Abstract
Aim:
The aim of the present study was to compare the undisturbed plaque formation on teeth bonded with Preadjusted (Captain Ortho, Libral Traders, Mumbai, India) and Begg Brackets (Captain Ortho, Libral Traders, Mumbai, India) with nonbonded control sites via a de novo plaque growth over a period of 7 days.
Materials and Methods:
A clinical trial with the split-mouth design was set up enrolling 10 dental students. Within each subject sites with (Preadjusted) (P-site), Begg brackets (B-site) and control sites were followed. Plaque index and gingival index were recorded on days 3 and 7. Supra-gingival and sub-gingival plaque samples were taken from the brackets and the teeth on days 3 and 7, and were sent for aerobic and anaerobic culturing. The total number of bacterial colony forming units (CFU) was assessed for each sample using a colony counter. Tukeys and Dunnett test then statistically analyzed data.
Results:
The mean plaque index and gingival index increased on P-site and B-site on the third and 7th day. The shift from aerobic to anaerobic species was observed earlier in P-sites than in B-sites. The CFU were significantly higher for all sites on day 7 when compared with day 3. The aerobe/anaerobe CFU ratio was significantly lower in P-sites than in B-sites and then control showing an increase in the number of anaerobic species on the 3rd and 7th day (P < 0.05). Based on observed means, the mean difference was significant (P < 0.05).
Conclusion:
The present data suggest that Preadjusted brackets accumulated more plaque than Begg brackets. Bracket design can have a significant impact on bacterial load and on periodontal parameters.
Keywords: Beggs, colony forming units, preadjusted
INTRODUCTION
Orthodontic fixed appliance therapy is the preferred mode of treatment for most types of malocclusions and the most commonly used orthodontic materials are brackets, tubes, band material, ligating materials and arch wires.[1] Prior to the introduction of the bonding technique, orthodontic brackets were attached to metal bands that were individually fitted and cemented to each tooth. Bonding of brackets using acid etching and composite resin has been a major advancement in orthodontics after introduction of edgewise principle. Bonded brackets have many advantages over bands such as better aesthetics, ease of placement and removal and accessibility for oral hygiene. However, design and surface characteristics of both bonded brackets and composite may influence plaque retention.[2] Magno et al. reported that fixed appliances promote continuous accumulation and retention of microbial growth.[3] They formed mechanical plaque biofilm traps and impaired plaque removal, proper oral hygiene, and gingival health, thus promoting specific alteration in the oral environment which include decreased pH, increased plaque accumulation and elevation of microbial counts in the saliva and the biofilm.[4,5]
Gingivitis may develop in patients who do not institute proper oral hygiene measures and can become an established lesion in 21 days. Patients often exhibit gingival hypertrophy, bleeding, increased plaque accumulation and calculus formation during orthodontic treatment.[6] It is also difficult to remove microbial growth or clean orthodontic appliances fixed at the critical sites leading to enamel decalcification and white spot lesion formation around orthodontic appliances.[7,8]
Transformation of oral biota by the presence of orthodontic fixed appliances poses a significant impact on the patient's oral and general health. It is also a common belief that plaque formation during treatment with fixed appliances is mainly attributed to the complexity of the bracket design and ligating methods. The quantity as well as the quality of the plaque is influenced by many factors, including surface characteristics, surface roughness, and surface-free energy.[9,10,11] As the tooth is a solid, non-shedding surface, the colonization of the tooth surface by bacteria will lead to rapid development of plaque. Electrostatic attractions and Vander Waal forces influence adhesion of microorganisms to surfaces. Although it is clear that the initial attachment is an important factor governing further colonization, the mechanism of attachment and those of subsequent adhesion may differ significantly. Decreased wettability may inhibit direct adhesion and colonization of bacteria onto the appliances.[12]
The most common site for bacterial adhesion and biofilm formation is at the bracket adhesive-enamel junction, an area that is difficult to clean with daily brushing. Oral biofilms at this junction not only damage oral tissues, but also weaken the bond strength of adhesives. Additionally, excessive adhesive around brackets especially provide a site for the rapid adhesion and growth of bacteria. Furthermore, the surface of an orthodontic adhesive is often rough, with a gap of around 10 um at the adhesive enamel interface due to polymerization shrinkage. This provides adhering bacteria with a protected site against oral cleansing forces.[13,14,15,16,17] The presence of gingival inflammation further increases plaque growth. The total amount of bacteria and the ratio between the aerobic and anaerobic bacteria is an important etiological factor in the development of gingivitis and periodontitis.
The variability in the design and material of orthodontic brackets may influence plaque adhesion and hence gingival disease.[18] Although a large number of studies have shown a shift in microbial populations in the presence of orthodontic fixed appliances, limited information is available as to which bracket material would be less prone to adhesion of bacterial species and plaque accumulation. The aim of this study was to compare the undisturbed plaque formation on teeth bonded with Preadjusted and Begg brackets with nonbonded control sites via a de novo plaque growth over a period of 7 days.
MATERIALS AND METHODS
The study protocol was approved by the Institutional Ethics Board Committee of Dr. D.Y. Patil University, Navi Mumbai, India. Ten dental students (seven males and three females) aged between 18 and 25 years with clinically healthy gingiva were selected at random from Dr. D.Y. Patil Dental College and Hospital, Navi Mumbai, India. Students were explained about the background of the study, its objectives, and their involvement. After screening for suitability and after good comprehension of the protocol, they all gave their consent. The students were also asked whether they already received an orthodontic treatment with fixed appliances, because this might have consequences for the smoothness of the buccal enamel[19] and on the microbial adhesion in the early formation of dental plaque biofilm.[20,21] Smokers and subjects with extensive dental restorations were excluded from the study.
Experimental Design
Eighty sites (20 Preadjusted sites, 20 Begg site, 40 control sites) were taken into this clinical trial with split-mouth design wherein within every subject 8 sites/subject were defined: Both premolars from each quadrant. (Preadjusted) (Captain Ortho, Libral Traders, Mumbai, India) and Begg Brackets (Captain Ortho, Libral Traders, Mumbai, India) were placed in contralateral antagonistic quadrants (first and third) and the second and the fourth quadrant premolars served as a control [Figure 1]. The sites which received Preadjusted brackets were denoted as P-site and the sites which received Begg brackets were denoted as B-site.
Figure 1.
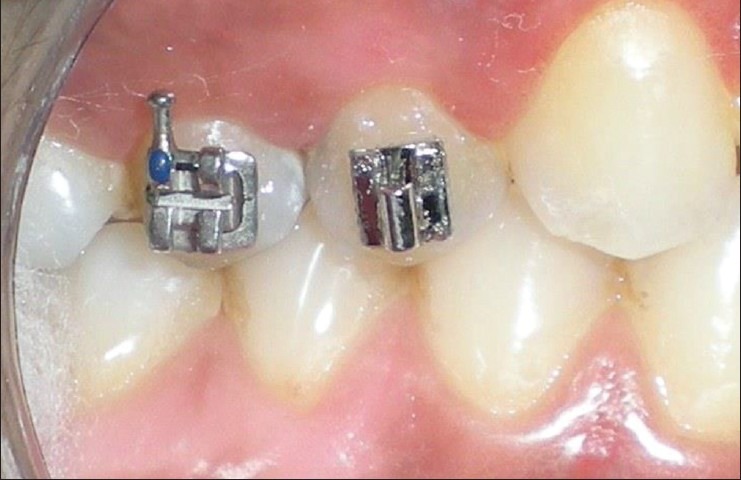
Placement of Beggs and Preadjusted brackets on premolars
The variables assessed in this study, were Modified Quigley Hein plaque index (PI)[22,23] and Loe and Silness gingival index (GI).[24]
Modified Quigley Hein Plaque Index
The Quigley Hein plaque index represents the broad surface area of the whole buccal or lingual surfaces, while giving focus to the gingival third of the tooth and grades plaque and debris on a scale 0-5 (0 = no plaque/debris, 5 = plaque covering two-thirds or more of the crown of the tooth). Modifications of the Quigley Hein plaque index include separating each buccal and lingual aspect into 3 surfaces (mesial, distal, and mid), using the line angles of the tooth to the contact point bordered by the gingival margin as guidelines for proximal regions, to give a total of 6 surfaces/tooth.
Loe and Silness Gingival Index
The Loe and Silness gingival index was created for the assessment of the gingival condition and records qualitative changes in the gingiva. It scores the marginal and interproximal tissues separately based on 0-3 condition and records qualitative changes in the gingiva. The bleeding is assessed by probing gently along the wall of soft tissue of the gingival sulcus. The scores of the four areas of the tooth can be summed and divided by four to give the GI for the tooth. The GI of the individual can be obtained by adding the values of each tooth and dividing by the number of teeth examined. The GI may be scored for all surfaces of all or selected teeth or for selected areas of all or selected teeth.
All the subjects underwent scaling, root planing and were refrained from brushing and other oral hygiene procedures on these bonded and control teeth during the course of the study. Immediately after scaling and root planing brackets were bonded on the first premolars. The subjects had to return on the 3rd day to record periodontal parameters, microbial sampling, and removal of brackets from first premolars. Subjects also reported on the 7th day to record periodontal parameters, microbial sampling, and removal of brackets from the second premolars followed by scaling and fluoride application on the bonded teeth.
Bracket Placement and Removal
The areas to bond were isolated with cotton rolls and saliva suction. The enamel was etched locally with 37% orthophosphoric acid (Total Etch, Ivoclar Vivadent, New Delhi, India) for 30 s and rinsed rigorously with water afterwards. The etching was accurately carried out by means of disposable mini sponge applicators (3M ESPE, St. Paul, USA). After rinsing with water and drying, the sites were inspected for the characteristic dull, white, frosted appearance of adequately etched enamel. By means of mini sponges, the bonding agent (Heliobond, Ivoclar Vivadent, New Delhi, India) was applied in such way that it fully covered the etched enamel and was light cured for 20 s. The nanocomposite (Ivoclar N Ceram, Ivoclar Vivadent, New Delhi, India) was then directly applied to the bracket base, pressed firmly onto the enamel surface, and any excess of adhesive was removed. Brackets were then placed along the long axis of the tooth 4 mm coronal to the gingival margin with the help of bonding pliers and pressure applied over the tooth. Excess flash was removed with a probe and then the bracket was light cured for 40 s. On the 7th day after complete isolation of the surrounding field, brackets were removed with sterile debonding pliers.
Collection of Plaque Samples
Sample sites were isolated using cotton rolls, and supragingival plaque was removed using sterile swabs to avoid sub-gingival plaque contamination [Figure 2]. Sub-gingival plaque was collected using sterile absorbable paper points [Figure 3] which were inserted into the gingival sulcus for 25-30 s and then transferred to preheated Robertson's cooked meat medium (Marine Chemicals, Kerala, India) (2 ml) for aerobic and anaerobic culturing.
Figure 2.
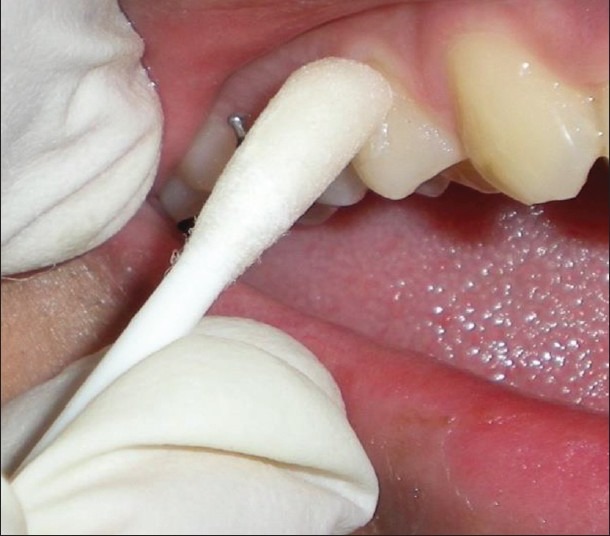
Supra-gingival plaque sampling
Figure 3.
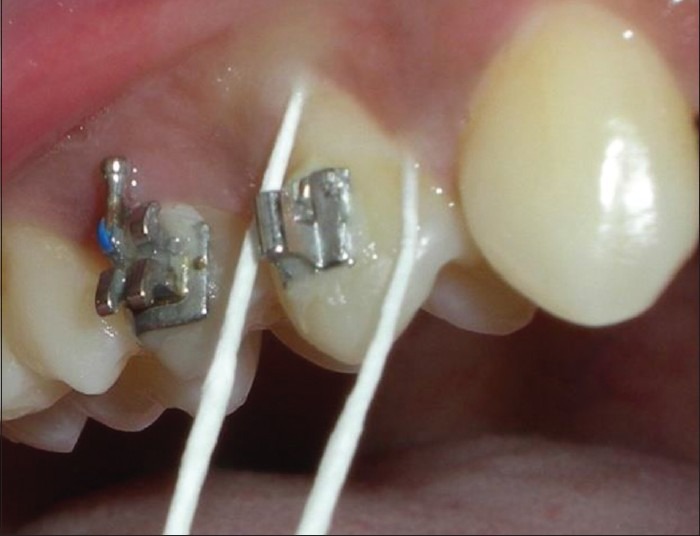
Subgingival plaque sampling
Aerobic and Anaerobic Culturing
Preheated Robertson cooked meat media and thioglycolate media were used to transport the paper points to the microbiological laboratory. For aerobic culturing[25] the trypticase soy agar plates were placed in an incubator and incubated at 37°C for 24 h. For anaerobic culturing[25] anaerobic condition was achieved using a Gaspak system. Subculture from Robertson cooked meat media and thioglycolate media was done using trypticase agar plate. The system comprised a transparent plastic body jar with an airtight lid fitted with a screened catalyst chamber containing palladium-sized aluminium pellets. Water was added to a disposable aluminium foil packet containing pellets of sodium borohydride, tartaric acid, and sodium bicarbonate. The packet was immediately put in a jar. Reactions then took place to supply hydrogen and carbon dioxide. The agar plates mounted on a metal stand were immediately placed in a jar and the lid was closed and clamped tightly. The jar was then placed in an incubator and incubated at 37°C for 48 h. After an incubation period, the plates were observed for microbial growth. The total number of colony forming units (CFU) for each plate was assessed for each sample by a blinded investigator using a colony counter [Figure 4]. The number of CFUs is related to the viable number of bacteria in the sample.
Figure 4.
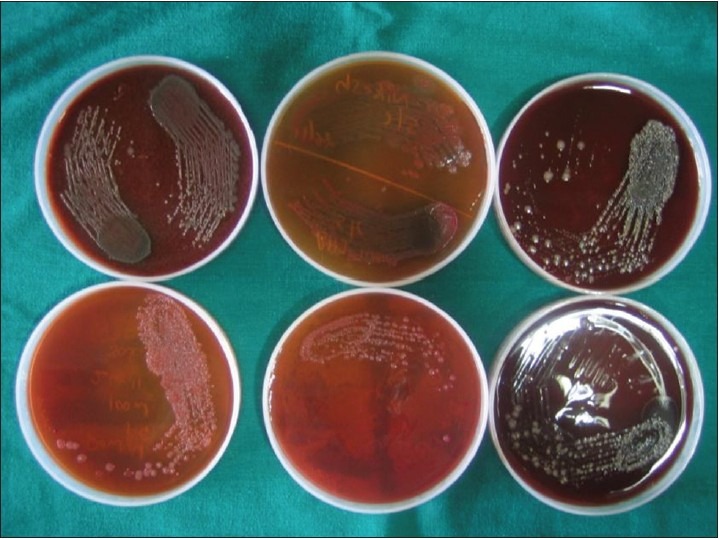
Growth of microorganisms on blood agar
Statistical Analysis
The results obtained with the aerobic and anaerobic culturing were analyzed descriptively by using the SPSS statistical software version 17 (SPSS Inc., Chicago, USA) to evaluate the level of contamination of the brackets by microorganisms and the level of significance was set at 0.05 (P < 0.05). A general linear model was used where repeated measures was used to fit the data ANCOVA tables were made to see whether there was an interaction effect between time and treatment and whether the separator material and day effects were significant. The following tests were performed like calculation of statistical parameters for parametric variables such as mean, standard deviation and 95% confidence interval of the mean aerobic and anaerobic CFU. Analysis of overall CFU was done by Tukey Honest Significant Difference (HSD) and Dunnett test. Tukeys test was based on the assumption that the observations being tested are independent, and there is equal within-group variance across the groups associated with each mean in the test. Dunnett test was used for comparing control to each of the other groups, but not comparing others to each other.
RESULTS
The mean plaque index and gingival index increased on B-site and P-site on the 3rd and 7th day. The mean plaque index was 1.40 at the P-site on the 3rd day and 2.12 on the 7th day. The mean plaque index was 1.13 at the B-site on the 3rd day and 1.85 on the 7th day, while on the control sites, it was 1.07 on the 3rd day and 1.79 on the 7th day. P-sites in general harbored more plaque formation than B-sites [Table 1].
Table 1.
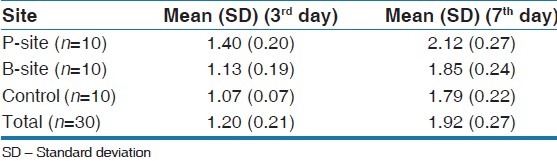
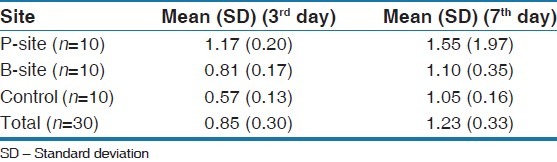
The mean gingival index was 1.17 at the P-site on the 3rd day and 1.55 on the 7th day while on the B-site it was 0.81 at 3rd day and 1.1 on the 7th day while at control sites it was 0.57 on the 3rd day and 1.05 on the 7th day [Table 2].
Table 2.
General linear model for Loe and Silness gingival index[24] by site by day
The number of aerobic and anaerobic CFU in plaque samples from the different sites showed significant differences. The CFU was significantly higher for all sites on day 7 when compared with day 3. P-sites showed significantly higher CFU than B-sites and both P and B-sites had higher values than control sites [Table 3]. The mean CFU in the P-site was 0.27 on the 3rd day and 0.10 on the 7th day. The mean CFU in the B-site was 0.45 on the 3rd day and 0.22 on the 7th day while on the control sites, it was 0.70 on the 3rd day and 0.62 on the 7th day [Table 4 and Figure 5].
Table 3.
Overall mean colony forming units ratio
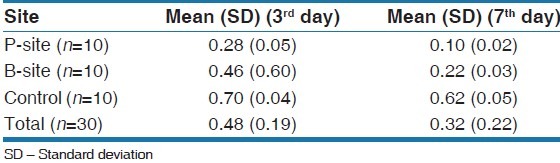
Table 4.
CFU (aerobic/anaerobic) ratio
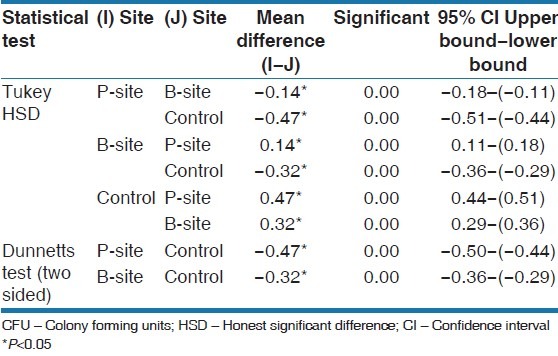
Figure 5.
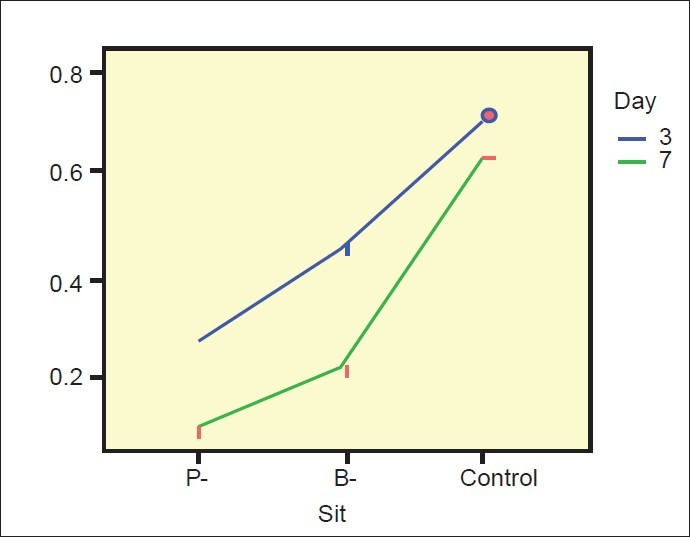
Estimated marginal means of colony forming units ratio
No differences in undisturbed plaque growth were seen. The interaction effect between day and site was borderline significant. On day 7, more anaerobic species were seen, and both P and B-sites showed significantly higher values than the control sites. Tukey HSD test and Dunnett test was used to assess the CFU and it was low for the P-site followed by B-site and control showing an increase in the number of anaerobic species on the 3rd and 7th day (P < 0.05). Based on observed means the mean difference was significant (P < 0.05) [Tables 3 and 4].
DISCUSSION
This plaque growth model detected some significant differences between the bonded Begg and Preadjusted brackets and the nonbonded control teeth. The use of subjects with good periodontal health as subjects for this study was important as several studies indicated an increased plaque accumulation in the presence of gingival inflammation.[26] The duration of the study was set at 7 days, mainly to assess the formation of early plaque biofilm. This was also positive for the compliance of the dental students, as longer periods would have resulted in a decrease in compliance of nonbrushing. The split-mouth study was selected to avoid any bias on the part of the study.
In the present study, sample sites were isolated using cotton rolls and supragingival plaque was harvested from both P- and B-sites of brackets using sterile swabs to avoid sub-gingival plaque contamination. Supragingival plaque biofilm was mostly located at the gingival margin, around orthodontic attachments and on indented surfaces. These findings can be explained by reduced shear forces in these regions, where bacteria are protected from mechanical and hydrodynamic effects such as tongue movement and saliva flow. Rough surfaces will promote plaque formation and maturation, and high-energy surfaces are known to collect more plaque, to bind the plaque more strongly and to select specific bacteria. Although both variables interact with each other, the influence of surface roughness overrules that of the surface-free energy.
The sub-gingival plaque was collected using sterile absorbable paper points, which were inserted into the gingival sulcus for 25-30 s. For the sub-gingival environment, with more facilities for microorganisms to survive, the importance of surface characteristics dramatically decreases. However, the influence of surface roughness and surface-free energy on supragingival plaque justifies the demand for smooth surfaces with a low surface-free energy in order to minimize plaque formation, thereby reducing the occurrence of caries and periodontitis.[27]
The results of the study indicated an increase in plaque and gingival index in both the Preadjusted and Begg site over a period of 7 days. However, the plaque accumulation was more on the Preadjusted site on the apical border of the brackets. The reason could be attributed to larger surface area than the Begg type of brackets and complicated design characteristics. Other reasons could be attributed to the higher binding force between bacteria and high-surface energy and the selectivity in the bacterial adhesion.[28] CFU ratio (aerobic/anaerobic) decreased significantly from day 3 to day 7 at P-site, suggesting that P-site harbored more anaerobic microflora.
Lee et al. found significant differences in the prevalence of putative periodontal pathogens in sub gingival dental plaque from gingivitis lesions in orthodontic patients. Tannerella forsythia, Treponema denticola and Prevotella nigrescens were significantly more common in the samples obtained from the orthodontic patients than in the samples obtained from the nonorthodontic control patients.[29] Papaioannou et al. have mentioned that the salivary pellicle seems to facilitate the adhesion of Porphyromonas gingivalis and biofilm formation on orthodontic brackets, while the material comprising the brackets does not significantly influence the number of bacteria.[30] This shows that the local changes associated with the wearing of orthodontic brackets may affect the prevalence of periodontal pathogens in dental plaque.
A similar study was conducted by van Gastel et al. where they assessed both anaerobic and aerobic CFU and found that anaerobe and aerobe CFU were higher in subjects with speed brackets than GAC brackets.[31] Clinically a gap was seen at the composite enamel junction around the bracket base in all specimens. Bacterial plaque accumulation was detected in all these gaps. The gap could be due to the setting shrinkage which is an inherent property of composite during polymerization and has been reported in various studies.[32] There have also been studies where glass ionomer cement has been used and have shown to release fluoride weekly which may be beneficial to prevent enamel demineralization and have an anticaries effect.[33]
Dental adhesives can have adverse effects on the gingiva as they are toxic to the gingival fibroblasts in vitro.[34] Particularly the residual monomers may cause gingival inflammation and irritation.[35] Both phosphoric acid and composite were carefully applied onto the tooth with complete isolation. The bonding material was directly polymerized and thus contact with the gingival margin was prevented. Control sites did not show any significant changes over a period of 7 days in relation to the CFU ratio.
The placement of both Preadjusted and Begg brackets had a significant impact on microbial and clinical variables. Preadjusted brackets had a higher rate on plaque adhesion than Begg brackets thus indicating that bracket design can have a significant impact on the periodontal parameters and bacterial load. A possible limitation of this study was its short duration of 1-week, therefore, long-term longitudinal studies and comparison between various types of brackets would give a clear picture on the plaque accumulation tendencies.
Recent research has renewed interest in the use of alternative antibacterial agents such as metallic nanoparticles, which have shown greatest biocidal activity against bacteria. In the oral cavity, the antibacterial properties of nanoparticles have been used through two broad mechanisms of combining dental materials with nanoparticles or coating surfaces with nanoparticles to prevent microbial adhesion, with the overall aim of reducing the biofilm formation.[36,37,38,39] Further research should be performed to visualize the potentially different periodontal complications of different orthodontic bracket systems used in the treatment with fixed appliances in such way that brackets can be designed to reduce plaque adhesion. Additional bacteriological studies using a split-mouth design would help delineate any possible relationships between bracket composition and the microbial flora that colonize them.
CONCLUSION
The present data seemed to indicate that Preadjusted brackets accumulated more plaque than Begg brackets. Bracket designs could have a significant adverse impact on the plaque microflora. The placement of brackets with different design can present different risks for periodontal disease at short time, and the long-term results are not elucidated so far. Clinical studies of dental and gingival health between patients with each bracket type would help determine any possible clinical significance of these subtle differences in plaque composition between different bracket types. It is necessary to understand periodontal complications of different orthodontic bracket systems used with fixed appliances to ensure that brackets are designed in such a way that plaque adhesion and maturation are prevented, and periodontal health is restored.
Footnotes
Source of Support: This study was supported by grants from Padmashree Dr. D. Y. Patil Dental College and Hospital, Nerul, Navi Mumbai, India
Conflict of Interest: None declared.
REFERENCES
- 1.Pandurangan H, Thillai SS, Varadhrajan K, Gnanamani A. Microbial adhesion on orthodontic ligating materials: An in vitro assessment. Adv Microbiol. 2013;3:108–14. [Google Scholar]
- 2.Boyd RL, Baumrind S. Periodontal considerations in the use of bonds or bands on molars in adolescents and adults. Angle Orthod. 1992;62:117–26. doi: 10.1043/0003-3219(1992)062<0117:PCITUO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Magno AF, Enoki C, Ito IY, Matsumoto MA, Faria G, Nelson-Filho P. In-vivo evaluation of the contamination of super slick elastomeric rings by Streptococcus mutans in orthodontic patients. Am J Orthod Dentofacial Orthop. 2008;133:S104–9. doi: 10.1016/j.ajodo.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 4.Naranjo AA, Triviño ML, Jaramillo A, Betancourth M, Botero JE. Changes in the subgingival microbiota and periodontal parameters before and 3 months after bracket placement. 2006;130:275. Am J Orthod Dentofacial Orthop. 2006;130:275.17–22. doi: 10.1016/j.ajodo.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Ai H, Lu HF, Liang HY, Wu J, Li RL, Liu GP, Xi Y. Influences of bracket bonding on mutans streptococcus in plaque detected by real time fluorescence-quantitative polymerase chain reaction. Chin Med J (Engl) 2005;118:2005–10. [PubMed] [Google Scholar]
- 6.Türkkahraman H, Sayin MO, Bozkurt FY, Yetkin Z, Kaya S, Onal S. Archwire ligation techniques, microbial colonization, and periodontal status in orthodontically treated patients. Angle Orthod. 2005;75:231–6. doi: 10.1043/0003-3219(2005)075<0227:ALTMCA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 7.Al-Fattani MA, Douglas LJ. Penetration of Candida biofilms by antifungal agents. Antimicrob Agents Chemother. 2004;48:3291–7. doi: 10.1128/AAC.48.9.3291-3297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sukontapatipark W, El-Agroudi MA, Selliseth NJ, Thunold K, Selvi KA. Bacterial colonization associated with fixed orthodontic appliances - A SEM study. Eur J Orthod. 1998;23:475–84. doi: 10.1093/ejo/23.5.475. [DOI] [PubMed] [Google Scholar]
- 9.Azmi MG, Al-Jasser N. The effect of fixed orthodontic appliance therapy on oral Candida carriage. Saudi Dent J. 2003;15:141–4. [Google Scholar]
- 10.Hägg U, Kaveewatcharanont P, Samaranayake YH, Samaranayake LP. The effect of fixed orthodontic appliances on the oral carriage of Candida species and Enterobacteriaceae. Eur J Orthod. 2004;26:623–9. doi: 10.1093/ejo/26.6.623. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Kho HS, Lee SW, Yang WS. Experimental salivary pellicles on the surface of orthodontic materials. Am J Orthod Dentofacial Orthop. 2001;119:59–66. doi: 10.1067/mod.2001.110583. [DOI] [PubMed] [Google Scholar]
- 12.Raju AS, Hegde NA, Reddy VP, Chandrashekar BS, Mahendra S, Harishkoushik SR. An in vivo study on bacterial colonization with metal, ceramic and self ligating brackets: A scanning electron microscopic study. J Indian Orthod Soc. 2013;47:88–96. [Google Scholar]
- 13.Mei L, Busscher HJ, van der Mei HC, Chen Y, de Vries J, Ren Y. Oral bacterial adhesion forces to biomaterial surfaces constituting the bracket-adhesive-enamel junction in orthodontic treatment. Eur J Oral Sci. 2009;117:419–26. doi: 10.1111/j.1600-0722.2009.00648.x. [DOI] [PubMed] [Google Scholar]
- 14.Sukontapatipark W, El-Agroudi MA, Selliseth NJ, Thunold K, Selvig KA. Bacterial colonization associated with fixed orthodontic appliances. A scanning electron microscopy study. Eur J Orthod. 2001;23:475–84. doi: 10.1093/ejo/23.5.475. [DOI] [PubMed] [Google Scholar]
- 15.Guzmán-Armstrong S, Chalmers J, Warren JJ. Ask us. White spot lesions: Prevention and treatment. Am J Orthod Dentofacial Orthop. 2010;138:690–6. doi: 10.1016/j.ajodo.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Ahn HB, Ahn SJ, Lee SJ, Kim TW, Nahm DS. Analysis of surface roughness and surface free energy characteristics of various orthodontic materials. Am J Orthod Dentofacial Orthop. 2009;136:668–74. doi: 10.1016/j.ajodo.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 17.Lee SP, Lee SJ, Lim BS, Ahn SJ. Surface characteristics of orthodontic materials and their effects on adhesion of Mutans Streptococci. Angle Orthod. 2009;79:353–60. doi: 10.2319/021308-88.1. [DOI] [PubMed] [Google Scholar]
- 18.Anhoury P, Nathanson D, Hughes CV, Socransky S, Feres M, Chou LL. Microbial profile on metallic and ceramic bracket materials. Angle Orthod. 2002;72:338–43. doi: 10.1043/0003-3219(2002)072<0338:MPOMAC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Eliades T, Gioka C, Eliades G, Makou M. Enamel surface roughness following debonding using two resin grinding methods. Eur J Orthod. 2004;26:333–8. doi: 10.1093/ejo/26.3.333. [DOI] [PubMed] [Google Scholar]
- 20.Quirynen M, Marechal M, Busscher HJ, Weerkamp AH, Arends J, Darius PL, et al. The influence of surface free-energy on planimetric plaque growth in man. J Dent Res. 1989;68:796–9. doi: 10.1177/00220345890680050801. [DOI] [PubMed] [Google Scholar]
- 21.Quirynen M, Marechal M, Busscher HJ, Weerkamp AH, Darius PL, Van SD. The influence of surface free energy and surface roughness on early plaque formation. An in vivo study in man. J Clin Periodontol. 1990;17:138–44. doi: 10.1111/j.1600-051x.1990.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 22.Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of vitamin C. J Periodontol. 1970;41:41–3. doi: 10.1902/jop.1970.41.41.41. [DOI] [PubMed] [Google Scholar]
- 23.Quigley GA, Hein JW. Comparative cleansing efficiency of manual and power brushing. J Am Dent Assoc. 1962;65:26–9. doi: 10.14219/jada.archive.1962.0184. [DOI] [PubMed] [Google Scholar]
- 24.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 25.McCartney M. Practical Medical Microbiology. 13th ed. Vol. 2. United Kingdom: Churchill Living Stone; 1986. pp. 126–30.pp. 644–5. [Google Scholar]
- 26.Rowshani B, Timmerman MF, Van der Velden U. Plaque development in relation to the periodontal condition and bacterial load of the saliva. J Clin Periodontol. 2004;31:214–8. doi: 10.1111/j.0303-6979.2004.00468.x. [DOI] [PubMed] [Google Scholar]
- 27.Quirynen M, Bollen CM. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J Clin Periodontol. 1995;22:1–14. doi: 10.1111/j.1600-051x.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 28.Van Pelt AW, Van der Mel HC, Busscher HJ, Arends J, Weerkamp AH. Surface free energies of oral streptococci. FEMS Microbiol Lett. 1984;25:279–82. [Google Scholar]
- 29.Lee SM, Yoo SY, Kim HS, Kim KW, Yoon YJ, Lim SH, et al. Prevalence of putative periodontopathogens in subgingival dental plaques from gingivitis lesions in Korean orthodontic patients. J Microbiol. 2005;43:260–5. [PubMed] [Google Scholar]
- 30.Papaioannou W, Panagopoulos A, Koletsi-Kounari H, Kontou E, Makou M. Adhesion of Porphyromonas gingivalis and biofilm formation on different types of orthodontic brackets. Int J Dent. 2012;2012:471380. doi: 10.1155/2012/471380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Gastel J, Quirynen M, Teughels W, Coucke W, Carels C. Influence of bracket design on microbial and periodontal parameters in vivo. J Clin Periodontol. 2007;34:423–31. doi: 10.1111/j.1600-051X.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 32.Jacobsen PH. Clinical aspects of composite restorative materials. Br Dent J. 1975;139:276–80. doi: 10.1038/sj.bdj.4803593. [DOI] [PubMed] [Google Scholar]
- 33.Vahid-Dastjerdi E, Borzabadi-Farahani A, Pourmofidi-Neistanak H, Amini N. An in-vitro assessment of weekly cumulative fluoride release from three glass ionomer cements used for orthodontic banding. Prog Orthod. 2012;13:49–56. doi: 10.1016/j.pio.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Huang TH, Tsai CY, Chen SL, Kao CT. An evaluation of the cytotoxic effects of orthodontic bonding adhesives upon a primary human oral gingival fibroblast culture and a permanent, human oral cancer-cell line. J Biomed Mater Res. 2002;63:814–21. doi: 10.1002/jbm.10412. [DOI] [PubMed] [Google Scholar]
- 35.Gioka C, Bourauel C, Hiskia A, Kletsas D, Eliades T, Eliades G. Light-cured or chemically cured orthodontic adhesive resins. A selection based on the degree of cure, monomer leaching, and cytotoxicity? Am J Orthod Dentofacial Orthop. 2005;127:413–9. doi: 10.1016/j.ajodo.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Borzabadi-Farahani A, Borzabadi E, Lynch E. Nanoparticles in orthodontics, a review of antimicrobial and anti-caries applications. Acta Odontol Scand. 2014;72:413–7. doi: 10.3109/00016357.2013.859728. [DOI] [PubMed] [Google Scholar]
- 37.Allaker RP. The use of nanoparticles to control oral biofilm formation. J Dent Res. 2010;89:1175–86. doi: 10.1177/0022034510377794. [DOI] [PubMed] [Google Scholar]
- 38.Hannig M, Kriener L, Hoth-Hannig W, Becker-Willinger C, Schmidt H. Influence of nanocomposite surface coating on biofilm formation in situ. J Nanosci Nanotechnol. 2007;7:4642–8. [PubMed] [Google Scholar]
- 39.Monteiro DR, Gorup LF, Takamiya AS, Ruvollo-Filho AC, DE Camargo ER, Barbosa DB. The growing importance of materials that prevent microbial adhesion: Antimicrobial effect of medical devices containing silver. Int J Antimicrob Agents. 2009;34:103–10. doi: 10.1016/j.ijantimicag.2009.01.017. [DOI] [PubMed] [Google Scholar]


