentiviral vector allowed up to a 100-fold increase in enzymatic activity in comparison to the levels observed with the AAV vectors. In Morquio A fibroblasts, lentiviral vectors produced higher enzymatic activities than those observed with AAV vectors with or without the use of IRES sequences, in addition to the normalization of β-hexosaminidase and β-galactosidase levels [108].
In summary, during the last decade, gene therapies for MPS have shown a favorable safety profile with long-term expression periods (up to 11 years) for the viral vectors and promising results for nonviral vectors. However, spine deformities seem to be difficult to correct even with neonatal therapy and supraphysiological levels of the enzymatic activity. Specifically for MPS IVA, the results show the feasibility to treat the disease by using AAV vectors with bone-targeting system and the need to use the coexpression with SUMF1 to produce therapeutic levels of the enzymatic activity in the bone. Nevertheless, further studies should focus on the long-term evaluation of the therapy with both the unmodified and the modified vectors, as well as the in vivo evaluation of the lentiviral vectors, which will show a great potential for the treatment of this disease.
3. Expert opinion
Morquio A syndrome is a prototype of severe progressive skeletal dysplasia, whose pathogenesis of the bone lesions remains unknown. In spite of unique clinical features including laxity of joints and normal intelligence, delay of diagnosis often happens because of ignorance of this rare disorder and false negative results of urine total GAG assay. Physicians, who take care of Morquio A patients, should be acquainted with the most common complications, diagnosis of the disease and an expert center. This will lead to earlier diagnosis for patients, providing better comprehensive therapy and avoidance of progression of irreversible damage.
A comprehensive assessment of individual patient at initial diagnosis should also be required by primary clinicians and experts since it leads to death in the second or third decade of life or severe handicaps in the absence of the proper orthopedic surgical procedure and respiratory care in an appropriate timing.
Although current treatments available do not cure the disease, they may provide the potential to improve the clinical phenotypes in the bone, especially if treatment starts at an early stage of the disease.
However, development of therapy for systemic bone dysplasia, especially in avascular growth plate region, remains an unmet challenge.
The advanced therapies described here show the potential on how to reach systemic bone disease by using the bone-targeting system. Novel targeting therapies with the current ERT or gene therapy along with newborn screening should be established for this disorder. Such strategy will facilitate to improve a quality of life in patients with Morquio A syndrome.
Article highlights.
Advanced therapies for MPS IVA are described.
Bone-targeting system can provide more impact.
Avascular cartilage region is a challenge tissue.
This box summarizes key points contained in the article.
Figure 2.
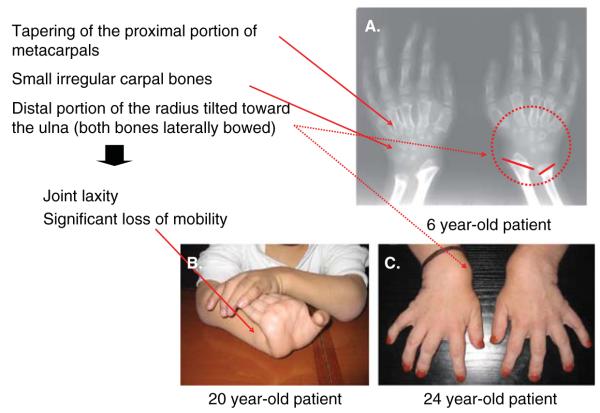
Skeletal/joint disease—Hands (copyright permission from International Morquio Organization). A. Bilateral hand radiographs in a patient aged 6 years. Note the tapering of the proximal portion of metacarpals 2 through 5 and small irregular carpal bones. Ossification is less and premature, suggesting that the radiographic bone age is substantially delayed compared with the chronological age. B. Hyper mobile joints are obvious by the age of 2 – 3 years. C. The hands in time take on a characteristic with tilting of the radial epiphysis toward the ulna, resulting from a combination of metaphyseal deformities, hypoplasia of the bones and degradation of connective tissues near the joint secondary to GAG accumulation.
Acknowledgments
Editorial assistance to the manuscript was provided by Michelle Stofa at Nemours/Alfred I. duPont Hospital for Children. S Tomatsu and CJ Alméciga-Díaz regarded as joint first authors.
Declaration of interest This work was supported by grants from the Austrian MPS Society, Jacob Randoll Foundation, Bennett Foundation, National MPS Society, and International Morquio Organization (Carol Ann Foundation). S Tomatsu and R Mason were supported by the National Institutes of Health grant P20 GM103464 08. CJ Alméciga-Díaz and LA Barrera were also supported by Colciencias and Pontificia Universidad Javeriana (ID PRY 003400 and 003577). The content of the article has not been influenced by the sponsors.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. 8th edition McGraw-Hill; New York: 2001. pp. 3421–52. [Google Scholar]
- 2.Tomatsu S, Orii KO, Vogler C, et al. Mouse model for Galns−/− produced by targeted disruption of the gene defective in Morquio A disease. Hum Mol Genet. 2003;12:3349–58. doi: 10.1093/hmg/ddg366. [DOI] [PubMed] [Google Scholar]
- 3.Tomatsu S, Gutiérrez MA, Nishioka T, et al. Development of MPS IVA mouse (Galns tm(hC79mC76)slu) tolerant hGALNS. Hum Mol Genet. 2005;14:3321–36. doi: 10.1093/hmg/ddi364. [DOI] [PubMed] [Google Scholar]
- 4.Tomatsu S, Vogler C, Montaño AM, et al. Murine model (Galns(tm(C76S)slu)) of MPS IVA with missense mutation at the active site cysteine conserved among sulfatase proteins. Mol Genet Metab. 2007;91:251–8. doi: 10.1016/j.ymgme.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Montaño AM, Tomatsu S, Gottesman GS, et al. International Morquio A registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007;30:165–74. doi: 10.1007/s10545-007-0529-7. • Summary of clinical phenotypes in Morquio A.
- 6.Tomatsu S, Montaño AM, Oikawa H, et al. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Cur Pharm Biotech. 2011;12:931–45. doi: 10.2174/138920111795542615. • Review for therapies of Morquio A.
- 7.Tomatsu S, Mackenzie WG, Theroux MC, et al. Current and emerging treatments and surgical interventions for Morquio A syndrome: a review. Res Rep Endocr Disord. 2012;2:65–77. doi: 10.2147/RRED.S37278. • Review for therapies of Morquio A.
- 8.Hendriksz CJ, Al-Jawad M, Berger KI, et al. Clinical overview and treatment options for non-skeletal manifestations of mucopolysaccharidosis type IVA. J Inherit Metab Dis. 2012;36:309–22. doi: 10.1007/s10545-012-9459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi-Nishioka T, Yokogawa K, Tomatsu S, et al. Targeted drug delivery to bone: pharmacokinetic and pharmacological properties of acidic oligopeptide-tagged drugs. Curr Drug Discov Technol. 2008;5:39–48. doi: 10.2174/157016308783769405. [DOI] [PubMed] [Google Scholar]
- 10.De Franceschi L, Roseti L, Desando G, et al. A molecular and histological characterization of cartilage from patients with Morquio syndrome. Osteoarthritis Cartilage. 2007;15:1311–17. doi: 10.1016/j.joca.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Bank RA, Groener JE, van Gemund JJ, et al. Deficiency in N-acetylgalactosamine-6-sulfate sulfatase results in collagen perturbations in cartilage of Morquio syndrome A patients. Mol Genet Metab. 2009;97:196–201. doi: 10.1016/j.ymgme.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Yasuda E, Fushimi K, Suzuki Y, et al. Pathogenesis of Morquio A syndrome: an autopsied case reveals systemic storage disorder. Mol Genet Metab. 2013;109:301–11. doi: 10.1016/j.ymgme.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Kakkis ED, Muenzer J, Tiller GE, et al. Enzyme-replacement therapy in mucopolysaccharidosis I. N Engl J Med. 2001;344:182–8. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- 14.Muenzer J, Wraith JE, Beck M, et al. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet Med. 2006;8:465–73. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 15.Muenzer J, Lamsa JC, Garcia A, et al. Enzyme replacement therapy in mucopolysaccharidosis type II (Hunter syndrome): a preliminary report. Acta Paediatr Suppl. 2002;91:98–9. doi: 10.1111/j.1651-2227.2002.tb03115.x. [DOI] [PubMed] [Google Scholar]
- 16.Harmatz P, Whitley CB, Waber L, et al. Enzyme replacement therapy in mucopolysaccharidosis VI (Maroteaux-Lamy syndrome) J Pediatr. 2004;144:574–80. doi: 10.1016/j.jpeds.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Harmatz P, Ketteridge D, Giugliani R, et al. Direct comparison of measures of endurance, mobility, and joint function during enzyme-replacement therapy of mucopolysaccharidosis VI (Maroteaux-Lamy syndrome): results after 48 weeks in a phase 2 open-label clinical study of recombinant human N-acetylgalactosamine 4-sulfatase. Pediatrics. 2005;115:e681–9. doi: 10.1542/peds.2004-1023. [DOI] [PubMed] [Google Scholar]
- 18.Harmatz P, Giugliani R, Schwartz I, et al. Enzyme replacement therapy for mucopolysaccharidosis VI: a phase 3, randomized, double-blind, placebo-controlled, multinational study of recombinant human N-acetylgalactosamine 4-sulfatase (recombinant human arylsulfatase B or rhASB) and follow-on, open-label extension study. J Pediatr. 2006;148:533–9. doi: 10.1016/j.jpeds.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Harmatz P, Giugliani R, Schwartz IV, et al. Long-term follow-up of endurance and safety outcomes during enzyme replacement therapy for mucopolysaccharidosis VI: final results of three clinical studies of recombinant human N-acetylgalactosamine 4-sulfatase. Mol Genet Metab. 2008;94:469–75. doi: 10.1016/j.ymgme.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Connock M, Juarez-Garcia A, Frew E, et al. A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type I. Health Technol Assess. 2006;10(20):iii–v.ix-113. doi: 10.3310/hta10200. [DOI] [PubMed] [Google Scholar]
- 21.Rohrbach M, Clarke JT. Treatment of lysosomal storage disorders: progress with enzyme replacement therapy. Drugs. 2007;67:2697–716. doi: 10.2165/00003495-200767180-00005. [DOI] [PubMed] [Google Scholar]
- 22.Chirino AJ, Mire-Sluis A. Characterizing biological products and assessing comparability following manufacturing changes. Nat Biotechnol. 2004;22:1383–91. doi: 10.1038/nbt1030. [DOI] [PubMed] [Google Scholar]
- 23.Parveen S, Sahoo SK. Nanomedicine: clinical applications of polyethylene glycol conjugated proteins and drugs. Clin Pharmacokinet. 2006;45:965–88. doi: 10.2165/00003088-200645100-00002. [DOI] [PubMed] [Google Scholar]
- 24.Dickson P, Peinovich M, McEntee M, et al. Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I. J Clin Invest. 2008;118:2868–76. doi: 10.1172/JCI34676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomatsu S, Montaño AM, Ohashi A, et al. Enzyme replacement therapy in a murine model of Morquio A syndrome. Hum Mol Genet. 2008;17:815–24. doi: 10.1093/hmg/ddm353. [DOI] [PubMed] [Google Scholar]
- 26.Tomatsu S, Montaño AM, DunG VC, et al. Enhancement of drug delivery: enzyme replacement therapy for murine Morquio A syndrome. Mol Ther. 2012;18:1094–102. doi: 10.1038/mt.2010.32. •• Bone targeting therapy for Morquio A syndrome.
- 27.Ellinwood NM, Vite CH, Haskins ME. Gene therapy for lysosomal storage diseases: the lessons and promise of animal models. J Gene Med. 2004;6:481–506. doi: 10.1002/jgm.581. [DOI] [PubMed] [Google Scholar]
- 28.Hodges BL, Cheng SH. Cell and gene-based therapies for the lysosomal storage diseases. Curr Gene Ther. 2006;6:227–41. doi: 10.2174/156652306776359522. [DOI] [PubMed] [Google Scholar]
- 29.Alméciga-Díaz CJ, Montaño AM, Tomatsu S, et al. Adeno-associated virus gene transfer in Morquio A disease: effect of promoters and sulfatase-modifying factor 1. FEBS J. 2010;277:3608–19. doi: 10.1111/j.1742-4658.2010.07769.x. [DOI] [PubMed] [Google Scholar]
- 30.Vellodi A, Young E, Cooper A, et al. Long-term follow-up following bone marrow transplantation for Hunter disease. J Inherit Metab Dis. 1999;22:638–48. doi: 10.1023/a:1005525931994. [DOI] [PubMed] [Google Scholar]
- 31.Nishioka T, Tomatsu S, Gutierrez MA, et al. Enhancement of drug delivery to bone: characterization of human tissue-nonspecific alkaline phosphatase tagged with an acidic oligopeptide. Mol Genet Metab. 2006;88:244–55. doi: 10.1016/j.ymgme.2006.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millán JL, Narisawa S, Lemire I, et al. Enzyme replacement therapy for murine hypophosphatasia. J Bone Miner Res. 2008;23:777–87. doi: 10.1359/JBMR.071213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whyte MP, Greenberg CR, Salman NJ, et al. Enzyme-replacement therapy in life-threatening hypophosphatasia. N Engl J Med. 2011;8:904–13. doi: 10.1056/NEJMoa1106173. [DOI] [PubMed] [Google Scholar]
- 34.Kasugai S, Fujisawa R, Waki Y, et al. Selective drug delivery system to bone: small peptide (Asp)6 conjugation. J Bone Miner Res. 2000;15:936–43. doi: 10.1359/jbmr.2000.15.5.936. [DOI] [PubMed] [Google Scholar]
- 35.Yokogawa K, Miya K, Sekido T, et al. Selective delivery of estradiol to bone by aspartic acid oligopeptide and its effects on ovariectomized mice. Endocrinology. 2001;142:1228–33. doi: 10.1210/endo.142.3.8024. [DOI] [PubMed] [Google Scholar]
- 36.Montaño A, Oikawa H, Tomatsu S, et al. Acidic amino acid tag enhances response to enzyme replacement in mucopolusaccharidosis type VII. Mol Genet Metab. 2008;94:178–89. doi: 10.1016/j.ymgme.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Theroux MC, Nerker T, Ditro C, et al. Anesthetic care and perioperative complications of children with Morquio syndrome. Paediatr Anaesth. 2012;22:901–7. doi: 10.1111/j.1460-9592.2012.03904.x. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi T, Katsuta S, Tamura Y, et al. Bone-targeting endogenous secretory RAGE rescues rheumatoid arthritis. Mol Med. 2013;19:183–94. doi: 10.2119/molmed.2012.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giugliani R, Federhen A, Rojas MV, et al. Mucopolysaccharidosis I, II, and VI: brief review and guidelines for treatment. Genet Mol Biol. 2010;33:589–604. doi: 10.1590/S1415-47572010005000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harmatz P. Enzyme replacement therapy with galsulfase for mucopolysaccharidosis VI: clinical facts and figures. Turk J Pediatr. 2010;52:443–9. [PubMed] [Google Scholar]
- 41.Tylki-Szymanska A, Marucha J, Jurecka A, et al. Efficacy of recombinant human alpha-L-iduronidase (laronidase) on restricted range of motion of upper extremities in mucopolysaccharidosis type I patients. J Inherit Metab Dis. 2010;33:151–7. doi: 10.1007/s10545-010-9059-9. [DOI] [PubMed] [Google Scholar]
- 42.Crawley AC, Niedzielski KH, Isaac EL, et al. Enzyme replacement therapy from birth in a feline model of mucopolysaccharidosis type VI. J Clin Investig. 1997;99:651–62. doi: 10.1172/JCI119208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simonaro CM, D’Angelo M, Haskins ME, et al. Joint and bone disease in mucopolysaccharidoses VI and VII: identification of new therapeutic targets and biomarkers using animal models. Pediatr Res. 2005;57:701–7. doi: 10.1203/01.PDR.0000156510.96253.5A. [DOI] [PubMed] [Google Scholar]
- 44.Jespan J. Clinical trial of enzyme replacement therapy for Morquio A. The 2nd annual Morquio Conference; Wilmington, DE. 19 – 21 July 2013. [Google Scholar]
- 45.Dierks T, Schmidt B, Borissenko LV, et al. Multiple sulfatase deficiency is caused by mutations in the gene encoding the human C (alpha)-formylglycine generating enzyme. Cell. 2003;113:435–44. doi: 10.1016/s0092-8674(03)00347-7. [DOI] [PubMed] [Google Scholar]
- 46.Cosma MP, Pepe S, Annunziata I, et al. The multiple sulfatase deficiency gene encodes an essential and limiting factor for the activity of sulfatases. Cell. 2003;113:445–56. doi: 10.1016/s0092-8674(03)00348-9. [DOI] [PubMed] [Google Scholar]
- 47.Ohashi A, Montaño AM, Colón JE, et al. Sacral dimple: incidental findings from newborn evaluation. Mucopolysaccharidosis IVA disease. Acta Paediatr. 2009;98:768–9. doi: 10.1111/j.1651-2227.2009.01134.x. [DOI] [PubMed] [Google Scholar]
- 48.Beck M, Braun S, Coerdt W, et al. Fetal presentation of Morquio disease type A. Prenat Diagn. 1992;12:1019–29. doi: 10.1002/pd.1970121207. [DOI] [PubMed] [Google Scholar]
- 49.Tylki-Szymanska A, Jurecka A, Zuber Z, et al. Enzyme replacement therapy for mucopolysaccharidosis II from 3 months of age: 3-year follow-up. Acta Paediatr. 2012;101:e42–7. doi: 10.1111/j.1651-2227.2011.02385.x. [DOI] [PubMed] [Google Scholar]
- 50.Rowan DJ, Tomatsu S, Grubb JH, et al. Long circulating enzyme replacement therapy rescues bone pathology in mucopolysaccharidosis VII murine model. Mol Genet Metab. 2012;107:161–72. doi: 10.1016/j.ymgme.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willyard C. Limb-saving medicines sought to prevent amputations. Nat Med. 2012;18:328. doi: 10.1038/nm0312-328. [DOI] [PubMed] [Google Scholar]
- 52.Gruber K. Europe gives gene therapy the green light. Lancet. 2012;380:e10. doi: 10.1016/s0140-6736(12)61992-8. [DOI] [PubMed] [Google Scholar]
- 53.Seregin SS, Amalfitano A. Gene therapy for lysosomal storage diseases: progress, challenges and future prospects. Curr Pharm Des. 2011;17:2558–74. doi: 10.2174/138161211797247578. [DOI] [PubMed] [Google Scholar]
- 54.Ponder K, Haskins M. Gene therapy for mucopolysaccharidosis. Expert Opin Biol Ther. 2007;7:1333–45. doi: 10.1517/14712598.7.9.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Available from: http://clinicaltrials.gov/
- 56.Osborn MJ, McElmurry RT, Peacock B, et al. Targeting of the CNS in MPS-IH using a nonviral transferrin-alpha-L-iduronidase fusion gene product. Mol Ther. 2008;16:1459–66. doi: 10.1038/mt.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Osborn MJ, McElmurry RT, Lees CJ, et al. Minicircle DNA-based gene therapy coupled with immune modulation permits long-term expression of alpha-L-iduronidase in mice with mucopolysaccharidosis type I. Mol Ther. 2011;19:450–60. doi: 10.1038/mt.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aronovich EL, Bell JB, Belur LR, et al. Prolonged expression of a lysosomal enzyme in mouse liver after Sleeping Beauty transposon-mediated gene delivery: implications for non-viral gene therapy of mucopolysaccharidoses. J Gene Med. 2007;9:403–15. doi: 10.1002/jgm.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aronovich EL, Bell JB, Khan SA, et al. Systemic correction of storage disease in MPS I NOD/SCID mice using the sleeping beauty transposon system. Mol Ther. 2009;17:1136–44. doi: 10.1038/mt.2009.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baldo G, Wozniak DF, Ohlemiller KK, et al. Retroviral-vector-mediated gene therapy to mucopolysaccharidosis I mice improves sensorimotor impairments and other behavioral deficits. J Inherit Metab Dis. 2013;36:499–512. doi: 10.1007/s10545-012-9530-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Traas AM, Wang P, Ma X, et al. Correction of clinical manifestations of canine mucopolysaccharidosis I with neonatal retroviral vector gene therapy. Mol Ther. 2007;15:1423–31. doi: 10.1038/sj.mt.6300201. [DOI] [PubMed] [Google Scholar]
- 62.Herati RS, Knox VW, O’Donnell P, et al. Radiographic evaluation of bones and joints in mucopolysaccharidosis I and VII dogs after neonatal gene therapy. Mol Genet Metab. 2008;95:142–51. doi: 10.1016/j.ymgme.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Herati RS, Ma X, Tittiger M, et al. Improved retroviral vector design results in sustained expression after adult gene therapy in mucopolysaccharidosis I mice. J Gene Med. 2008;10:972–82. doi: 10.1002/jgm.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Metcalf JA, Ma X, Linders B, et al. A self-inactivating gamma-retroviral vector reduces manifestations of mucopolysaccharidosis I in mice. Mol Ther. 2010;18:334–42. doi: 10.1038/mt.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Visigalli I, Delai S, Politi LS, et al. Gene therapy augments the efficacy of hematopoietic cell transplantation and fully corrects mucopolysaccharidosis type I phenotype in the mouse model. Blood. 2010;116:5130–9. doi: 10.1182/blood-2010-04-278234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ellinwood NM, Ausseil J, Desmaris N, et al. Safe, efficient, and reproducible gene therapy of the brain in the dog models of Sanfilippo and Hurler syndromes. Mol Ther. 2011;19:251–9. doi: 10.1038/mt.2010.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolf DA, Lenander AW, Nan Z, et al. Direct gene transfer to the CNS prevents emergence of neurologic disease in a murine model of mucopolysaccharidosis type I. Neurobiol Dis. 2011;43:123–33. doi: 10.1016/j.nbd.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Friso A, Tomanin R, Zanetti A, et al. Gene therapy of Hunter syndrome: evaluation of the efficiency of muscle electro gene transfer for the production and release of recombinant iduronate-2-sulfatase (IDS) Biochim Biophys Acta. 2008;1782:574–80. doi: 10.1016/j.bbadis.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 69.Jung SC, Park ES, Choi EN, et al. Characterization of a novel mucopolysaccharidosis type II mouse model and recombinant AAV2/8 vector-mediated gene therapy. Mol Cells. 2010;30:13–18. doi: 10.1007/s10059-010-0083-2. [DOI] [PubMed] [Google Scholar]
- 70.Heldermon CD, Qin EY, Ohlemiller KK, et al. Disease correction by combined neonatal intracranial AAV and systemic lentiviral gene therapy in Sanfilippo Syndrome type B mice. Gene Ther. 2013 doi: 10.1038/gt.2013.14. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fu H, Kang L, Jennings JS, et al. Significantly increased lifespan and improved behavioral performances by rAAV gene delivery in adult mucopolysaccharidosis IIIB mice. Gene Ther. 2007;14:1065–77. doi: 10.1038/sj.gt.3302961. [DOI] [PubMed] [Google Scholar]
- 72.Fu H, Dirosario J, Killedar S, et al. Correction of neurological disease of mucopolysaccharidosis IIIB in adult mice by rAAV9 trans-blood-brain barrier gene delivery. Mol Ther. 2011;19:1025–33. doi: 10.1038/mt.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heldermon CD, Ohlemiller KK, Herzog ED, et al. Therapeutic efficacy of bone marrow transplant, intracranial AAV-mediated gene therapy, or both in the mouse model of MPS IIIB. Mol Ther. 2010;18:873–80. doi: 10.1038/mt.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ruzo A, Marco S, Garcia M, et al. Correction of pathological accumulation of glycosaminoglycans in central nervous system and peripheral tissues of MPSIIIA mice through systemic AAV9 gene transfer. Hum Gene Ther. 2012;23:1237–46. doi: 10.1089/hum.2012.029. [DOI] [PubMed] [Google Scholar]
- 75.Fu H, DiRosario J, Kang L, et al. Restoration of central nervous system alpha-N-acetylglucosaminidase activity and therapeutic benefits in mucopolysaccharidosis IIIB mice by a single intracisternal recombinant adeno-associated viral type 2 vector delivery. J Gene Med. 2010;12:624–33. doi: 10.1002/jgm.1480. [DOI] [PubMed] [Google Scholar]
- 76.Di DC, Villani GR, Di ND, et al. Intracranial gene delivery of LV-NAGLU vector corrects neuropathology in murine MPS IIIB. Am J Med Genet A. 2009;149A:1209–18. doi: 10.1002/ajmg.a.32861. [DOI] [PubMed] [Google Scholar]
- 77.McIntyre C, Byers S, Anson DS. Correction of mucopolysaccharidosis type IIIA somatic and central nervous system pathology by lentiviral-mediated gene transfer. J Gene Med. 2010;12:717–28. doi: 10.1002/jgm.1489. [DOI] [PubMed] [Google Scholar]
- 78.McIntyre C, Derrick RAL, Ranieri E, et al. Lentiviral-mediated gene therapy for murine mucopolysaccharidosis type IIIA. Mol Genet Metab. 2008;93:411–18. doi: 10.1016/j.ymgme.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 79.Lau AA, Hopwood JJ, Kremer EJ, et al. SGSH gene transfer in mucopolysaccharidosis type IIIA mice using canine adenovirus vectors. Mol Genet Metab. 2010;100:168–75. doi: 10.1016/j.ymgme.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 80.Lau AA, Rozaklis T, Ibanes S, et al. Helper-dependent canine adenovirus vector-mediated transgene expression in a neurodegenerative lysosomal storage disorder. Gene. 2012;491:53–7. doi: 10.1016/j.gene.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Cotugno G, Annunziata P, Tessitore A, et al. Long-term amelioration of feline mucopolysaccharidosis VI after AAV-mediated liver gene transfer. Mol Ther. 2011;19:461–9. doi: 10.1038/mt.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferla R, O’Malley T, Calcedo R, et al. Gene therapy for mucopolysaccharidosis type VI is effective in cats without pre-existing immunity to AAV8. Hum Gene Ther. 2013;24:163–9. doi: 10.1089/hum.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Calcedo R, Vandenberghe LH, Gao G, et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–90. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ponder KP, O’Malley TM, Wang P, et al. Neonatal gene therapy with a gamma retroviral vector in mucopolysaccharidosis VI cats. Mol Ther. 2012;20:898–907. doi: 10.1038/mt.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Byers S, Rothe M, Lalic J, et al. Lentiviral-mediated correction of MPS VI cells and gene transfer to joint tissues. Mol Genet Metab. 2009;97:102–8. doi: 10.1016/j.ymgme.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 86.Richard M, Arfi A, Seguin J, et al. Widespread biochemical correction of murine mucopolysaccharidosis type VII pathology by liver hydrodynamic plasmid delivery. Gene Ther. 2009;16:746–56. doi: 10.1038/gt.2009.36. [DOI] [PubMed] [Google Scholar]
- 87.Smith LJ, Martin JT, O’Donnell P, et al. Effect of neonatal gene therapy on lumbar spine disease in mucopolysaccharidosis VII dogs. Mol Genet Metab. 2012;107:145–52. doi: 10.1016/j.ymgme.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xing EM, Knox VW, O’Donnell PA, et al. The effect of neonatal gene therapy on skeletal manifestations in mucopolysaccharidosis VII dogs after a decade. Mol Genet Metab. 2013;109:183–93. doi: 10.1016/j.ymgme.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Macsai CE, Derrick-Roberts AL, Ding X, et al. Skeletal response to lentiviral mediated gene therapy in a mouse model of MPS VII. Mol Genet Metab. 2012;106:202–13. doi: 10.1016/j.ymgme.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 90.Chen YH, Claflin K, Geoghegan JC, et al. Sialic acid deposition impairs the utility of AAV9, but not peptide-modified AAVs for brain gene therapy in a mouse model of lysosomal storage disease. Mol Ther. 2012;20:1393–9. doi: 10.1038/mt.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Husain T, Passini MA, Parente MK, et al. Long-term AAV vector gene and protein expression in mouse brain from a small pan-cellular promoter is similar to neural cell promoters. Gene Ther. 2009;16:927–32. doi: 10.1038/gt.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bielicki J, McIntyre C, Anson DS. Comparison of ventricular and intravenous lentiviral-mediated gene therapy for murine MPS VII. Mol Genet Metab. 2010;101:370–82. doi: 10.1016/j.ymgme.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 93.Toietta G, Severini G, Traversari C, et al. Various cells retrovirally transduced with N-acetylgalactosoamine-6-sulfate sulfatase correct Morquio skin fibroblasts in vitro. Hum Gene Ther. 2001;12:2007–16. doi: 10.1089/104303401753204571. [DOI] [PubMed] [Google Scholar]
- 94.Alméciga-Díaz C, Montaño AM, Tomatsu S, et al. Adeno-associated virus gene transfer on Morquio A: effect of promoters and sulfatase-modifying factor 1. FEBS J. 2010;277:3608–19. doi: 10.1111/j.1742-4658.2010.07769.x. [DOI] [PubMed] [Google Scholar]
- 95.Alméciga-Díaz C, Rueda-Paramo M, Espejo A, et al. Effect of elongation factor 1α promoter and SUMF1 over in-vitro expression of N-acetylgalactosamine-6-sulfate sulfatase. Mol Biol Rep. 2009;36:1863–70. doi: 10.1007/s11033-008-9392-3. [DOI] [PubMed] [Google Scholar]
- 96.Gutierrez M, Garcia-Vallejo F, Tomatsu S, et al. Construction of an adenoassociated virus-derived vector for the treatment of Morquio A disease. Biomedica. 2008;28:448–59. [PubMed] [Google Scholar]
- 97.Papadakis E, Nicklin S, Baker A, et al. Promoters and control elements: designing expression cassettes for gene therapy. Curr Gene Ther. 2004;4:89–113. doi: 10.2174/1566523044578077. [DOI] [PubMed] [Google Scholar]
- 98.Alméciga-Díaz C, Montaño A, Tomatsu S, et al. Adeno-associated virus mediated gene therapy in a murine model of Morquio syndrome type A. Mol Genet Metab. 2009;96:S13. [Google Scholar]
- 99.Buning H, Ried MU, Perabo L, et al. Receptor targeting of adeno-associated virus vectors. Gene Ther. 2003;10:1142–51. doi: 10.1038/sj.gt.3301976. [DOI] [PubMed] [Google Scholar]
- 100.Loiler SA, Conlon TJ, Song S, et al. Targeting recombinant adeno-associated virus vectors to enhance gene transfer to pancreatic islets and liver. Gene Ther. 2003;10:1551–8. doi: 10.1038/sj.gt.3302046. [DOI] [PubMed] [Google Scholar]
- 101.Choi VW, McCarty DM, Samulski RJ. AAV hybrid serotypes: improved vectors for gene delivery. Curr Gene Ther. 2005;5:299–310. doi: 10.2174/1566523054064968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.White K, Buning H, Kritz A, et al. Engineering adeno-associated virus 2 vectors for targeted gene delivery to atherosclerotic lesions. Gene Ther. 2008;15:443–51. doi: 10.1038/sj.gt.3303077. [DOI] [PubMed] [Google Scholar]
- 103.Alméciga-Díaz C, Cuaspa R, Barrera L. Gene delivery systems: tailoring vectors to reach specific tissues. In: Yuan X, editor. Non-viral gene therapy. InTech; Rijeka; Croatia: 2011. pp. 51–76. [Google Scholar]
- 104.Alméciga-Díaz C, Montaño A, Tomatsu S, et al. Contribución colombiana al conocimiento de la enfermedad de Morquio. Medicina (Mex) 2012;34:221–41. [Google Scholar]
- 105.Tomatsu S, Montaño AM, Alméciga-Díaz C, et al. Delivery of therapeutic agents to the bone. 2010. US20100008979.
- 106.Tomatsu S, Montaño AM, Oikawa H, et al. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Cur Pharm Biotech. 2012;12(6):931–45. doi: 10.2174/138920111795542615. [DOI] [PubMed] [Google Scholar]
- 107.Tomatsu S, Mackenzie WG, Theroux MC, et al. Current and emerging treatments and surgical interventions for Morquio A syndrome: a review. Res Rep Endocr Dis. 2012;2:65–77. doi: 10.2147/RRED.S37278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alméciga-Díaz C, Herrera J, Barbosa H, et al. New viral vectors for Morquio syndrome type A gene therapy. Mol Genet Metab. 2013;108:S19. [Google Scholar]
- 109.Ma X, Liu Y, Tittiger M, et al. Improvements in mucopolysaccharidosis I mice after adult retroviral vector-mediated gene therapy with immunomodulation. Mol Ther. 2007;15:889–902. doi: 10.1038/sj.mt.6300112. [DOI] [PubMed] [Google Scholar]