Abstract
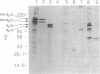
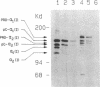
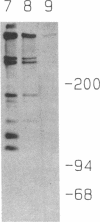
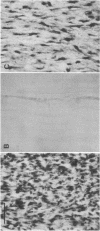
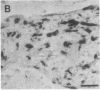
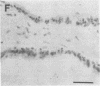
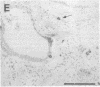
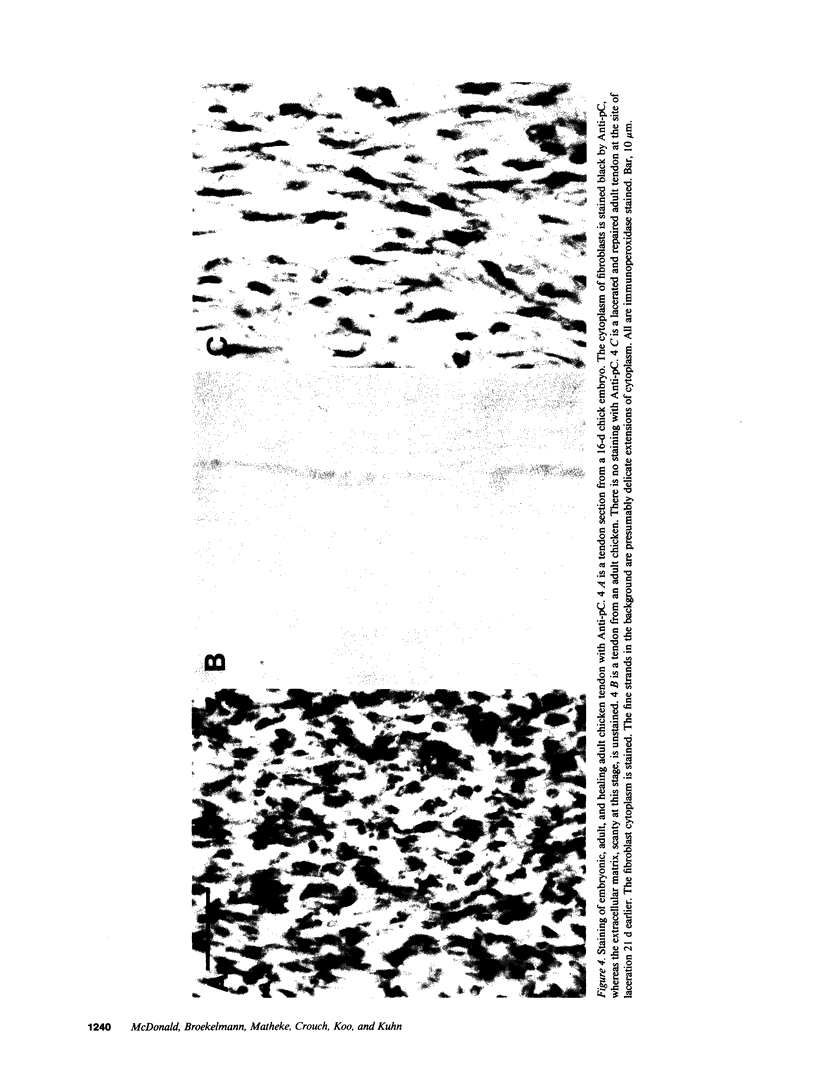
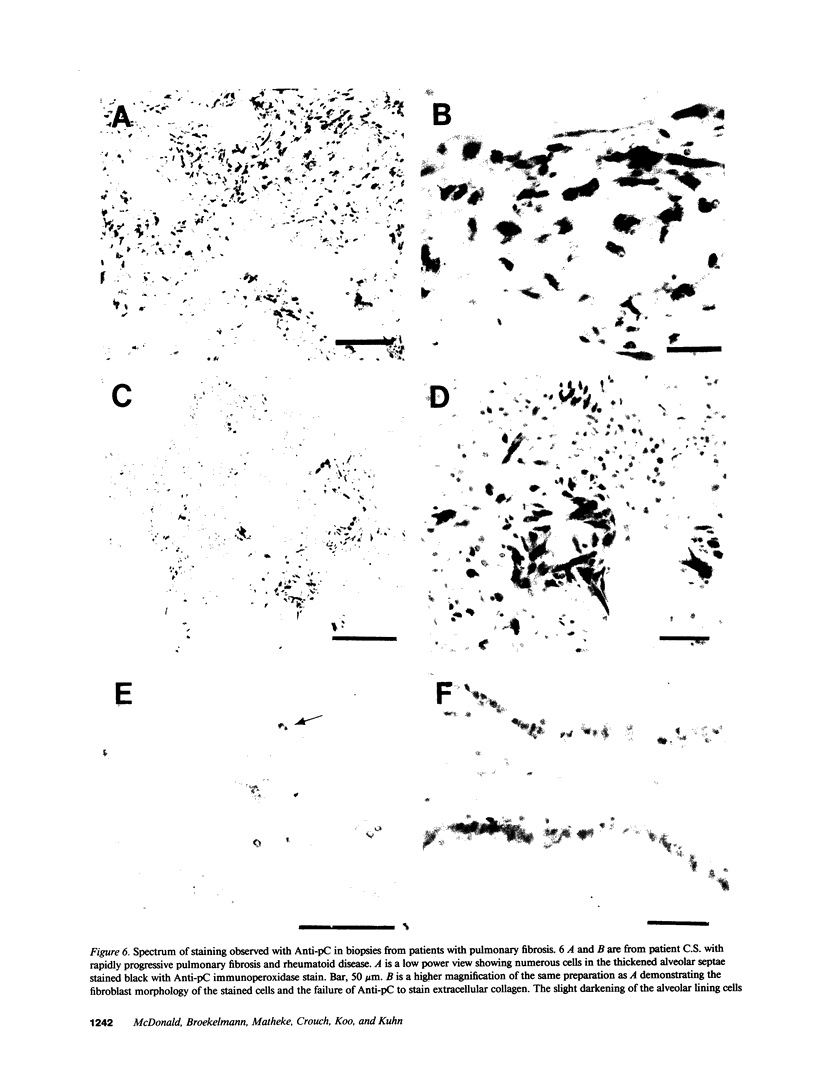
Excessive collagen deposition plays a critical role in the development of fibrosis, and early or active fibrosis may be more susceptible to therapeutic intervention than later stages of scarring. However, at present there is no simple method for assessing the collagen-synthesizing and secreting activity of fibroblasts in human tissues. Type I procollagen carboxyterminal domains are proteolytically removed during collagen secretion. Thus, antibodies to these domains should stain fibroblasts synthesizing type I collagen but not extracellular collagen fibrils which could mask the signal from the cells. We developed and characterized a monoclonal antibody (Anti-pC) specific for the carboxyterminal propeptide of type I procollagen. To determine the relationship between Anti-pC staining and collagen synthesis, we stained embryonic and adult chicken tendon. Embryonic chick tendon fibroblasts actively synthesizing type I collagen stained heavily with Anti-pC, while quiescent adult tendon fibroblasts did not stain with Anti-pC. Wounded adult tendons developed fibroblasts that stained with Anti-pC at the wound site. Thus, Anti-pC specifically visualized fibroblasts actively synthesizing collagen. Lung biopsies from patients with fibrotic lung disease were stained with Anti-pC. Interstitial and intraalveolar fibroblasts in biopsies from patients with active fibrosis stained intensely with Anti-pC, while normal human lung was unstained. The absence of staining in normal lung supports the hypothesis that fibrosis is associated with an altered collagen-synthesizing phenotype of tissue fibroblasts. Anti-pC may provide a useful clinical tool for assessing fibrogenic activity at sites of tissue injury.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bateman E. D., Turner-Warwick M., Haslam P. L., Adelmann-Grill B. C. Cryptogenic fibrosing alveolitis: prediction of fibrogenic activity from immunohistochemical studies of collagen types in lung biopsy specimens. Thorax. 1983 Feb;38(2):93–101. doi: 10.1136/thx.38.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsell D. C., Tustanoff E. R., Kindsay W. K. Collagen production in regenerating tendon. Plast Reconstr Surg. 1966 Jun;37(6):504–511. doi: 10.1097/00006534-196606000-00005. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Click E. M., Harper E., Bornstein P. Interchain disulfide bonds in procollagen are located in a large nontriple-helical COOH-terminal domain. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3009–3013. doi: 10.1073/pnas.72.8.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. G., Kuhn C., 3rd, McDonald J. A., Mecham R. P. Lung connective tissue. Int Rev Connect Tissue Res. 1983;10:249–331. doi: 10.1016/b978-0-12-363710-9.50011-3. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Fessler J. H., Fessler L. I. Biosynthesis of procollagen. Annu Rev Biochem. 1978;47:129–162. doi: 10.1146/annurev.bi.47.070178.001021. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Olsen B. R., Timpl R., Perlish J. S., Lovelace O. Collagen fibril formation during embryogenesis. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3354–3358. doi: 10.1073/pnas.80.11.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmajer R., Timpl R., Tuderman L., Raisher L., Wiestner M., Perlish J. S., Graves P. N. Ultrastructural identification of extension aminopropeptides of type I and III collagens in human skin. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7360–7364. doi: 10.1073/pnas.78.12.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulmer J. D., Bienkowski R. S., Cowan M. J., Breul S. D., Bradley K. M., Ferrans V. J., Roberts W. C., Crystal R. G. Collagen concentration and rates of synthesis in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1980 Aug;122(2):289–301. doi: 10.1164/arrd.1980.122.2.289. [DOI] [PubMed] [Google Scholar]
- Galambos M. R., Collins D. C., Galambos J. T. A radioimmunoassay procedure for type III procollagen: its use in the detection of hepatic fibrosis. Hepatology. 1985 Jan-Feb;5(1):38–42. doi: 10.1002/hep.1840050109. [DOI] [PubMed] [Google Scholar]
- Goldberg B. D., Phelps R. G., Kessler E., Klein M. J., Taubman M. B. The carboxyl fragment released by bacterial collagenase from human type I procollagen: antibodies to the propeptide determinants. Coll Relat Res. 1985 Nov;5(5):393–404. doi: 10.1016/s0174-173x(85)80027-3. [DOI] [PubMed] [Google Scholar]
- Hølund B., Clausen P. P., Clemmensen I. The influence of fixation and tissue preparation on the immunohistochemical demonstration of fibronectin in human tissue. Histochemistry. 1981;72(2):291–299. doi: 10.1007/BF00517142. [DOI] [PubMed] [Google Scholar]
- Kao W. W., Berg R. A., Prockop D. J. Kinetics for the secretion of procollagen by freshly isolated tendon cells. J Biol Chem. 1977 Dec 10;252(23):8391–8397. [PubMed] [Google Scholar]
- Kirk J. M., Bateman E. D., Haslam P. L., Laurent G. J., Turner-Warwick M. Serum type III procollagen peptide concentration in cryptogenic fibrosing alveolitis and its clinical relevance. Thorax. 1984 Oct;39(10):726–732. doi: 10.1136/thx.39.10.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk J. M., Da Costa P. E., Turner-Warwick M., Littleton R. J., Laurent G. J. Biochemical evidence for an increased and progressive deposition of collagen in lungs of patients with pulmonary fibrosis. Clin Sci (Lond) 1986 Jan;70(1):39–45. doi: 10.1042/cs0700039. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Low R. B., Cutroneo K. R., Davis G. S., Giancola M. S. Lavage type III procollagen N-terminal peptides in human pulmonary fibrosis and sarcoidosis. Lab Invest. 1983 Jun;48(6):755–759. [PubMed] [Google Scholar]
- McDonald J. A., Broekelmann T. J., Kelley D. G., Villiger B. Gelatin-binding domain-specific anti-human plasma fibronectin Fab' inhibits fibronectin-mediated gelatin binding but not cell spreading. J Biol Chem. 1981 Jun 10;256(11):5583–5587. [PubMed] [Google Scholar]
- McDonald J. A., Kelley D. G., Broekelmann T. J. Role of fibronectin in collagen deposition: Fab' to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J Cell Biol. 1982 Feb;92(2):485–492. doi: 10.1083/jcb.92.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. A., Kelley D. G. Specific binding of fibronectin--antifibronectin immune complexes to procollagen: a new pitfall in immunostaining. J Cell Biol. 1984 Mar;98(3):1042–1047. doi: 10.1083/jcb.98.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris N. P., Fessler L. I., Fessler J. H. Procollagen propeptide release by procollagen peptidases and bacterial collagenase. J Biol Chem. 1979 Nov 10;254(21):11024–11032. [PubMed] [Google Scholar]
- Phelps R. G., Martinez-Hernandez A., Goldberg B. D. Ultrastructural immunocytochemical localization of type I procollagen in cultured human fibroblasts. Coll Relat Res. 1985 Nov;5(5):405–414. doi: 10.1016/s0174-173x(85)80028-5. [DOI] [PubMed] [Google Scholar]
- Pratt D. S., Schwartz M. I., May J. J., Dreisin R. B. Rapidly fatal pulmonary fibrosis: the accelerated variant of interstitial pneumonitis. Thorax. 1979 Oct;34(5):587–593. doi: 10.1136/thx.34.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara K., Igarashi S., Hatahara T. Localization of type III procollagen aminopeptide antigenicity in hepatocytes from cirrhotic human liver. Virchows Arch A Pathol Anat Histopathol. 1985;408(2-3):219–228. doi: 10.1007/BF00707984. [DOI] [PubMed] [Google Scholar]
- Savolainen E. R., Goldberg B., Leo M. A., Velez M., Lieber C. S. Diagnostic value of serum procollagen peptide measurements in alcoholic liver disease. Alcohol Clin Exp Res. 1984 Jul-Aug;8(4):384–389. doi: 10.1111/j.1530-0277.1984.tb05684.x. [DOI] [PubMed] [Google Scholar]
- Schwarz R. I. Procollagen secretion meets the minimum requirements for the rate-controlling step in the ascorbate induction of procollagen synthesis. J Biol Chem. 1985 Mar 10;260(5):3045–3049. [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Taubman M. B., Goldberg B. The processing of procollagen in cultures of human and mouse fibroblasts. Arch Biochem Biophys. 1976 Apr;173(2):490–494. doi: 10.1016/0003-9861(76)90286-1. [DOI] [PubMed] [Google Scholar]
- Torikata C., Villiger B., Kuhn C., 3rd, McDonald J. A. Ultrastructural distribution of fibronectin in normal and fibrotic human lung. Lab Invest. 1985 Apr;52(4):399–408. [PubMed] [Google Scholar]
- Uitto J., Booth B. A., Polak K. L. Collagen biosynthesis by human skin fibroblasts. II. Isolation and further characterization of type I and type III procollagens synthesized in culture. Biochim Biophys Acta. 1980 Aug 21;624(2):545–561. doi: 10.1016/0005-2795(80)90095-1. [DOI] [PubMed] [Google Scholar]
- Watters L. C., King T. E., Schwarz M. I., Waldron J. A., Stanford R. E., Cherniack R. M. A clinical, radiographic, and physiologic scoring system for the longitudinal assessment of patients with idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1986 Jan;133(1):97–103. doi: 10.1164/arrd.1986.133.1.97. [DOI] [PubMed] [Google Scholar]
- Zapol W. M., Trelstad R. L., Coffey J. W., Tsai I., Salvador R. A. Pulmonary fibrosis in severe acute respiratory failure. Am Rev Respir Dis. 1979 Apr;119(4):547–554. doi: 10.1164/arrd.1979.119.4.547. [DOI] [PubMed] [Google Scholar]