Abstract
Background and Aims:
The aim was to evaluate efficacy of optic nerve sheath diameter (ONSD) by ultrasound as a noninvasive method for detecting raised intracranial pressure (ICP) in intensive care unit, to compare with computed tomography/magnetic resonance imaging (MRI) findings of raised ICP and to prognosticate ONSD value with treatment.
Materials and Methods:
We conducted a prospective, observational study on 101 adults by including 41 healthy individuals in group A as control and 60 patients in group B admitted with fever, headache, vomiting, and altered sensorium. We examined them in supine position using 10 MHz linear array probe on closed eyelid. ONSD was measured 3 mm behind the globe in each eye. A mean binocular ONSD > 4.6 mm in female and 4.8 mm in male was considered abnormal. Midline shift, edema, effacement or ONSD > 5.0 mm on T2 MRI suggestive of elevated ICP was used to evaluate ONSD accuracy.
Results:
Group A mean ONSD was 4.6 mm in females and 4.8 mm in males. Group B mean ONSD for 17 females was 5.103 ± 0.6221 mm (P = 0.002) and for 43 males 5.081 ± 0.5799 mm (P = 0.032). Radiological sign of raised ICP was confirmed in 35 patients (females = 11 and males = 24) with high ONSD value. Sensitivity of detecting raised ICP by ONSD was 84.6% in females and 75% in males while specificity was 100% in both genders. Out of 25 patients without radiological signs of raised ICP 10 patients showed high ONSD (females = 4.735 mm and males = 4.907 mm). ONSD was well prognosticated with treatment modalities.
Conclusion:
Bedside ocular ultrasonography for measuring ONSD can be used an early test for diagnosing raised ICP as it is a noninvasive, cost effective bedside test, which can be repeated for re-evaluation.
Keywords: Computed tomography, intensive care unit, magnetic resonance imaging, mannitol, optic nerve sheath diameter, raised intracranial pressure, ultrasonography
Introduction
Raised intracranial pressure (ICP) is usually associated with increased morbidity, mortality, and poor neurological outcomes. The etiology could be varied viz stroke, liver failure, meningitis, meningoencephalitis, metabolic encephalopathy and postresuscitation syndrome.[1,2,3,4,5,6] Early detection and prompt treatment of raised ICP in such situations are essential. However, it may pose challenges at the same time.[4,7] Invasive ICP monitoring is the gold standard. It is associated with complications such as infection, bleeding and being expensive. Regular assessment and comparison by computed tomography (CT)/magnetic resonance imaging (MRI) in these critically ill-patients is fought with dangers of transporting to radiology.[8]
The optic nerve, as a part of the central nervous system, is surrounded by a subarachnoid space and experiences the same pressure change as the intracranial compartment.[9,10,11,12] The intraorbital part of the sheath, and particularly its retrobulbar segment, can distend when ICP is elevated.
The use of bedside ocular ultrasonography (USG) in measuring optic nerve sheath diameter (ONSD) can be a useful method for detecting raised ICP. It has the advantage of being a noninvasive, portable, easily performed at bedside in minimum time. It can be repeated for re-evaluation without risk of radiation.
Keeping this in view, we conducted the bedside study of a noninvasive measurement of ONSD as predictor for detecting raised ICP. We also followed up the ONSD trends during the treatment and monitor its usefulness.
Materials and Methods
We conducted a prospective observational study on 101 adult individuals after Institutional Review Board's permission over a period between May 2013 and August 2013. They were divided into two groups A and B. Group A were, 41 healthy controls of which 20 were female and 21 male. A total of 60 patients were admitted during this period with symptoms of fever, headache, vomiting and altered sensorium with possibility of elevated ICP, were included in group B. They were 17 female and 43 male. All these patients were examined in the supine position using a 10 MHz phased linear array probe on the closed eyelids [Figure 1]. The structures of the eye were visualized to align the optic nerve directly opposite the probe, with the ONSD width perpendicular to the vertical axis of the scanning plane. A single ONSD was measured 3.00 mm behind the globe [Figure 2] in both the eyes. The ONSD measurements were obtained averaging three readings from each eye to create a binocular ONSD measurement.[11,12]
Figure 1.

Occular sonography: High frequency 10 MHz linear array probe placed on the gel on the closed eyelid
Figure 2.
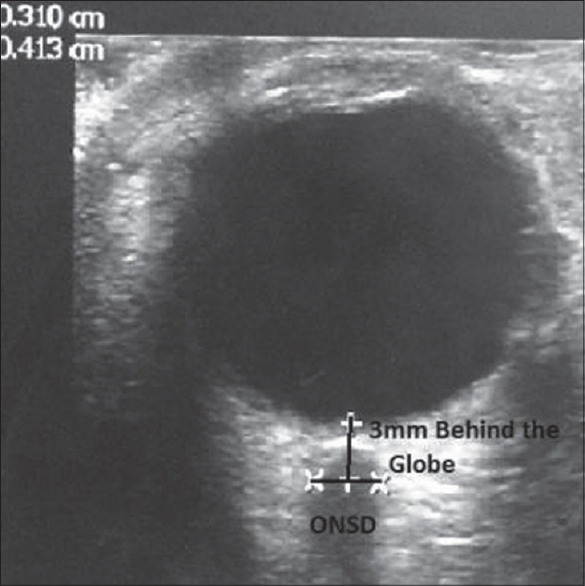
The optic nerve sheath diameter (ONSD) measurement: Optic nerve appears homogeneous with low internal reflectivity compared with the high reflectivity of the nerve sheath. ONSD measured 3 mm behind the globe using an electronic caliper with an angle perpendicular to the eye ball
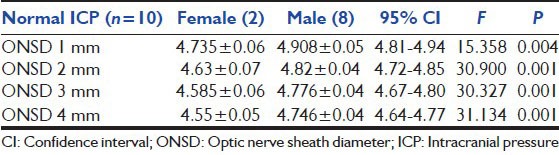
The individuals in the control group were between 18 and 40 years. In these controls a mean binocular ONSD was 4.6 mm and 4.8 mm in females and males respectively. The measurements above 4.6 mm and 4.8 mm in females and males were considered to have increased ICP.[13,14,15] Imaging of the head CT/MRI was done as per requirement in group B patients only. The finding of CT/MRI was reported by the on-site radiologist, and they were correlated with bedside ONSD measurement.[16] The patient's imaging result was considered to be positive for raised ICP if the radiologist's impression described findings, suggestive of elevated ICP such as significant cerebral edema, midline shift, mass effect, effacement of sulci, collapse of ventricles, compression of cisterns and ONSD > 5.0 mm on T2 MRI.
The waiver of the individual consent was requested as the intervention was completely harmless, nonchargeable to the patient and no patient identification was used. Security and confidentiality of data was preserved and analyzed in aggregate. Patients with a history of optic neuritis, arachnoid cyst of the optic nerve, high myopic, optic nerve trauma, and anterior orbital or cavernous sinus mass are excluded from the study.
The variables used were age, sex, diagnosis, heart rate, blood pressure, respiratory rate, oxygen saturation, temperature, Glasgow Coma Scale (GCS), ONSD, signs of raised ICP on imaging, serum osmolarity, serum creatinine, mannitol, and management.
Statistical analysis
Statistical analyses were performed using standard statistical software. Categorical variables were summarized through the calculation of frequency and relative frequency. Continuous variables were summarized through the calculation of mean and standard error. Statistically assessed the observed differences among various ONSD groups in the distribution of categorical variables, the Pearson χ2 or Fisher exact test of association was used. Statistics to test baseline differences between the study group and controls was performed with the Mann-Whitney U-test. The Wilcoxon matched pairs signed ranks test was used. P < 0.05 (two-tailed) were considered statistically significant.
Results
We conducted prospective observational study on 101 adult individuals. In group A were 41 (females = 20 and males = 21) as a control. In group B there were 60 (females = 17 and males = 43) patients.
The mean ONSD of control and study group for female was 4.627 ± 0.09 mm and 5.103 ± 0.62 mm and for male was 4.8 ± 0.10 mm and 5.081 ± 0.58 mm respectively. The mean age of the control and study group was 27.44 ± 3.31 and 56.15 ± 18.86 years respectively. Demographic profile of our study population as shown in Table 1.
Table 1.
Demographic profile of the study patient

Out of 60 patients that were admitted, 35 cases showed raised ICP on imaging and their ONSD 5.43 ± 0.53 mm. The other 25 patients did not show a raised ICP on imaging and ONSD was 4.61 ± 0.19 mm. It showed a significant difference between the two sets of patients with a P < 0.001. However, 10 out of their 25 showed a rise in ONSD for a female 4.735 mm > 4.6 mm and male 4.907 mm > 4.8 mm which required reduction of ICP. The mean GCS for raised ICP and normal ICP on imaging was 10.5 ± 0.12 and 12.04 ± 2.11 respectively. The mean temperature 99.31 ± 0.75°F and 98.89 ± 0.55°F respectively for raised ICP and normal ICP. It showed statistically significant as P value for GCS and temperature was 0.04 (<0.05) and 0.02 (<0.05) respectively. Other variables were also analyzed and did not show any statistically significant difference as shown in Table 2.
Table 2.
Association of ICP with clinical profile

Receiver operator characteristic (ROC) curve (area under the curve [AUC]) for mean ONSD 4.716 mm is 98.6% (95% CI: 96.5-100%) with sensitivity of 77.8% and specificity of 100%. For female ROC curve (AUC) for ONSD > 4.6 mm is 100% (95% CI: 100%), with sensitivity of 84.6% and specificity of 100% whereas for male ROC curve (AUC) for ONSD > 4.8 mm is 97.4% (95% CI: 93.5-100%) with sensitivity of 75.0% and specificity of 100% as shown in Figures 3 and 4.
Figure 3.

Receiving operating characteristic curve for detecting raised intracranial pressure by ultrasonography optic nerve sheath diameter for female
Figure 4.

Receiving operating characteristic curve for detecting raised intracranial pressure by ultrasonography optic nerve sheath diameter for male
All these patients received treatment with mannitol, antiepileptics, antiplatelet, antibiotics, and antiviral depending on cerebrospinal fluid (CSF) analysis.
The trend of ONSD in patients with raised ICP was recorded from day 1 to day 4. In the female population ONSD on day 1 was 5.405 ± 0.5729 mm and by day 4 it decreased to 4.868 ± 0.4417 mm. Similarly in male ONSD on day1 was 5.442 ± 0.5233 mm and by day 4 it decreased to 5.065 ± 0.3730 mm. In 10 cases that did not show raised ICP on imaging but had increased ONSD on day 1 for female 4.735 ± 0.064 mm and male 4.907 ± 0.054 mm. Reduction of ICP was noted on 2nd day as ONSD decreased to 4.630 ± 0.071 mm in female and to 4.823 ± 0.038 mm in male. It showed significant difference as P < 0.05 [Tables 3a and b].
Table 3a.
Trend of ONSD with treatment

Table 3b.
Trend of ONSD with treatment
About 35 patients had raised ICP on imaging and ONSD of 5.430 ± 0.5311 mm, 21 cases diagnosed as infective (meningitis, meningoencephalities), 12 cases were cerebrovascular accident (stroke, intracranial hemorrhage), and 2 cases were metabolic encephalopathy. Whereas out of 25 cases that did not show raised ICP radiologically 2 cases were infective (meningitis, meningoencephalities), 18 cases were cerebrovascular accident (stroke, intracranial hemorrhage) and 5 cases were metabolic encephalopathy. But 10 cases did not show raised ICP radiologically but had mean ONSD for two female as 4.735 mm and eight male as 4.907 mm. They were 8 cerebrovascular accident, 1 metabolic encephalopathy, and 1 infective.
In the study group out of 60 patients, 28 patients had high ONSD and signs of raised ICP on imaging received mannitol as definitive treatment. However, three patients who showed only high ONSD and did not show raised ICP on imaging also received mannitol and they showed a decrease in ONSD after supportive therapy.
Totally 26 patients required intubation and controlled mechanical ventilation with sedation. In the study group, 21 patients had high ONSD and signs of raised ICP on imaging, but the other five patients had high ONSD, but did not show signs of raised ICP on imaging. It showed significant difference with P = 0.014 < 0.05 in the management. Total 5 cases expired, 2 with ICP increased, 3 without ICP and it did not find any statistical significant.
Discussion
Raised ICP is, usually, associated with conditions such as stroke, liver failure, meningitis, meningoencephalitis, metabolic encephalopathy, and postresuscitation syndrome. The cranium and the vertebral canal along with the relatively inelastic dura form a rigid container, such that the increase in any of its contents, that is, brain, blood, or CSF, will tend to increase the ICP. The Monro-Kelliy doctrine relationship states that small increases in brain volume do not lead to immediate increase in ICP due to the ability of the CSF to be displaced into the spinal canal, as well as the slight ability to stretch the falx cerebri between the hemispheres and the tentorium between the hemispheres and the cerebellum. However, once the ICP reaches around 20-25 mmHg, any small increase in brain volume can lead to marked elevations in ICP due to failure of intracranial compliance.[17]
In stage 1, there is a minimal increase in ICP. In stage 2, there is a drastic increase in ICP as change in volume is > 100-200 ml. Stage 3 is characterized by a sustained increased ICP, with dramatic changes in ICP with small changes in volume. As the ICP approaches the mean arterial pressure, it becomes more and more difficult to squeeze blood into the intracranial space, leading to widespread reduction in cerebral flow and perfusion, eventually leading to ischemia and brain infarction. Because of hypoxia and hyper apnea, patients present with decreased level of consciousness (LOC), Cheyne-Stokes respiration, hyperventilation, sluggish or dilated pupils and widened pulse pressure.[18] Generally when there is a rise in ICP, common symptoms and signs include headache, vomiting without nausea, and altered LOC. The headache is worse on coughing/sneezing/bending, and progressively worsens over time.[19,20]
The optic nerve sheath (ONS) is anatomically continuous with the dura mater and has a trabeculated arachnoid space through which CSF slowly percolates.[21] On ultrasound examination, optic nerve appears homogeneous with low internal reflectivity compared with the high reflectivity of the nerve sheath; this was utilized by Ossoinig when he performed the first ultrasound measurement of the optic nerve using an A-scan technique, and subsequently described standardized A-scanning.[21]
Using these echography techniques several groups have investigated the relation between the ONSD as measured by A-scan and the ICP Cennamo et al.,[22] Gangemi et al.,[23] and Tamburelli et al.[24] each demonstrated a positive linear relation between these two variables in neurosurgical patients and in particular, an immediate change in ONSD with change in ICP. A position 3 mm behind the globe was chosen because the ultrasound contrast is greatest, the results are more reproducible, and anatomically the anterior nerve is most distensible. Hansen et al. presented data using a transorbital B-scan approach for the measurement of ONSD,[25] this approach allowed them to select a distance behind the globe to consistently measure the nerve, something difficult to attain with A-scan techniques. Helmke and Hansen[9,10,11] demonstrated in cadaver studies that the ONSD increased by up to 60% at a distance of 3 mm behind the globe compared with only 35% at 10 mm thus confirming Liu and Kahn's[26] observations. Furthermore, they went on to show that the optimal experimental scanning position was longitudinal (axial) where the least interobserver variability was found although there was no significant difference in measurement by lateral, axial, or transverse projection.
In our study, the average ONSD in the control group aged between 18 and 40 years was 4.6 mm and 4.8 mm in female and male respectively. It has been proved by Dubourg et al. in a systematic review and meta-analysis of a ultrasonographic of ONSD of detection of raised ICP, a ONSD in a adult < 5 mm, pediatrics (1-15 years) < 4.5 mm, infant < 4 mm was considered to be a normal. They also concluded that ONSD > 5.00-5.70 mm had a raised ICP > 20 mm with diagnostic odds ratio of 51, sensitivity of 90% (95% CI: 80-95%) and specificity 85% (95% CI: 73-93%).[13] In a study conducted by Dubost et al. to detect the incidence of raised ICP in preeclampatic patients, concluded that ONSD was 5.4 mm in preeclampatic patients when compared to healthy pregnant women which was 4.5 mm.[14] Similar study conducted by Rajajee et al. who concluded that bedside measurement of ONSD is an accurate noninvasive method to identify ICP > 20 mmHg in a heterogeneous group of patients with acute brain injury with ONSD > 4.8 mm has greatest accuracy.[15,27] In 60 admitted patients, 35 cases had raised ICP on radiological findings, the ONSD for female was 5.405 mm and male was 5.442 mm. About 10 (40%) individuals did not have raised ICP on imaging, but their ONSD was found to be more than the control for female 4.735 ± 0.064 mm and male 4.907 ± 0.054 mm. We found for mean ONSD of 4.716 mm, sensitivity for detecting raised ICP was 77.8% (95% CI: 83-100%) and specificity 100%. In female sensitivity for raised ICP is 84.6% and specificity 100%. In male sensitivity for raised ICP was 75.0% and specificity was 100%. Beare et al. evaluated ONS ultrasound as a noninvasive method of detecting raised ICP in African children. They concluded that sensitivity and specificity of detecting raised ICP on CT for ONSD 4.2 mm was 100% and 86% respectively.[28] Tayal et al. conducted a prospective blinded observational study on adult head injury patients in an emergency department. They correlated ONSD by USG with CT findings for raised ICP. The sensitivity for detecting raised ICP was 100%, and specificity was 63%.[29] It has been proved by Dubourg et al. in a systemic review and meta-analysis of a ultrasonographic measurement of ONS diameter of detection of raised ICP, that ONSD > 5.00 mm had a raised ICP > 20 mm, pooled sensitivity 90% (95% CI: 80-95%), specificity 85% (95% CI: 73-93%). The patients with raised ICP are 51 times more likely to have a positive ONSD.[13,15] It was also noted that papilledema was not found in the acute situation as it takes hours to days to develop.[30] Acute rise in ICP can be difficult to diagnose because the symptoms are nonspecific, and direct measurement of ICP has the attendant risks of intracranial hemorrhage and infection. In our analysis, 35 patients who had raised ICP on CT/MRI only temperature and GCS had a significant difference [Table 2].
We feel that the results of the present study are encouraging. About 35 cases had raised ICP on imaging and high ONSD on the 1st day of admission and after starting the appropriate treatment ONSD was measured subsequently on days 2, 3 and 4. We found a significant reduction of ONSD for female and male after 72 h of treatment[14] [Table 3a]. About 25 cases did not have increased ICP on imaging. However 10 cases out of 25 had ONSD higher than the cutoff value for female 4.735 ± 0.064 mm and male 4.907 ± 0.054 mm [Table 3b]. After supportive treatment, their ONSD on day 2 for female and male was almost returned to normal value.
The early detection of raised ICP can be very difficult when invasive devices are not available. Clinical signs of raised ICP such as headache, vomiting and drowsiness are not specific and often difficult to interpret. In sedated patients, clinical signs of raised ICP frequently appear late, when ischemic brain injury is already established.[20] Furthermore, a normal CT scan does not exclude a raised ICP.[31,32,33]
Conclusion
Bedside measurement of ONSD is a useful test to identify raised ICP, being noninvasive, can be repeated multiple times, devoid of ionizing radiation and can be applied in a broad range of settings. Further studies are required to validate its usefulness in raised ICP patients.
Acknowledgments
We gratefully acknowledge the Department of Neurology, Department of Radiology, respiratory technicians and nurses, and management of the hospital for their valuable support. We are thankful to the statistician of National Institute of Nutrition, Hyderabad for a valuable support in analyzing the data. We are also grateful to all the patients and volunteers who were part of this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–22. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]
- 2.Ware AJ, D'Agostino AN, Combes B. Cerebral edema: A major complication of massive hepatic necrosis. Gastroenterology. 1971;61:877–84. [PubMed] [Google Scholar]
- 3.Quagliarello V, Scheld WM. Bacterial meningitis: Pathogenesis, pathophysiology, and progress. N Engl J Med. 1992;327:864–72. doi: 10.1056/NEJM199209173271208. [DOI] [PubMed] [Google Scholar]
- 4.Newton CR, Crawley J, Sowumni A, Waruiru C, Mwangi I, English M, et al. Intracranial hypertension in Africans with cerebral malaria. Arch Dis Child. 1997;76:219–26. doi: 10.1136/adc.76.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen M, Brandt CT, Knudsen GM, Ostergaard C, Skinhøj P, Frimodt-Møller N, et al. Cerebral blood flow autoregulation in early experimental S. pneumoniae meningitis. J Appl Physiol (1985) 2007;102:72–8. doi: 10.1152/japplphysiol.00697.2006. [DOI] [PubMed] [Google Scholar]
- 6.Bergman R, Tjan DH, Adriaanse MW, van Vugt R, van Zanten AR. Unexpected fatal neurological deterioration after successful cardio-pulmonary resuscitation and therapeutic hypothermia. Resuscitation. 2008;76:142–5. doi: 10.1016/j.resuscitation.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Calvo A, Hernández P, Spagnuolo E, Johnston E. Surgical treatment of intracranial hypertension in encephalic cryptococcosis. Br J Neurosurg. 2003;17:450–5. doi: 10.1080/02688690310001611242. [DOI] [PubMed] [Google Scholar]
- 8.Beckmann U, Gillies DM, Berenholtz SM, Wu AW, Pronovost P. Incidents relating to the intra-hospital transfer of critically ill patients. An analysis of the reports submitted to the Australian Incident Monitoring Study in Intensive Care. Intensive Care Med. 2004;30:1579–85. doi: 10.1007/s00134-004-2177-9. [DOI] [PubMed] [Google Scholar]
- 9.Hansen HC, Helmke K. The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheath. Surg Radiol Anat. 1996;18:323–8. doi: 10.1007/BF01627611. [DOI] [PubMed] [Google Scholar]
- 10.Helmke K, Hansen HC. Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension. I. Experimental study. Pediatr Radiol. 1996;26:701–5. doi: 10.1007/BF01383383. [DOI] [PubMed] [Google Scholar]
- 11.Helmke K, Hansen HC. Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension II. Patient study. Pediatr Radiol. 1996;26:706–10. doi: 10.1007/BF01383384. [DOI] [PubMed] [Google Scholar]
- 12.Hansen HC, Helmke K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: Ultrasound findings during intrathecal infusion tests. J Neurosurg. 1997;87:34–40. doi: 10.3171/jns.1997.87.1.0034. [DOI] [PubMed] [Google Scholar]
- 13.Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: A systematic review and meta-analysis. Intensive Care Med. 2011;37:1059–68. doi: 10.1007/s00134-011-2224-2. [DOI] [PubMed] [Google Scholar]
- 14.Dubost C, Le Gouez A, Jouffroy V, Roger-Christoph S, Benhamou D, Mercier FJ, et al. Optic nerve sheath diameter used as ultrasonographic assessment of the incidence of raised intracranial pressure in preeclampsia: A pilot study. Anesthesiology. 2012;116:1066–71. doi: 10.1097/ALN.0b013e318246ea1a. [DOI] [PubMed] [Google Scholar]
- 15.Rajajee V, Vanaman M, Fletcher JJ, Jacobs TL. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 2011;15:506–15. doi: 10.1007/s12028-011-9606-8. [DOI] [PubMed] [Google Scholar]
- 16.Geeraerts T, Newcombe VF, Coles JP, Abate MG, Perkes IE, Hutchinson PJ, et al. Use of T2-weighted magnetic resonance imaging of the optic nerve sheath to detect raised intracranial pressure. Crit Care. 2008;12:R114. doi: 10.1186/cc7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology. 2001;56(12):1746–8. doi: 10.1212/wnl.56.12.1746. [DOI] [PubMed] [Google Scholar]
- 18.Kelly G. Appearances observed in the dissection of two individuals; death from cold and congestion of the brain. Trans Med Chir Sci Edinb. 1824;1:84–169. [PMC free article] [PubMed] [Google Scholar]
- 19.Steiner LA, Andrews PJ. Monitoring the injured brain: ICP and CBF. Br J Anaesth. 2006;97:26–38. doi: 10.1093/bja/ael110. [DOI] [PubMed] [Google Scholar]
- 20.Ghajar J. Traumatic brain injury. Lancet. 2000;356:923–9. doi: 10.1016/S0140-6736(00)02689-1. [DOI] [PubMed] [Google Scholar]
- 21.Ossoinig KC. Standardized echography: Basic principles, clinical applications, and results. Int Ophthalmol Clin. 1979;19:127–210. [PubMed] [Google Scholar]
- 22.Cennamo G, Gangemi M, Stella L. The correlation between endocranial pressure and optic nerve diameter: An ultrasonographic study. Ophthal Echography. 1987;7:603–6. [Google Scholar]
- 23.Gangemi M, Cennamo G, Maiuri F, D'Andrea F. Echographic measurement of the optic nerve in patients with intracranial hypertension. Neurochirurgia (Stuttg) 1987;30:53–5. doi: 10.1055/s-2008-1053656. [DOI] [PubMed] [Google Scholar]
- 24.Tamburelli C, Aricle C, Mangiola A. CSF dynamic parameters and changes of optic nerve diameters measured by standardised echography. Ophthal Echography. 1993;13:101–9. [Google Scholar]
- 25.Hansen HC, Helmke K, Kunze K. Optic nerve sheath enlargement in acute intracranial hypertension. Neuroophthalmology. 1994;14:345–54. [Google Scholar]
- 26.Liu D, Kahn M. Measurement and relationship of subarachnoid pressure of the optic nerve to intracranial pressures in fresh cadavers. Am J Ophthalmol. 1993;116:548–56. doi: 10.1016/s0002-9394(14)73195-2. [DOI] [PubMed] [Google Scholar]
- 27.Dutton JJ. Optic nerve sheath meningiomas. Surv Ophthalmol. 1992;37:167–83. doi: 10.1016/0039-6257(92)90135-g. [DOI] [PubMed] [Google Scholar]
- 28.Beare NA, Kampondeni S, Glover SJ, Molyneux E, Taylor TE, Harding SP, et al. Detection of raised intracranial pressure by ultrasound measurement of optic nerve sheath diameter in African children. Trop Med Int Health. 2008;13:1400–4. doi: 10.1111/j.1365-3156.2008.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tayal VS, Neulander M, Norton HJ, Foster T, Saunders T, Blaivas M. Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med. 2007;49:508–14. doi: 10.1016/j.annemergmed.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 30.Johnson LN, Hepler RS, Bartholomew MJ. Accuracy of papilledema and pseudopapilledema detection: A multispecialty study. J Fam Pract. 1991;33:381–6. [PubMed] [Google Scholar]
- 31.O'Sullivan MG, Statham PF, Jones PA, Miller JD, Dearden NM, Piper IR, et al. Role of intracranial pressure monitoring in severely head-injured patients without signs of intracranial hypertension on initial computerized tomography. J Neurosurg. 1994;80:46–50. doi: 10.3171/jns.1994.80.1.0046. [DOI] [PubMed] [Google Scholar]
- 32.Winkler F, Kastenbauer S, Yousry TA, Maerz U, Pfister HW. Discrepancies between brain CT imaging and severely raised intracranial pressure proven by ventriculostomy in adults with pneumococcal meningitis. J Neurol. 2002;249:1292–7. doi: 10.1007/s00415-002-0844-8. [DOI] [PubMed] [Google Scholar]
- 33.Hiler M, Czosnyka M, Hutchinson P, Balestreri M, Smielewski P, Matta B, et al. Predictive value of initial computerized tomography scan, intracranial pressure, and state of autoregulation in patients with traumatic brain injury. J Neurosurg. 2006;104:731–7. doi: 10.3171/jns.2006.104.5.731. [DOI] [PubMed] [Google Scholar]


