Abstract
Introduction:
Mupirocin (pseudomonic acid A) is a topical antimicrobial agent with excellent antistaphylococcal and antistreptococcal activity. A nasal formulation is approved by the United States Food and Drug Administration for eradicating nasal carriage in adult patients as well as in health care personnel. Resistance to mupirocin has already been reported worldwide. The increasing prevalence of mupirocin resistance among Staphylococcus aureus and coagulase-negative Staphylococcus (CoNS) species could be an important threat to the future use of mupirocin against methicillin-resistant S. aureus (MRSA). Thus, this study was carried out to find the prevalence of mupirocin resistance in S. aureus and CoNS by disc diffusion and to determine the rates of high-level and low-level mupirocin resistance in S. aureus and CoNS by disc diffusion.
Materials and Methods:
A total of 140 healthcare workers (HCWs) (doctor, nursing staff, housekeeping staff) were randomly selected. S. aureus and CoNS isolates were tested for mupirocin resistance by the disk diffusion method using 5 μg and 200 μg mupirocin discs. MRSA isolates were tested for antibiotics by Kirby-Bauer disc-diffusion method as per Clinical and Laboratory Standards Institute guidelines.
Results:
Out of 140 nasal swabs collected from HCWs, S. aureus was isolated in 38 (27.14%), and CoNS was isolated in 73 (52.14%). MRSA was isolated in 20 (14.28%) and methicillin-resistant coagulase-negative Staphylococci (MRCoNS) in 34 (24.29%. Methicillin-sensitive S. aureus (MSSA) and MSCoNS isolates were 100% sensitive to mupirocin, but two isolates from MRSA (1.43%) and five from MRCoNS (3.57%) were mupirocin resistant.
Conclusion:
The presence of mupirocin resistance in MRSA and MRCoNS is a cause for concern. It could be limited by regular surveillance and effective infection control initiatives so to inform health care facilities to guide therapeutic and prophylactic use of mupirocin.
Keywords: Antibiotic resistance, methicillin-resistant coagulase-negative Staphylococci, methicillin-resistant Staphylococcus aureus, mupirocin resistance
Introduction
Nasal carriage of Staphylococcus aureus plays a key role in the epidemiology and pathogenesis of infection and is a major risk factor for the development of both community-acquired and nosocomial infections.[1] Currently, the health problems associated with this microorganism have become more serious due to the increasing incidence of methicillin-resistant S. aureus (MRSA).[2] Several studies worldwide have reported the rate of nasal carriage of S. aureus strains, varying from 16.8% to 90%.[3,4,5,6] A causal relationship between S. aureus nasal carriage and infection is supported by the fact that the nasal strain and the infecting strain share the same genotype.[7]
In recent years, nosocomial outbreaks of MRSA have become a major infection control problem. MRSA strains may spread readily in hospitals from colonized or infected persons. Colonized employees are generally asymptomatic, although they are a potential reservoir of infections acquired by patients.[8] Colonized or infected hospital personnel (HCWs) may serve as reservoir and disseminator of MRSA in hospitals.[9]
Nasal mupirocin has an important role to play in the eradication of MRSA carriage. It acts by binding specifically to the bacterial isoleucyl-tRNA synthetase (IRS) enzyme and inhibits its protein synthesis. With the increased use of mupirocin, both low and high level resistance has been reported during treatment with nasal mupirocin.[10] Mupirocin was first introduced in the UK in 1985 and was used to treat staphylococcal and streptococcal wound infections and to eradicate nasal carriage of S. aureus including MRSA.[11] Within 2 years after its introduction, mupirocin resistance among MRSA isolates emerged in the UK and since then in Ireland 2%, New Zealand 12.4%, the USA 24%, and in Trinidad and Tobago 44.1%.[12,13,14,15,16]
Although no performance standards or interpretive criteria have been published for mupirocin susceptibility testing, two mupirocin resistance phenotypes namely low level (MuL) and high level (MuH) mupirocin resistance are defined in Staphylococci.
Low-level resistance (minimal inhibitory concentration [MICs], 8-256 μg/ml) is usually associated with point mutations in the chromosomally encoded ileS gene whereas high-level resistance (MICs, ≥512 μg/ml) is generally due to a plasmid-mediated gene, mupA (also referred to as ileS2), which encodes an additional modified IRS[17] and is typically located on mobile genetic elements, which likely facilitates the dissemination of this resistance mechanism. The mupA gene is typically plasmid mediated, and some of these plasmids are conjugative. MupB is a new high level mupirocin resistance mechanism in S. aureus.[18]
Various studies suggest that during mupirocin prophylaxis transfer of mupA gene from normal commensal flora of the skin such as Staphylococcus epidermidis to MRSA is responsible for the emergence of mupirocin resistance.[19] The risk of the emergence of such resistance appears to be greater among methicillin-resistant strains of S. aureus than among methicillin-susceptible strains.
Detection and differentiation of both types has important clinical implications. The presence of high-level mupirocin resistance (MuH) excludes its clinical use, however low-level mupirocin resistance (MuL) can be overcome by recommending higher than usual dosage.
Therefore, it is essential not only to discriminate between susceptible and resistant strains but also to determine the level of resistance. The true extent of mupirocin-resistant among HCWs in our area is unknown. Thus, this study was carried out to with the following aims and objectives.
Aims and objectives
To find the prevalence of S. aureus from the nasal swabs of HCWs
To find the prevalence of mupirocin resistance in S. aureus and coagulase-negative Staphylococcus (CoNS) spp. by disc diffusion
To determine the rates of MuH and MuL in S. aureus and CoNS spp. by disc diffusion
To know the antimicrobial susceptibility patterns of S. aureus.
Materials and Methods
A prospective cross-sectional study was carried out from the period of December 2013 to April 2014. Approval was obtained from the Ethical Committee for carrying out the study. Written informed consent was obtained from participants.
A total of 140 HCWs (doctor, nursing staff, housekeeping staff) were randomly selected. The age, sex, designation, duration of working in the critical care unit and other relevant information were obtained in a proforma which was made for this purpose. Participants were HCWs in the intensive care unit and operation theatre for more than 1 year. Eight HCWs were not included as they were having upper respiratory tract infection.
Nasal swabs from both nostrils were collected by rotating a sterile cotton swab pre-wetted with sterile saline 5 times on the vestibule of both anterior nares. The swabs were immediately placed in test tubes for further processing in the laboratory.
Nasal swabs from both nostrils were streaked on blood agar for 24 h at 37°C. Identification of S. aureus was done by standard biochemical techniques.[20]
All the confirmed S. aureus strains were subsequently tested for methicillin resistance using cefoxitin disc (30 μg). The isolates were considered methicillin-resistant if the zone of inhibition was 21 mm or less.[20]
The isolates of S. aureus were then tested for mupirocin resistance. This was done by the disk diffusion method using 5 μg and 200 μg mupirocin discs to determine low and high level resistance respectively.[20]
Criteria of zone diameter breakpoints for susceptible and resistant isolates were set at > 14 mm and < 13 mm respectively.[21] Three different phenotypes are:
Mupirocin susceptible: A zone diameter of ≥ 14 mm for both 5 μg and 200 μg discs
Low-level resistance: Isolates that showed zone diameters < 14 mm in the 5 μg disc but more than or equal to 14 mm in the 200 μg disc
High-level resistance: Isolates with zone diameters < 14 mm for both 5 μg and 200 μg.
Staphylococcus aureus isolates was then subjected to antimicrobial susceptibility testing by modified Kirby-Bauer's disc diffusion method on Mueller-Hinton agar plates using as per Clinical and Laboratory Standards Institute guidelines[20] except for fusidic acid where the French Society of Microbiology recommendations were used.
The following antibiotics were tested amikacin (30 μg), erythromycin (15 μg), clindamycin (2 μg), linezolid (30 μg), gentamycin (30 μg), fusidic acid (10 μg), trimethoprim-sulfamethoxazole (25 μg), teicoplanin (30 μg), rifampicin (10 μg) and vancomycin (30 μg), mupirocin (5 μg) and mupirocin (200 μg) (discs were procured from Hi-media Laboratories, Mumbai, India and Mast group, UK).
Results
Out of 140 HCWs, S. aureus was isolated in 38 (27.14%) out of which MRSA and methicillin-sensitive S. aureus (MSSA) were 20 (14.28%) and 18 (12.86%) respectively. CoNS was isolated in 73 (52.14%) workers, among them methicillin-resistant coagulase-negative Staphylococci (MRCoNS) was found in 34 (24.29%) and methicillin-sensitive coagulase negative Staphylococci (MSCoNS) 39 (27. 86%) respectively [Table 1]. The prevalence of the S. aureus nasal carriage was higher among female HCWs 21 (55.26%) than males 17 (44.74%). There is statistically no significance between colonization of female HCW and male HCW [Table 2].
Table 1.
Culture results of 140 nasal swabs of HCW

Table 2.
Gender-wise prevalence of S. aureus and MRSA isolates

In relation to the professional category, Doctors have presented the lowest prevalence of colonization (21.05%), followed by the Housekeeping staff (31.58%) and the high prevalence was found in nursing staff (47.37%) [Table 3].
Table 3.
Category-wise prevalence of S. aureus and MRSA isolates

In our study among the 140 health care workers, MSSA and MSCoNS isolates were 100% sensitive to mupirocin but two isolates from MRSA (1.43%) and five from MRCoNS (3.57%) showed mupirocin resistance. [Table 4 and Figure 1].
Table 4.
Mupirocin resistance in Staphylococcus spp

Figure 1.
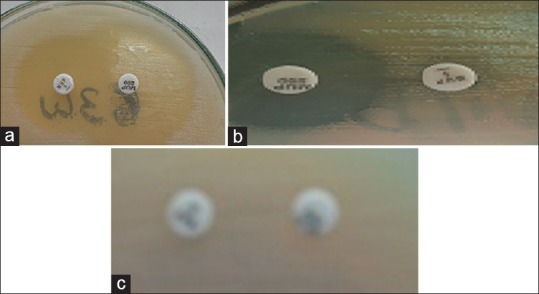
(a) Mupirocin susceptible (b) Low-level resistance (c) High-level resistance
Discussion
Methicillin-resistant S. aureus has been recognized as an important nosocomial pathogen worldwide because of the increased rate of multidrug resistant strains among the hospital acquired MRSA. MRSA colonization precedes infection, anterior nares being the ecological niches of S. aureus.
The prevalence of S. aureus in a nasal carriage of HCWs in our study was 38 (27.14%). Similar were the findings of Golia et al. (24.84%)[22] and Rongpharpi et al. (22.22%).[23]
The prevalence of MRSA and MSSA in our study was 20 (14.28%) and 18 (12. 86%) Golia et al. noted to the tune of 13.37% and 11.46%.[22] Other workers reported the prevalence of MRSA of 11.43%, and 28.6% respectively.[23,24,25]
Recent studies have shown that the prevalence of MRSA colonization among health staff changes according to the location and with the characteristics of each institution.
In this study, female HCWs were more colonized 21 (55.26) when compared to male HCWs 17 (44.74). Similar were the findings of Shakya et al. who observed males HCWs (42%) and females HCWs (57%).[25]
In relation to the professional category, doctors have presented the lowest prevalence of colonization (21.05%), followed by the housekeeping staff (31.58%) and the high prevalence was found in nursing staff (47.37%) [Table 3]. Other workers reported the prevalence of colonization among doctors to the tune of 25% and 22.7%.[23,24]
Silva et al. observe that to develop activities like respiratory therapists or laboratory technicians represent a risk factor for the colonization by S. aureus. These individual possesses 4.57 times greater probability of being colonized when compared to doctors. And the author believe that the high prevalence among nursing staff probably occurs because these professionals are less provided with information related to risks, for the team and for the patients, caused by health staff, colonized by pathogenic microorganisms.[26]
Mupirocin is the cornerstone of decolonization regimens, a successful strategy to prevent healthcare-associated staphylococcal infections. However, new MRSA colonization has been reported even after the use of mupirocin.
Shakya et al. observed 0% resistance to mupirocin in the HCW's whereas in our study among the 140 HCW's, MSSA and MSCoNS isolates were 100% sensitive to mupirocin but two isolates from MRSA (1.43%) and five from MRCoNS (3.57%) were mupirocin resistance [Table 4 and Figure 1].[25]
Prolonged, widespread or uncontrolled use and multiple courses of mupirocin are all associated with the development of mupirocin resistance[14] exposure of CoNS on skin surfaces during prolonged or repeated topical application of mupirocin may lead to the development of a reservoir of high-level resistance determinants in CoNS which may then be transferred to S. aureus in patients on mupirocin therapy.
Oommen et al. observed the rate of mupirocin resistance in S. aureus as 1.02% but the rate of mupirocin resistance in CoNS (16%). In a study by Gadepalli et al. which first documented the extent of mupirocin resistance in an Indian hospital, it was found that 1% of 200 S. aureus isolates (including 0.9% of MRSA and 1.1% of MSSA) showed low-level resistance and 5% showed high-level resistance (8.2% of MRSA and 1.1% of MSSA isolates).[27,28]
In a study that included Staphylococci from 19 European hospitals, the prevalence of MuH was found to be 1.6% in S. aureus and 5.6% in CoNS.[29]
In our study higher percentage of antibiotic resistance was noted to erythromycin (66.67%), gentamicin (50%), clindamycin (47.61%), trimethoprim-sulfamethoxazole (42.85%) and lower resistance was observed for rifampicin (2.38%) and fusidic acid (11.90%). None of our isolates showed resistance to teicoplanin, vancomycin and linezolid [Table 5].
Table 5.
Antibiotic resistance pattern of S. aureus isolates

O'Neill et al. reported that MRSA strains with mupirocin resistance were often more susceptible to other antimicrobial agents, such as tetracycline and trimethoprim-sulfamethoxazole. In contrast, mupirocin-resistant isolates were more likely to be resistant to fusidic acid. It is speculated by the author that the fusB determinant, which is responsible for fusidic acid resistance is on the same plasmid as the mupA gene in isolates with MuH.[30] However, there are studies that demonstrate that some isolate bacterial of S. aureus already present genes resistant to mupirocin, compromising the therapeutic value of the latter.
If stable chromosomal MuH were to become prevalent, reducing the antibiotic selection pressure may not lead to a reduction in rates of resistance and control measures will have to rely on prevention of transmission.
Conclusions
Mupirocin is a topical antibiotic used for treating MRSA associated skin and soft tissue infections and eliminating nasal colonization of MRSA among patients and medical staff.
The presence of mupirocin resistance in MRSA and MRCoNS is a cause for concern. Hospitals need to develop more stringent hospital infection control policies, but also to create awareness among housekeeping and nursing staff by educating them to eradicate MRSA carriage.
Mupirocin nasal ointment should be reserved for the eradication (in both patients and staff) of nasal carriage of MRSA. Alternative preparations such as chlorhexidine and neomycin cream (Naseptin-Manufactured by Alliance Pharmaceuticals) should be considered if colonization persists after two courses of mupirocin or if swabs confirm mupirocin resistance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Yazgi H, Ertek M, Ozbek A, Kadanali A. Nasal carriage of Staphylococcus aureus in hospital personnel and the normal population and antibiotic resistance of the isolates. Mikrobiyol Bul. 2003;37:137–42. [PubMed] [Google Scholar]
- 2.Sousa-Junior FC, Silva-Carvalho MC, Fernandes MJ, Vieira MF, Pellegrino FL, Figueiredo AM, et al. Genotyping of methicillin-resistant Staphylococcus aureus isolates obtained in the Northeast region of Brazil. Braz J Med Biol Res. 2009;42:877–81. doi: 10.1590/s0100-879x2009005000018. [DOI] [PubMed] [Google Scholar]
- 3.Heininger U, Datta F, Gervaix A, Schaad UB, Berger C, Vaudaux B, et al. Prevalence of nasal colonization with methicillin-resistant Staphylococcus aureus (MRSA) in children a multicenter cross-sectional study. Pediatr Infect Dis J. 2007;26:544–6. doi: 10.1097/INF.0b013e31804d244a. [DOI] [PubMed] [Google Scholar]
- 4.Morange-Saussier V, Giraudeau B, van der Mee N, Lermusiaux P, Quentin R. Nasal carriage of methicillin-resistant Staphylococcus aureus in vascular surgery. Ann Vasc Surg. 2006;20:767–72. doi: 10.1007/s10016-006-9088-x. [DOI] [PubMed] [Google Scholar]
- 5.Creech CB, 2nd, Kernodle DS, Alsentzer A, Wilson C, Edwards KM. Increasing rates of nasal carriage of methicillin-resistant Staphylococcus aureus in healthy children. Pediatr Infect Dis J. 2005;24:617–21. doi: 10.1097/01.inf.0000168746.62226.a4. [DOI] [PubMed] [Google Scholar]
- 6.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–62. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 7.Halablab MA, Hijazi SM, Fawzi MA, Araj GF. Staphylococcus aureus nasal carriage rate and associated risk factors in individuals in the community. Epidemiol Infect. 2010;138:702–6. doi: 10.1017/S0950268809991233. [DOI] [PubMed] [Google Scholar]
- 8.Peacock JE, Jr, Moorman DR, Wenzel RP, Mandell GL. Methicillin-resistant Staphylococcus aureus: Microbiologic characteristics, antimicrobial susceptibilities, and assessment of virulence of an epidemic strain. J Infect Dis. 1981;144:575–82. doi: 10.1093/infdis/144.6.575. [DOI] [PubMed] [Google Scholar]
- 9.Goyal R, Das S, Mathur M. Colonisation of methicillin resistant Staphylococcus aureus among health care workers in a tertiary care hospital of Delhi. Indian J Med Sci. 2002;56:321–4. [PubMed] [Google Scholar]
- 10.Palepou MF, Johnson AP, Cookson BD, Beattie H, Charlett A, Woodford N. Evaluation of disc diffusion and Etest for determining the susceptibility of Staphylococcus aureus to mupirocin. J Antimicrob Chemother. 1998;42:577–83. doi: 10.1093/jac/42.5.577. [DOI] [PubMed] [Google Scholar]
- 11.Laupland KB, Conly JM. Treatment of Staphylococcus aureus colonization and prophylaxis for infection with topical intranasal mupirocin: An evidence-based review. Clin Infect Dis. 2003;37:933–8. doi: 10.1086/377735. [DOI] [PubMed] [Google Scholar]
- 12.Rahaman M, Noble WC, Cookson B. Mupirocin-resistant Staphylococcus aureus. Lancet. 1987;2:387–8. [PubMed] [Google Scholar]
- 13.Moorhouse E, Fenelon L, Hone R, Smyth E, McGahon J, Dillon M. Staphylococcus aureus sensitivity to various antibiotics-a national survey in Ireland 1993. Ir J Med Sci. 1996;165:40–3. doi: 10.1007/BF02942801. [DOI] [PubMed] [Google Scholar]
- 14.Upton A, Lang S, Heffernan H. Mupirocin and Staphylococcus aureus: Recent paradigm of emerging antibiotic resistance. J Antimicrob Chemother. 2003;51:613–7. doi: 10.1093/jac/dkg127. [DOI] [PubMed] [Google Scholar]
- 15.Vasquez JE, Walker ES, Franzus BW, Overbay BK, Reagan DR, Sarubbi FA. The epidemiology of mupirocin resistance among methicillin-resistant Staphylococcus aureus at a Veterans' Affairs hospital. Infect Control Hosp Epidemiol. 2000;21:459–64. doi: 10.1086/501788. [DOI] [PubMed] [Google Scholar]
- 16.Orrett FA. The emergence of mupirocin resistance among clinical isolates of methicillin-resistant Staphylococcus aureus in Trinidad: A first report. Jpn J Infect Dis. 2008;61:107–10. [PubMed] [Google Scholar]
- 17.Udo EE, Jacob LE, Mathew B. Genetic analysis of methicillin-resistant Staphylococcus aureus expressing high- and low-level mupirocin resistance. J Med Microbiol. 2001;50:909–15. doi: 10.1099/0022-1317-50-10-909. [DOI] [PubMed] [Google Scholar]
- 18.Seah C, Alexander DC, Louie L, Simor A, Low DE, Longtin J, et al. MupB, a new high-level mupirocin resistance mechanism in Staphylococcus aureus. Antimicrob Agents Chemother. 2012;56:1916–20. doi: 10.1128/AAC.05325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurdle JG, O'Neill AJ, Mody L, Chopra I, Bradley SF. In vivo transfer of high-level mupirocin resistance from Staphylococcus epidermidis to methicillin-resistant Staphylococcus aureus associated with failure of mupirocin prophylaxis. J Antimicrob Chemother. 2005;56:1166–8. doi: 10.1093/jac/dki387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical and Laboratory Standard Institute. Performance standards for antimicrobial disc susceptibility tests, twentieth supplement. 2012;32:M100–S21. [Google Scholar]
- 21.Finlay JE, Miller LA, Poupard JA. Interpretive criteria for testing susceptibility of Staphylococci to mupirocin. Antimicrob Agents Chemother. 1997;41:1137–9. doi: 10.1128/aac.41.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golia S, Hittinahalli V, Kamath AS, Nirmala AR. A study of nasal carriage of MRSA among the health care workers of a tertiary care hospital, Bangalore. Int J Basic Appl Med Sci. 2013;3:3–7. [Google Scholar]
- 23.Rongpharpi SR, Hazarika NK, Kalita H. The prevalence of nasal carriage of Staphylococcus aureus among healthcare workers at a tertiary care hospital in Assam with special reference to MRSA. J Clin Diagn Res. 2013;7:257–60. doi: 10.7860/JCDR/2013/4320.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinodhkumaradithyaa A, Uma A, Shirivasan M, Ananthalakshmi I, Nallasivam P, Thirumalaikolundusubramanian P. Nasal carriage of methicillin-resistant Staphylococcus aureus among surgical unit staff. Jpn J Infect Dis. 2009;62:228–9. [PubMed] [Google Scholar]
- 25.Shakya B, Shrestha S, Mitra T. Nasal carriage rate of methicillin resistant Staphylococcus aureus among at National Medical College Teaching Hospital, Birgunj, Nepal. Nepal Med Coll J. 2010;12:26–9. [PubMed] [Google Scholar]
- 26.Silva EC, Antas Md, Monteiro B Neto A, Rabelo MA, Melo FL, Maciel MA. Prevalence and risk factors for staphylococcus aureus in health care workers at a university hospital of Recife-PE. Braz J Infect Dis. 2008;12:504–8. doi: 10.1590/s1413-86702008000600012. [DOI] [PubMed] [Google Scholar]
- 27.Oommen SK, Appalaraju B, Jinsha K. Mupirocin resistance in clinical isolates of Staphylococci in a tertiary care centre in south India. Indian J Med Microbiol. 2010;28:372–5. doi: 10.4103/0255-0857.71825. [DOI] [PubMed] [Google Scholar]
- 28.Gadepalli R, Dhawan B, Mohanty S, Kapil A, Das BK, Chaudhry R, et al. Mupirocin resistance in Staphylococcus aureus in an Indian hospital. Diagn Microbiol Infect Dis. 2007;58:125–7. doi: 10.1016/j.diagmicrobio.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz FJ, Jones ME. Antibiotics for treatment of infections caused by MRSA and elimination of MRSA carriage. What are the choices? Int J Antimicrob Agents. 1997;9:1–19. doi: 10.1016/s0924-8579(97)00027-7. [DOI] [PubMed] [Google Scholar]
- 30.O'Neill AJ, McLaws F, Kahlmeter G, Henriksen AS, Chopra I. Genetic basis of resistance to fusidic acid in Staphylococci. Antimicrob Agents Chemother. 2007;51:1737–40. doi: 10.1128/AAC.01542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]


