Abstract
Purpose:
The typical toxidrome in organophosphate (OP) poisoning comprises of the Salivation, Lacrimation, Urination, Defecation, Gastric cramps, Emesis (SLUDGE) symptoms. However, several other manifestations are described. We review the spectrum of symptoms and signs in OP poisoning as well as the different approaches to clinical features in these patients.
Materials and Methods:
Articles were obtained by electronic search of PubMed® between 1966 and April 2014 using the search terms organophosphorus compounds or phosphoric acid esters AND poison or poisoning AND manifestations.
Results:
Of the 5026 articles on OP poisoning, 2584 articles pertained to human poisoning; 452 articles focusing on clinical manifestations in human OP poisoning were retrieved for detailed evaluation. In addition to the traditional approach of symptoms and signs of OP poisoning as peripheral (muscarinic, nicotinic) and central nervous system receptor stimulation, symptoms were alternatively approached using a time-based classification. In this, symptom onset was categorized as acute (within 24-h), delayed (24-h to 2-week) or late (beyond 2-week). Although most symptoms occur with minutes or hours following acute exposure, delayed onset symptoms occurring after a period of minimal or mild symptoms, may impact treatment and timing of the discharge following acute exposure. Symptoms and signs were also viewed as an organ specific as cardiovascular, respiratory or neurological manifestations. An organ specific approach enables focused management of individual organ dysfunction that may vary with different OP compounds.
Conclusions:
Different approaches to the symptoms and signs in OP poisoning may better our understanding of the underlying mechanism that in turn may assist with the management of acutely poisoned patients.
Keywords: Intermediate syndrome, manifestations, organophosphate, poisoning
Introduction
Organophosphate (OP) poisoning continues to be a frequent reason for admission to hospitals and Intensive Care Units in developing countries.[1,2,3] The traditional approach to clinical features in acute OP poisoning has centered on receptor specific effects on muscarinic, nicotinic and central nervous system (CNS) receptors that result in diverse symptoms and signs.[4,5] This conventional classification of clinical features is useful given that muscarinic effects are reversed by atropine whilst nicotinic neuromuscular effects are not.[6] It is also known that drugs that cross the blood-brain barrier (e.g. atropine) are more likely to reverse CNS symptoms and signs than drugs that do not cross the blood-brain barrier.[7] An alternate approach to clinical features may be in terms of the time of onset of symptoms. In general, following OP exposure, Salivation, Lacrimation, Urination, Defecation, Gastric cramps, Emesis (SLUDGE) symptoms occur acutely within minutes to hours. However, some patients develop delayed effects either after an initial period of intense cholinergic symptoms and signs or after a period of minimal or no clinical features. Further symptoms and signs may occur as a continuum, wherein patients with acute symptoms involving one neuronal sub-system (e.g. neuromuscular weakness) may progress to develop delayed symptoms and signs of other neuronal sub-systems (e.g. extra-pyramidal). The third approach, an organ specific approach, have focused on neurologic,[8,9] respiratory[10,11] or cardiovascular[12,13,14] effects of OP. This review was thus undertaken to detail different classifications of the clinical features of OP poisoning and discuss mechanisms for the occurrence of these manifestations.
Materials and Methods
We performed a literature search (1966 to April 2014) using PubMed® with the search terms organophosphorus compounds or phosphoric acid esters medical subject heading (MESH) AND poison or poisoning (MESH) AND manifestations or symptoms that included neuromuscular or neurobehavioral or neurologic manifestations or tremor or skin or oral or eye manifestations or chorea or muscle weakness or fasciculation or dystonia or shock or respiratory failure [Table 1]. We also reviewed our personal files and records as well as references from other studies to identify additional articles. The focus was to provide different classifications of all symptoms and signs reported in OP poisoning.
Table 1.
Search strategy used for identifying articles on manifestations in organophosphate poisoning

The clinical features were classified (a) as receptor specific manifestations, (b) based on time of occurrence and (c) nature of organ system involvement. Mechanisms for the occurrence of specific manifestations, as well as the time of symptom onset, were explored from published literature.
Results
Of the 5026 articles on OP poisoning identified by literature search, 2584 articles were in humans; 452 articles pertaining to clinical manifestations of OP poisoning in humans were retrieved for detailed assessment [Table 1]. Articles were categorized based on whether the manifestations were approached as receptor-based or time-based or organ system involved. A descriptive review was undertaken based on the published articles.
Receptor based manifestations were categorized as nicotinic and muscarinic receptor manifestations [Table 2]. Irreversible binding of OP to acetylcholinesterase in the cholinergic synapses in the CNS and peripheral nervous system (PNS) results in high concentrations of acetylcholine in the synaptic clefts that cause initial excessive stimulation and later, blockade of synaptic transmission.[6] The peripheral muscarinic SLUDGE symptoms are due to actions on the relevant glands whilst central muscarinic effects result in symptoms such as confusion, coma and convulsions. Nicotinic effects are motor and sympathetic[5] and result in fasciculations, muscle weakness, tachycardia and hypertension. In a retrospective study of OP poisoning,[15] muscarinic symptoms and signs were the most frequent (84%) followed by CNS (78%) and nicotinic (17%).
Table 2.
Symptoms and signs of organophosphate poisoning based on receptors involved

Using the time-based approach, symptoms are traditionally categorized as acute (minutes to hours) and delayed or late (days to weeks); late and delayed being used interchangeably. Since symptom onset and mechanism of delayed manifestations (e.g. intermediate syndrome, delayed onset coma that typically occur within 2-week) are dissimilar to late manifestations (e.g. organophosphate induced delayed polyneuropathy [OPIDP] that typically occurs after 2-3 weeks), we propose [Table 3] that symptom onset is categorized as acute (within 24-h), delayed (24-h to 2-week) and late (beyond 2-week).
Table 3.
Symptoms and signs of organophosphate poisoning based on time of manifestation

Symptoms and signs were also categorized as organ-specific manifestations as neurologic [Table 4], cardiac [Table 5] and respiratory manifestations and manifestations of other systems.
Table 4.
Neurological manifestations of organophosphate poisoning

Table 5.
Cardiac effects of organophosphate poisoning

Discussion
Receptor specific manifestations
Organophosphate compounds bind irreversibly to acetylcholinesterase in the plasma, red cells and cholinergic synapses [Figure 1] in the CNS and the PNS. Reduced red cell or plasma cholinesterase activity suggests OP exposure. Red cell cholinesterase activity is better correlated with the severity of exposure than plasma cholinesterase activity.[16,17,18]
Figure 1.
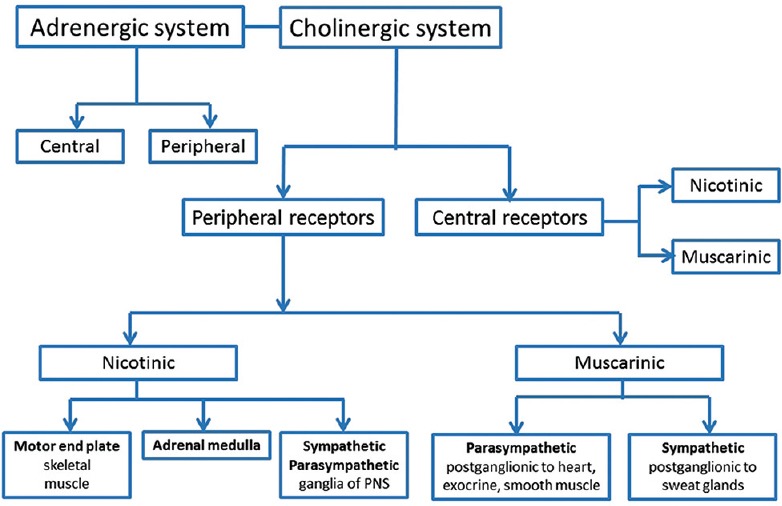
The cholinergic system - cholinergic synapses are present in the central nervous system (CNS) and the peripheral nervous system (PNS). Both nicotinic and muscarinic receptors are found in the CNS. The peripheral nicotinic receptors are present in the neuromuscular junction, adrenal medulla and the sympathetic and parasympathetic ganglia of the PNS. Peripheral parasympathetic muscarinic innervation is postganglionic to the heart, exocrine glands and smooth muscle and sympathetic postganglionic fibres innervate the sweat glands
The central nicotinic receptors are of the neuronal subtype (Nn or N2); this subtype is also present in the adrenal medulla and sympathetic and para-sympathetic ganglia of the PNS.[19,20] The peripheral nicotinic receptors (N1 or Nm) are present in the neuromuscular junction.[19] All 5 (M1 to M5) muscarinic receptor subunits[20,21] are present in the CNS [Figure 2]. Peripheral parasympathetic muscarinic innervation is postganglionic to the heart, exocrine glands and smooth muscle, while sympathetic postganglionic fibers innervate the sweat glands.[20,21,22]
Figure 2.
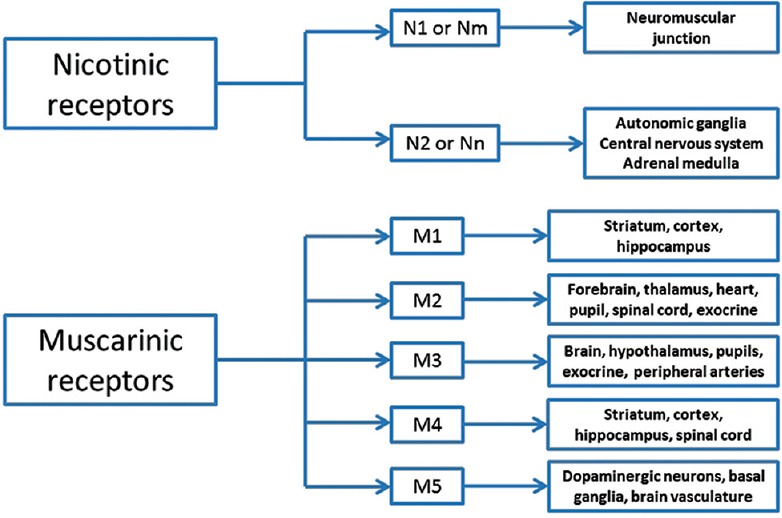
Subtypes of muscarinic and nicotinic receptors - the peripheral nicotinic receptors at the neuromuscular junction are of the N1 or Nm type and the central nicotinic receptors are of the neuronal nicotinic acetylcholinesterase subtype (Nn or N2). All five (M1 to M5) muscarinic receptor subunits are present in the central nervous system. The peripheral muscarinic receptors are predominantly of the M3 subunit although the M2 subunit is also represented in the heart and exocrine glands
Most symptoms and signs in OP poisoning are the result of excessive muscarinic receptor stimulation. Features such as tachycardia and high blood pressure, which are sometimes observed in acute poisoning and not readily explained is postulated to be due to overwhelming cholinergic effects on the CNS, sympathetic ganglionic synapses or the adrenal medulla.[6]
The traditional approach offers insight on the possible site(s) of action of the OP compound in patients with muscle weakness. Wadia et al. reported that in the so-called Type I paralysis, weakness appeared within 24-h and some responded to atropine.[5] In contrast, in Type II paralysis, weakness appeared after 24-h with concomitant atropine being administered in large doses, usually, 30-mg or more.[5] Recent electrophysiological studies have suggested possible reasons for this differential effect. Patients with early respiratory failure had normal repetitive nerve stimulation studies suggesting a predominant central muscarinic mechanism, highlighting the importance of rapid atropinization while patients with late respiratory failure had evidence of neuromuscular dysfunction.[23] Patients with moderate muscle weakness had an initial decrement-increment pattern on electrophysiology at high rates of stimulation progressing to decrement-increment patterns at intermediate-and low-frequency situations. Further progression was characterized by decrement-increment and repetitive fade patterns.[24] These electrophysiological abnormalities may thus help in the continued assessment and treatment (e.g. atropine, oximes) of neuromuscular weakness in poisoned patients.
Overstimulation of central receptors may contribute to early death. In animal models, OP causes excitatory electroencephalographic changes in the respiratory control regions of the brain.[25,26] In addition, focal respiratory center seizures result initially in an increase in phrenic nerve output followed by sudden cessation of activity.[26,27] Pretreatment of animals with centrally acting agents such as atropine or diazepam, dramatically increases 24-h survival of rats administered dichlorvos, while peripherally acting drugs such as ipratropium or glycopyrrolate did not impact outcome.[28] These results further support the hypothesis that early paralysis in OP poisoning may be centrally mediated.
Possible therapeutic implications of a receptor based approach
The choice of anticholinergic depends on the targeted receptor – central, peripheral or both. While atropine is the logical choice, as it acts on central and peripheral cholinergic receptors, adverse effects or allergic reactions may preclude its use.[7] In such situations glycopyrrolate or scopolamine are advocated.[7] Atropine and glycopyrrolate appear to be equally effective.[29] However, as glycopyrrolate does not cross the blood-brain barrier, a benzodiazepine or a specific antimuscarinic drug with good CNS penetration such as scopolamine may be needed to counter central effects.[7] In a case report, rapid reversal of severe extra-pyramidal signs was seen with intravenous scopolamine in chlorpyrifos poisoning.[30] However given the selective action, scopolamine is considered inferior to atropine and caramiphen.[31,32]
Given the irreversible binding of OP to acetylcholinesterase, the choice of muscle relaxant in OP poisoning is also important. Several studies[33,34,35,36] have reported prolonged neuromuscular blockade and apnea in the setting of acute or chronic exposure to OP due to reduced succinylcholine metabolism as a result of cholinesterase inhibition by the insecticide.[33]
In some patients with mega-dose OP intoxication, refractoriness to high dose atropine therapy (100-mg/h) with an inadequate heart rate response may be observed. In such situations, the addition of small doses of an adrenergic agent (e.g. adrenaline 1-2 mcg/min) improves heart rate with a dramatic reduction in atropine requirements (personal observations). The lack of response to atropine may be explained by sympathetic ganglionic dysfunction or blockade with inadequate adrenergic output at the postganglionic neuronal level or by inhibition of the sympathetic fibers of the adrenal gland.
The use of oximes in OP poisoning that has been extensively reviewed in other publications, merit mention for completion. Oximes are nucleophilic agents that cleave covalently bound OP off the OP-acetylcholinesterase conjugate thereby releasing the acetylcholinesterase.[37] Oxime therapy in OP poisoning has been the subject of numerous trials and meta-analysis. Although there is a pharmacological basis of use of oximes in OP poisoning, recent systematic reviews suggest that the current evidence is insufficient to indicate if oximes are beneficial.[38,39]
Symptoms based on time of occurrence
The time of occurrence of symptoms and signs depend on the route of exposure, poison load and chemical nature and solubility characteristics of the compound. Traditionally, symptoms are categorized as acute (minutes to hours) and delayed or late (days to weeks).[40,41,42] The time of onset and mechanism of delayed manifestations such as intermediate syndrome,[43] delayed onset coma[44] and extrapyramidal manifestation[45] are different to that of late manifestations such as organophosphate induced delayed polyneuropathy (OPIDP) that typically occurs after 2-3 weeks[46] and up to 4-week post exposure.[42] Thus, we propose [Table 3] that symptom onset is categorized as acute (within 24-h), delayed (24-h to 2-week) and late (beyond 2-week).
Acute onset symptoms
The acute symptoms and signs are due to muscarinic, nicotinic and central receptor effects. Muscarinic symptoms of salivation and bronchorrhea that dominate initially may cause drowsy patients to drown in their secretions. Acute muscarinic effects on the heart (bradycardia, hypotension) can be life-threatening. Nicotinic effects of muscle weakness contribute to respiratory distress whilst the acute central effects of restlessness, agitation, confusion and sometimes convulsions further compromise airway and breathing and increase aspiration risk and hypoxia. Since many of these effects are reversed by atropine, early and appropriate medical attention is vital. In developing countries, where OP poisoning is common, quick access to medical care is more problematic than early recognition.
Implications of route of exposure on onset of symptoms
The route of exposure determines the rapidity of symptom onset. Common routes of exposure are inhalational, skin and ingestional. The inhalational route has the fastest onset, generally within a few minutes of exposure. In the terrorist attacks in Japan with the nerve gas agent Sarin,[47] instantaneous death by respiratory arrest was suggested in 4 victims.[48] In farmers, inhalation exposure resulting in rapid symptom onset may occur with a sudden change in the wind direction during insecticide spraying.
In skin exposure, the volume of exposure, intactness of the skin and solubility characteristics of the OP determines lag-time. In one report, nausea, abdominal cramping, arm and leg weakness occurred within 30-min of dermal exposure of chlorpyrifos, a lipid soluble OP.[49] Although leg weakness improved, weakness of muscles at the site of skin exposure persisted beyond 2-week. In another report, symptom onset occurred at 3-h following the exposure to water soluble OP, monocrotophos, through a skin laceration.[50] Symptoms of poisoning have also occurred after 4-h and 24-h after application of a home-made shampoo contaminated with an OP.[51] In a rare situation of subcutaneous chlorpyrifos self-injection,[52] delayed cholinergic phase, prolonged coma and severe permanent neurologic injury were observed. Delayed and prolonged effects were attributed to the adipose and muscle tissue acting as reservoirs.[52]
In ingestional poisoning, symptom onset would depend on the poison load and absorption characteristics. In general, symptoms occur within a few minutes to hours. However, the first symptom in parathion poisoning may be delayed by up to 24-h as parathion must first be converted from the thion to the oxon form to be physiologically active. Many organothiophosphates readily undergo conversion from thions to oxons. This conversion occurs due to the substitution of oxygen for sulfur in the environment under the influence of oxygen and light, and in the body chiefly by the action of liver microsomes.[53] Oxons are generally more toxic than thions, but oxons break down more readily.
Delayed onset symptoms
With adequate atropinization,[54] the acute cholinergic symptoms abate within a few hours, but some patients develop delayed effects. Several recent publications [Figure 3] strengthen the case for its recognition as a distinct clinical entity.
Figure 3.
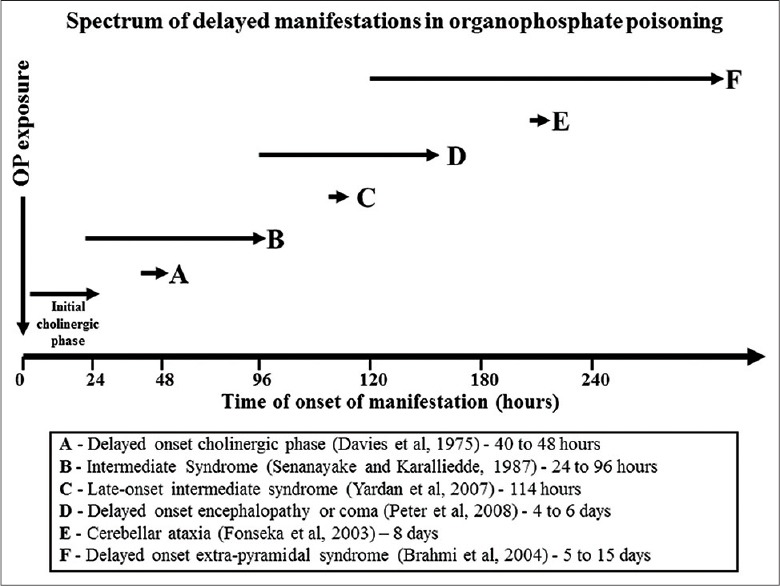
Spectrum of delayed manifestations in organophosphate poisoning - delayed onset cholinergic symptoms are reported to occur 40-48 h following poisoning (a). Intermediate syndrome (b) typically occurs 24-96 h following poisoning although it may be delayed up to 114-h (c). Delayed onset coma or encephalopathy (d) occurs about 4-day after poisoning, generally after a period of normal conscious state. Cerebellar ataxia (e) has been reported to occur 8-day after poisoning and extrapyramidal manifestations (f) after 5-15 days (reproduced with permission)
Although acute cholinergic manifestations typically occur within 24-h of exposure, late onset cholinergic symptoms and signs have been observed 40-48 h after dichlofenthion poisoning.[55]
Intermediate syndrome, the best described delayed manifestation, is characterized by paralysis of proximal limb muscles, neck flexors, motor cranial nerves and respiratory muscles 24-96 h after poisoning, after the cholinergic phase had settled down, with weakness lasting for up to 18-day.[56] A neuromuscular junctional defect has been demonstrated in electromyography studies.[57] Delayed onset intermediate syndrome has been reported 114-h after methamidophos poisoning.[58] Since methamidophos is highly lipophilic and persists in fat stores, re-distribution and re-inhibition of cholinesterase may have delayed symptom onset.[58]
Although intermediate syndrome involves muscle groups, focal weakness has also been reported; in particular, laryngeal paralysis,[59,60,61,62] either acute[61] or delayed by 4-14 days[59,60] presenting as “failed extubation.” Laryngeal electromyography was consistent with bilateral laryngeal paralysis although standard needle electromyography was normal.[60] Severe and prolonged diaphragmatic paralysis has also been reported with Malathion poisoning.[63]
Coma is seen in 17-29% of patients and can last for hours to days.[16,64] OP poisoning may also present as brainstem stroke.[65] However, some patients manifest altered consciousness or coma days after poisoning, particular after a period of “normal” consciousness. This clinical entity termed delayed organophosphate encephalopathy (DOPE) or “CNS intermediate” is probably akin to type II paralysis. Coma with absent brainstem reflexes or encephalopathy has been reported after 4-day of normal consciousness and spontaneously resolved after another 4-day.[44,66] The clinical distinguishing feature between “brain death” and this “mimic” was “small miosed pupils” in patients with DOPE. The delay in coma onset was attributed to the slow release and re-distribution of the lipid soluble OP compounds with saturation of the CNS receptors over time rather than immediately. Since OP compounds cause irreversible binding, if the rate of regeneration of acetylcholinesterase receptors was slower than that of inhibition, then symptoms could persist or worsen over time. This hypothesis is supported by the persistently low pseudocholinesterase levels and increasing atropine requirements during coma.[44] The electroencephalogram in patients with late-onset coma showed features consistent with encephalopathy. Mitochondrial dysfunction, reported with chronic exposure to dichlorvos[67] may also play a role in delayed coma. Delayed onset extrapyramidal signs are not uncommon. In the earliest report[68] six patients manifested dystonia, rest tremor, cog-wheel rigidity and choreo-athetosis, 4-40 days after poisoning and disappeared spontaneously in 1-4 weeks. More recently,[45] similar features were described in 4 patients between 5 and 15-day, with complete recovery. Cerebellar ataxia has also been described as a delayed presentation.[69]
Late onset symptoms
The classical late onset neuropathy in OP poisoning, OPIDP is characterized by distal weakness that occurs 2-4 weeks after OP exposure. In a retrospective patient cohort, OPIDP developed in 34.2% between the 14th and 22nd-day following poisoning and was characterized by cramping pain and paresthesias of the extremities followed by weakness of the distal limb muscles, especially in the legs.[70] The molecular target for OPIDP is considered to be the neuropathy target esterase which is inhibited by OPs.[46,71] Electrophysiological changes include reduced amplitude of the compound muscle potential, increased distal latencies and normal or slightly reduced nerve conduction velocities.[71] Nerve biopsy may show features of axonal degeneration with secondary demyelination.[71] Recovery is, usually, complete, particularly in the young. However, mild weakness with increase in vibration threshold may persist for 2-year following acute poisoning.[72] Other late onset features reported include cerebellar ataxia, developing about 5-week after acute exposure to an OP[73] and extrapyramidal symptoms at 40-day.[68]
Organ specific manifestations
An organ specific approach enables focused attention and support of specific organ dysfunction. Given that OP compounds are neurotoxic insecticides, the dominant organ involved in acute and chronic exposure is the nervous system. The spectrum of neurological manifestations is summarized in Table 4.
Neurological manifestations
Three types of paralysis are described. Type I paralysis, characterized by weakness, fasciculations, cramps and twitching, occurs acutely with the cholinergic symptoms. Type II paralysis, seen in 80-49%,[74,75,76] occurs more insidiously 24-96 h following poisoning[56] and has a predilection to proximal, neck and respiratory muscles and cranial nerves with recovery in 1-2 weeks. Type III paralysis characterized by distal weakness occurs 2-3 weeks after poisoning with recovery in weeks to months.[70] Weakness of specific muscle groups at sites of dermal exposure,[49] cranial nerve palsies,[77] supra nuclear gaze palsy,[78] isolated laryngeal paralysis[59,60,61,62] and diaphragmatic paralysis[63] are all reported.
Restlessness, delirium, agitation, convulsions or coma may occur with acute exposure while neuropsychiatric symptoms and signs [Table 4] termed chronic organophosphate induced neuropsychiatric disorder may occur with chronic exposure.[79] Extrapyramidal manifestations,[45,68] ocular signs,[78,80,81,82,83] ototoxicity,[84] presentation as a Guillain-Barre syndrome[85] and sphincter involvement[86] are also described [Table 4].
Cardiovascular manifestations
Cardiac manifestations are observed in about two-thirds of patients with OP poisoning [Table 5].[13,14] Common electrocardiographic findings are QTc prolongation, ST-T segment changes and T wave abnormalities.[13,14,87,88,89,90] Other cardiac manifestations include sinus bradycardia or tachycardia, hypotension or hypertension, supraventricular and ventricular arrhythmias and ventricular premature complexes and noncardiogenic pulmonary edema [Table 5].[91]
Death due to cardiac causes in OP poisoning occurs either due to arrhythmias[13] or severe and refractory hypotension.[92] Although shock is primarily vasodilatory,[92,93,94] circumferential endocardial ischemia with cardiogenic shock and leading to death has also been reported with Malathion poisoning.[95] Necropsy of patients who died following OP poisoning has revealed cardiac discoloration or blotchiness, patchy pericarditis, auricular thrombus and right ventricular hypertrophy and dilatation.[12] Myocardial interstitial edema, vascular congestion, patchy interstitial inflammation, mural thrombus and patchy myocarditis were the histological findings.[12] OP poisoning presenting as cardiac arrest[96] and late onset, prolonged asystole 12-day following poisoning[97] have been described.
Respiratory symptoms
Respiratory symptoms are common in OP poisoning. Muscarinic effects of salivation, rhinorrhea, bronchorrhea and bronchospasm contributed to hypoxemia and increased work of breathing. Nicotinic effects result in muscle weakness and paralysis and predispose to hypercapnic respiratory failure. Central effects of agitation, restlessness and seizures further compromise respiratory function.
In large cohorts, respiratory failure is reported to occur in 24-66% of patients.[3,10,98,99] Severity of poisoning was the primary determinant of respiratory failure.[99] Other factors contributing to respiratory failure include pneumonia,[98,99] cardiovascular collapse,[99] acute pulmonary edema[100] and acute respiratory distress syndrome.[101]
The mechanism of respiratory failure has been explored in experimental models. As described earlier, OP compounds cause excitatory changes in the respiratory control regions with an initial increase in phrenic nerve output and subsequent sudden cessation of activity.[25,26,27] More recently, in a rodent model, exposure to dichlorvos caused a rapid lethal central apnea[102] that was potentiated by hypoxia[103] and protected by vagally mediated feedback signals.[104] In animals sustained with mechanical ventilation, following central apnea, there was progressive pulmonary insufficiency.[102] Brief central apnea and complete acetylcholinesterase inhibition of the brainstem has also been reported with crotylsarin, another OP compound.[105] In other studies, paraoxon failed to produce apnea in a rat model, although postinjection and throughout the study, there was a significant decrease in the respiratory frequency and a significant increase in the expiratory time without modifications in the inspiratory time.[106]
Other features
Gastrointestinal symptoms [Table 1] occur early in OP poisoning and are rapidly reversed with atropine therapy. There are concerns that atropine slows down intestinal transit time and prolongs OP toxicity. In one series, persistence of the OP in the gut was demonstrated 10-day after poisoning.[107] Atropine therapy may also preclude early enteral feeding in OP poisoned patients. However, in a pilot study, early administration (by 48-h) of hypocaloric feeds was associated with gastric stasis in only 6.9% of patients receiving enteral feeds.[108]
Pancreatitis is not uncommon in OP poisoning[109,110,111,112] and reported in 12.8%.[112] Metabolic complications such as hyperglycemia and glycosuria[6,113] and OP intoxication presenting as diabetic ketoacidosis[114] are also described.
Conclusions
Three facets of approach to the symptoms and signs in OP poisoning have been presented. Although all OP compounds are generally considered within a single group entity, it is recognized that di-methyl and diethyl OP poisoning have different outcomes.[3] Each individual compound also has unique characteristics and outcomes.[115] Other differences such as lipid solubility, biochemical characteristics (oxon-thion), WHO class[116] and nature of solvent used further make each OP compound unique. These need to be kept in mind when approaching a patient with OP poisoning.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Senarathna L, Jayamanna SF, Kelly PJ, Buckley NA, Dibley MJ, Dawson AH. Changing epidemiologic patterns of deliberate self poisoning in a rural district of Sri Lanka. BMC Public Health. 2012;12:593. doi: 10.1186/1471-2458-12-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balme KH, Roberts JC, Glasstone M, Curling L, Rother HA, London L, et al. Pesticide poisonings at a tertiary children's hospital in South Africa: An increasing problem. Clin Toxicol (Phila) 2010;48:928–34. doi: 10.3109/15563650.2010.534482. [DOI] [PubMed] [Google Scholar]
- 3.Peter JV, Jerobin J, Nair A, Bennett A, Samuel P, Chrispal A, et al. Clinical profile and outcome of patients hospitalized with dimethyl and diethyl organophosphate poisoning. Clin Toxicol (Phila) 2010;48:916–23. doi: 10.3109/15563650.2010.528425. [DOI] [PubMed] [Google Scholar]
- 4.Peter JV, Cherian AM. Organic insecticides. Anaesth Intensive Care. 2000;28:11–21. doi: 10.1177/0310057X0002800102. [DOI] [PubMed] [Google Scholar]
- 5.Wadia RS, Sadagopan C, Amin RB, Sardesai HV. Neurological manifestations of organophosphorous insecticide poisoning. J Neurol Neurosurg Psychiatry. 1974;37:841–7. doi: 10.1136/jnnp.37.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Namba T. Cholinesterase inhibition by organophosphorus compounds and its clinical effects. Bull World Health Organ. 1971;44:289–307. [PMC free article] [PubMed] [Google Scholar]
- 7.Robenshtok E, Luria S, Tashma Z, Hourvitz A. Adverse reaction to atropine and the treatment of organophosphate intoxication. Isr Med Assoc J. 2002;4:535–9. [PubMed] [Google Scholar]
- 8.Singh G, Khurana D. Neurology of acute organophosphate poisoning. Neurol India. 2009;57:119–25. doi: 10.4103/0028-3886.51277. [DOI] [PubMed] [Google Scholar]
- 9.Singh S, Sharma N. Neurological syndromes following organophosphate poisoning. Neurol India. 2000;48:308–13. [PubMed] [Google Scholar]
- 10.Eddleston M, Mohamed F, Davies JO, Eyer P, Worek F, Sheriff MH, et al. Respiratory failure in acute organophosphorus pesticide self-poisoning. QJM. 2006;99:513–22. doi: 10.1093/qjmed/hcl065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noshad H, Ansarin K, Ardalan MR, Ghaffari AR, Safa J, Nezami N. Respiratory failure in organophosphate insecticide poisoning. Saudi Med J. 2007;28:405–7. [PubMed] [Google Scholar]
- 12.Anand S, Singh S, Nahar Saikia U, Bhalla A, Paul Sharma Y, Singh D. Cardiac abnormalities in acute organophosphate poisoning. Clin Toxicol (Phila) 2009;47:230–5. doi: 10.1080/15563650902724813. [DOI] [PubMed] [Google Scholar]
- 13.Karki P, Ansari JA, Bhandary S, Koirala S. Cardiac and electrocardiographical manifestations of acute organophosphate poisoning. Singapore Med J. 2004;45:385–9. [PubMed] [Google Scholar]
- 14.Saadeh AM, Farsakh NA, al-Ali MK. Cardiac manifestations of acute carbamate and organophosphate poisoning. Heart. 1997;77:461–4. doi: 10.1136/hrt.77.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee P, Tai DY. Clinical features of patients with acute organophosphate poisoning requiring intensive care. Intensive Care Med. 2001;27:694–9. doi: 10.1007/s001340100895. [DOI] [PubMed] [Google Scholar]
- 16.Brahmi N, Mokline A, Kouraichi N, Ghorbel H, Blel Y, Thabet H, et al. Prognostic value of human erythrocyte acetyl cholinesterase in acute organophosphate poisoning. Am J Emerg Med. 2006;24:822–7. doi: 10.1016/j.ajem.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Bobba R, Venkataraman BV, Pais P, Joseph T. Correlation between the severity of symptoms in organophosphorus poisoning and cholinesterase activity (RBC and plasma) in humans. Indian J Physiol Pharmacol. 1996;40:249–52. [PubMed] [Google Scholar]
- 18.Eddleston M, Eyer P, Worek F, Sheriff MH, Buckley NA. Predicting outcome using butyrylcholinesterase activity in organophosphorus pesticide self-poisoning. QJM. 2008;101:467–74. doi: 10.1093/qjmed/hcn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atri A, Chang MS, Strichartz GR. Cholinergic pharmacology. In: Golan DE, Tashjian AH, Armstrong EJ, Armstrong AW, editors. Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. 3rd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2011. pp. 110–31. [Google Scholar]
- 20.Kalamida D, Poulas K, Avramopoulou V, Fostieri E, Lagoumintzis G, Lazaridis K, et al. Muscle and neuronal nicotinic acetylcholine receptors. Structure, function and pathogenicity. FEBS J. 2007;274:3799–845. doi: 10.1111/j.1742-4658.2007.05935.x. [DOI] [PubMed] [Google Scholar]
- 21.Sellin AK, Shad M, Tamminga C. Muscarinic agonists for the treatment of cognition in schizophrenia. CNS Spectr. 2008;13:985–96. doi: 10.1017/s1092852900014048. [DOI] [PubMed] [Google Scholar]
- 22.Abrams P, Andersson KE, Buccafusco JJ, Chapple C, de Groat WC, Fryer AD, et al. Muscarinic receptors: Their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol. 2006;148:565–78. doi: 10.1038/sj.bjp.0706780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayawardane P, Senanayake N, Buckley NA, Dawson AH. Electrophysiological correlates of respiratory failure in acute organophosphate poisoning: Evidence for differential roles of muscarinic and nicotinic stimulation. Clin Toxicol (Phila) 2012;50:250–3. doi: 10.3109/15563650.2012.670875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayawardane P, Senanayake N, Dawson A. Electrophysiological correlates of intermediate syndrome following acute organophosphate poisoning. Clin Toxicol (Phila) 2009;47:193–205. doi: 10.1080/15563650902832608. [DOI] [PubMed] [Google Scholar]
- 25.McDonough JH, Jr, Clark TR, Slone TW, Jr, Zoeffel D, Brown K, Kim S, et al. Neural lesions in the rat and their relationship to EEG delta activity following seizures induced by the nerve agent soman. Neurotoxicology. 1998;19:381–91. [PubMed] [Google Scholar]
- 26.Rickett DL, Glenn JF, Beers ET. Central respiratory effects versus neuromuscular actions of nerve agents. Neurotoxicology. 1986;7:225–36. [PubMed] [Google Scholar]
- 27.Chang FC, Foster RE, Beers ET, Rickett DL, Filbert MG. Neurophysiological concomitants of soman-induced respiratory depression in awake, behaving guinea pigs. Toxicol Appl Pharmacol. 1990;102:233–50. doi: 10.1016/0041-008x(90)90023-n. [DOI] [PubMed] [Google Scholar]
- 28.Dickson EW, Bird SB, Gaspari RJ, Boyer EW, Ferris CF. Diazepam inhibits organophosphate-induced central respiratory depression. Acad Emerg Med. 2003;10:1303–6. doi: 10.1111/j.1553-2712.2003.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 29.Bardin PG, Van Eeden SF. Organophosphate poisoning: Grading the severity and comparing treatment between atropine and glycopyrrolate. Crit Care Med. 1990;18:956–60. [PubMed] [Google Scholar]
- 30.Kventsel I, Berkovitch M, Reiss A, Bulkowstein M, Kozer E. Scopolamine treatment for severe extra-pyramidal signs following organophosphate (chlorpyrifos) ingestion. Clin Toxicol (Phila) 2005;43:877–9. doi: 10.1080/15563650500357636. [DOI] [PubMed] [Google Scholar]
- 31.Weissman BA, Raveh L. Therapy against organophosphate poisoning: The importance of anticholinergic drugs with antiglutamatergic properties. Toxicol Appl Pharmacol. 2008;232:351–8. doi: 10.1016/j.taap.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Weissman BA, Raveh L. Multifunctional drugs as novel antidotes for organophosphates' poisoning. Toxicology. 2011;290:149–55. doi: 10.1016/j.tox.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Sener EB, Ustun E, Kocamanoglu S, Tur A. Prolonged apnea following succinylcholine administration in undiagnosed acute organophosphate poisoning. Acta Anaesthesiol Scand. 2002;46:1046–8. doi: 10.1034/j.1399-6576.2002.460821.x. [DOI] [PubMed] [Google Scholar]
- 34.Pérez Guillermo F, Martinez Pretel CM, Tarín Royo F, Peña Macias MJ, Alvarez Ossorio R, Alvarez Gómez JA, et al. Prolonged suxamethonium-induced neuromuscular blockade associated with organophosphate poisoning. Br J Anaesth. 1988;61:233–6. doi: 10.1093/bja/61.2.233. [DOI] [PubMed] [Google Scholar]
- 35.Jaksa RJ, Palahniuk RJ. Attempted organophosphate suicide: A unique cause of prolonged paralysis during electroconvulsive therapy. Anesth Analg. 1995;80:832–3. doi: 10.1097/00000539-199504000-00032. [DOI] [PubMed] [Google Scholar]
- 36.Weeks DB, Ford D. Prolonged suxamethonium-induced neuromuscular block associated with organophosphate poisoning. Br J Anaesth. 1989;62:237. doi: 10.1093/bja/62.2.237-a. [DOI] [PubMed] [Google Scholar]
- 37.Peter JV, Moran JL, Graham PL. Advances in the management of organophosphate poisoning. Expert Opin Pharmacother. 2007;8:1451–64. doi: 10.1517/14656566.8.10.1451. [DOI] [PubMed] [Google Scholar]
- 38.Buckley NA, Eddleston M, Li Y, Bevan M, Robertson J. Oximes for acute organophosphate pesticide poisoning. Cochrane Database Syst Rev. 2011:CD005085. doi: 10.1002/14651858.CD005085.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Eddleston M, Buckley NA, Eyer P, Dawson AH. Management of acute organophosphorus pesticide poisoning. Lancet. 2008;371:597–607. doi: 10.1016/S0140-6736(07)61202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marrs TC. Organophosphate poisoning. Pharmacol Ther. 1993;58:51–66. doi: 10.1016/0163-7258(93)90066-m. [DOI] [PubMed] [Google Scholar]
- 41.Rusyniak DE, Nañagas KA. Organophosphate poisoning. Semin Neurol. 2004;24:197–204. doi: 10.1055/s-2004-830907. [DOI] [PubMed] [Google Scholar]
- 42.Faiz MS, Mughal S, Memon AQ. Acute and late complications of organophosphate poisoning. J Coll Physicians Surg Pak. 2011;21:288–90. [PubMed] [Google Scholar]
- 43.Karalliedde L, Baker D, Marrs TC. Organophosphate-induced intermediate syndrome: Aetiology and relationships with myopathy. Toxicol Rev. 2006;25:1–14. doi: 10.2165/00139709-200625010-00001. [DOI] [PubMed] [Google Scholar]
- 44.Peter JV, Prabhakar AT, Pichamuthu K. Delayed-onset encephalopathy and coma in acute organophosphate poisoning in humans. Neurotoxicology. 2008;29:335–42. doi: 10.1016/j.neuro.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Brahmi N, Gueye PN, Thabet H, Kouraichi N, Ben Salah N, Amamou M. Extrapyramidal syndrome as a delayed and reversible complication of acute dichlorvos organophosphate poisoning. Vet Hum Toxicol. 2004;46:187–9. [PubMed] [Google Scholar]
- 46.Jokanovic M, Stukalov PV, Kosanovic M. Organophosphate induced delayed polyneuropathy. Curr Drug Targets CNS Neurol Disord. 2002;1:593–602. doi: 10.2174/1568007023338879. [DOI] [PubMed] [Google Scholar]
- 47.Okudera H. Clinical features on nerve gas terrorism in Matsumoto. J Clin Neurosci. 2002;9:17–21. doi: 10.1054/jocn.2001.1020. [DOI] [PubMed] [Google Scholar]
- 48.Yanagisawa N, Morita H, Nakajima T. Sarin experiences in Japan: Acute toxicity and long-term effects. J Neurol Sci. 2006;249:76–85. doi: 10.1016/j.jns.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 49.Meggs WJ. Permanent paralysis at sites of dermal exposure to chlorpyrifos. J Toxicol Clin Toxicol. 2003;41:883–6. doi: 10.1081/clt-120025357. [DOI] [PubMed] [Google Scholar]
- 50.Peiris JB, Fernando R, De Abrew K. Respiratory failure from severe organophosphate toxicity due to absorption through the skin. Forensic Sci Int. 1988;36:251–3. doi: 10.1016/0379-0738(88)90151-x. [DOI] [PubMed] [Google Scholar]
- 51.Sadaka Y, Broides A, Tzion RL, Lifshitz M. Organophosphate acetylcholine esterase inhibitor poisoning from a home-made shampoo. J Emerg Trauma Shock. 2011;4:433–4. doi: 10.4103/0974-2700.83893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soummer A, Megarbane B, Boroli F, Arbelot C, Saleh M, Moesch C, et al. Severe and prolonged neurologic toxicity following subcutaneous chlorpyrifos self-administration: A case report. Clin Toxicol (Phila) 2011;49:124–7. doi: 10.3109/15563650.2011.552066. [DOI] [PubMed] [Google Scholar]
- 53.Sams C, Mason HJ, Rawbone R. Evidence for the activation of organophosphate pesticides by cytochromes P450 3A4 and 2D6 in human liver microsomes. Toxicol Lett. 2000;116:217–21. doi: 10.1016/s0378-4274(00)00221-6. [DOI] [PubMed] [Google Scholar]
- 54.Eddleston M, Buckley NA, Checketts H, Senarathna L, Mohamed F, Sheriff MH, et al. Speed of initial atropinisation in significant organophosphorus pesticide poisoning - A systematic comparison of recommended regimens. J Toxicol Clin Toxicol. 2004;42:865–75. doi: 10.1081/clt-200035223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davies JE, Barquet A, Freed VH, Haque R, Morgade C, Sonneborn RE, et al. Human pesticide poisonings by a fat-soluble organophosphate insecticide. Arch Environ Health. 1975;30:608–13. doi: 10.1080/00039896.1975.10666790. [DOI] [PubMed] [Google Scholar]
- 56.Senanayake N, Karalliedde L. Neurotoxic effects of organophosphorus insecticides. An intermediate syndrome. N Engl J Med. 1987;316:761–3. doi: 10.1056/NEJM198703263161301. [DOI] [PubMed] [Google Scholar]
- 57.Jayawardane P, Dawson AH, Weerasinghe V, Karalliedde L, Buckley NA, Senanayake N. The spectrum of intermediate syndrome following acute organophosphate poisoning: A prospective cohort study from Sri Lanka. PLoS Med. 2008;5:e147. doi: 10.1371/journal.pmed.0050147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yardan T, Baydin A, Aygun D, Karatas AD, Deniz T, Doganay Z. Late-onset intermediate syndrome due to organophosphate poisoning. Clin Toxicol (Phila) 2007;45:733–4. doi: 10.1080/15563650701502733. [DOI] [PubMed] [Google Scholar]
- 59.Indudharan R, Win MN, Noor AR. Laryngeal paralysis in organophosphorous poisoning. J Laryngol Otol. 1998;112:81–2. doi: 10.1017/s0022215100139969. [DOI] [PubMed] [Google Scholar]
- 60.Jin YH, Jeong TO, Lee JB. Isolated bilateral vocal cord paralysis with intermediate syndrome after organophosphate poisoning. Clin Toxicol (Phila) 2008;46:482–4. doi: 10.1080/15563650701704842. [DOI] [PubMed] [Google Scholar]
- 61.Thompson JW, Stocks RM. Brief bilateral vocal cord paralysis after insecticide poisoning. A new variant of toxicity syndrome. Arch Otolaryngol Head Neck Surg. 1997;123:93–6. doi: 10.1001/archotol.1997.01900010103016. [DOI] [PubMed] [Google Scholar]
- 62.Vaidya SR, Salvi MM, Karnik ND, Sunder U, Yeolekar ME. Life threatening stridor due to bilateral recurrent laryngeal nerve palsy as an isolated manifestation of intermediate syndrome. J Assoc Physicians India. 2002;50:454–5. [PubMed] [Google Scholar]
- 63.Rivett K, Potgieter PD. Diaphragmatic paralysis after organophosphate poisoning. A case report. S Afr Med J. 1987;72:881–2. [PubMed] [Google Scholar]
- 64.Tsai JR, Sheu CC, Cheng MH, Hung JY, Wang CS, Chong IW, et al. Organophosphate poisoning: 10 years of experience in southern Taiwan. Kaohsiung J Med Sci. 2007;23:112–9. doi: 10.1016/S1607-551X(09)70385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hollis GJ. Organophosphate poisoning versus brainstem stroke. Med J Aust. 1999;170:596–7. doi: 10.5694/j.1326-5377.1999.tb127909.x. [DOI] [PubMed] [Google Scholar]
- 66.Peter JV, Prabhakar AT, Pichamuthu K. In-laws, insecticide - and a mimic of brain death. Lancet. 2008;371:622. doi: 10.1016/S0140-6736(08)60273-1. [DOI] [PubMed] [Google Scholar]
- 67.Kaur P, Radotra B, Minz RW, Gill KD. Impaired mitochondrial energy metabolism and neuronal apoptotic cell death after chronic dichlorvos (OP) exposure in rat brain. Neurotoxicology. 2007;28:1208–19. doi: 10.1016/j.neuro.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 68.Senanayake N, Sanmuganathan PS. Extrapyramidal manifestations complicating organophosphorus insecticide poisoning. Hum Exp Toxicol. 1995;14:600–4. doi: 10.1177/096032719501400708. [DOI] [PubMed] [Google Scholar]
- 69.Fonseka MM, Medagoda K, Tillakaratna Y, Gunatilake SB, de Silva HJ. Self-limiting cerebellar ataxia following organophosphate poisoning. Hum Exp Toxicol. 2003;22:107–9. doi: 10.1191/0960327103ht341cr. [DOI] [PubMed] [Google Scholar]
- 70.Aygun D, Onar MK, Altintop BL. The clinical and electrophysiological features of a delayed polyneuropathy developing subsequently after acute organophosphate poisoning and it's correlation with the serum acetylcholinesterase. Electromyogr Clin Neurophysiol. 2003;43:421–7. [PubMed] [Google Scholar]
- 71.Lotti M, Moretto A. Organophosphate-induced delayed polyneuropathy. Toxicol Rev. 2005;24:37–49. doi: 10.2165/00139709-200524010-00003. [DOI] [PubMed] [Google Scholar]
- 72.Miranda J, McConnell R, Wesseling C, Cuadra R, Delgado E, Torres E, et al. Muscular strength and vibration thresholds during two years after acute poisoning with organophosphate insecticides. Occup Environ Med. 2004;61:e4. [PMC free article] [PubMed] [Google Scholar]
- 73.Michotte A, Van Dijck I, Maes V, D'Haenen H. Ataxia as the only delayed neurotoxic manifestation of organophosphate insecticide poisoning. Eur Neurol. 1989;29:23–6. doi: 10.1159/000116371. [DOI] [PubMed] [Google Scholar]
- 74.Samuel J, Thomas K, Jeyaseelan L, Peter JV, Cherian AM. Incidence of intermediate syndrome in organophosphorous poisoning. J Assoc Physicians India. 1995;43:321–3. [PubMed] [Google Scholar]
- 75.De Bleecker J, Van den Neucker K, Colardyn F. Intermediate syndrome in organophosphorus poisoning: A prospective study. Crit Care Med. 1993;21:1706–11. doi: 10.1097/00003246-199311000-00020. [DOI] [PubMed] [Google Scholar]
- 76.He F, Xu H, Qin F, Xu L, Huang J, He X. Intermediate myasthenia syndrome following acute organophosphates poisoning - An analysis of 21 cases. Hum Exp Toxicol. 1998;17:40–5. doi: 10.1177/096032719801700107. [DOI] [PubMed] [Google Scholar]
- 77.Narendra J, Chethankumar JG, Rao BB. Cranial nerve palsies in organophosphorus poisoning. J Assoc Physicians India. 1989;37:732–3. [PubMed] [Google Scholar]
- 78.Liang TW, Balcer LJ, Solomon D, Messé SR, Galetta SL. Supranuclear gaze palsy and opsoclonus after Diazinon poisoning. J Neurol Neurosurg Psychiatry. 2003;74:677–9. doi: 10.1136/jnnp.74.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jokanovic M, Kosanovic M. Neurotoxic effects in patients poisoned with organophosphorus pesticides. Environ Toxicol Pharmacol. 2010;29:195–201. doi: 10.1016/j.etap.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 80.Ishikawa S, Miyata M, Aoki S, Hanai Y. Chronic intoxication of organophosphorus pesticide and its treatment. Folia Med Cracov. 1993;34:139–51. [PubMed] [Google Scholar]
- 81.De Bleecker JL. Transient opsoclonus in organophosphate poisoning. Acta Neurol Scand. 1992;86:529–31. doi: 10.1111/j.1600-0404.1992.tb05138.x. [DOI] [PubMed] [Google Scholar]
- 82.Tripathi AK, Misra UK. Ophthalmoplegia in dimethoate poisoning. J Assoc Physicians India. 1996;44:225. [PubMed] [Google Scholar]
- 83.Wang AG, Liu RS, Liu JH, Teng MM, Yen MY. Positron emission tomography scan in cortical visual loss in patients with organophosphate intoxication. Ophthalmology. 1999;106:1287–91. doi: 10.1016/S0161-6420(99)00710-1. [DOI] [PubMed] [Google Scholar]
- 84.Damasceno A, França MC, Jr, Nucci A. Chronic acquired sensory neuron diseases. Eur J Neurol. 2008;15:1400–5. doi: 10.1111/j.1468-1331.2008.02332.x. [DOI] [PubMed] [Google Scholar]
- 85.Fisher JR. Guillain-Barré syndrome following organophosphate poisoning. JAMA. 1977;238:1950–1. [PubMed] [Google Scholar]
- 86.Patial RK, Bansal SK, Sehgal VK, Chander B. Sphincteric involvement in organophosphorus poisoning. J Assoc Physicians India. 1991;39:492–3. [PubMed] [Google Scholar]
- 87.Ludomirsky A, Klein HO, Sarelli P, Becker B, Hoffman S, Taitelman U, et al. Q-T prolongation and polymorphous ("torsade de pointes") ventricular arrhythmias associated with organophosphorus insecticide poisoning. Am J Cardiol. 1982;49:1654–8. doi: 10.1016/0002-9149(82)90242-9. [DOI] [PubMed] [Google Scholar]
- 88.Vijayakumar S, Fareedullah M, Ashok Kumar E, Mohan Rao K. A prospective study on electrocardiographic findings of patients with organophosphorus poisoning. Cardiovasc Toxicol. 2011;11:113–7. doi: 10.1007/s12012-011-9104-4. [DOI] [PubMed] [Google Scholar]
- 89.Taira K, Aoyama Y, Kawamata M. Long QT and ST-T change associated with organophosphate exposure by aerial spray. Environ Toxicol Pharmacol. 2006;22:40–5. doi: 10.1016/j.etap.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 90.Yurumez Y, Yavuz Y, Saglam H, Durukan P, Ozkan S, Akdur O, et al. Electrocardiographic findings of acute organophosphate poisoning. J Emerg Med. 2009;36:39–42. doi: 10.1016/j.jemermed.2007.08.063. [DOI] [PubMed] [Google Scholar]
- 91.Kiss Z, Fazekas T. Arrhythmias in organophosphate poisonings. Acta Cardiol. 1979;34:323–30. [PubMed] [Google Scholar]
- 92.Davies J, Roberts D, Eyer P, Buckley N, Eddleston M. Hypotension in severe dimethoate self-poisoning. Clin Toxicol (Phila) 2008;46:880–4. doi: 10.1080/15563650802172063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Buckley NA, Dawson AH, Whyte IM. Organophosphate poisoning: Peripheral vascular resistance - A measure of adequate atropinization. J Toxicol Clin Toxicol. 1994;32:61–8. doi: 10.3109/15563659409000431. [DOI] [PubMed] [Google Scholar]
- 94.Asari Y, Kamijyo Y, Soma K. Changes in the hemodynamic state of patients with acute lethal organophosphate poisoning. Vet Hum Toxicol. 2004;46:5–9. [PubMed] [Google Scholar]
- 95.Mdaghri YA, Mossadeq A, Faroudy M, Sbihi A. Cardiac complications associated with organophosphate poisoning. Ann Cardiol Angeiol (Paris) 2010;59:114–7. doi: 10.1016/j.ancard.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 96.Teague B, Peter JV, O'Fathartaigh M, Peisach AR. An unusual cause for cardiac arrest. Crit Care Resusc. 1999;1:362–5. [PubMed] [Google Scholar]
- 97.Chacko J, Elangovan A. Late onset, prolonged asystole following organophosphate poisoning: A case report. J Med Toxicol. 2010;6:311–4. doi: 10.1007/s13181-010-0095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang CY, Wu CL, Tsan YT, Hsu JY, Hung DZ, Wang CH. Early onset pneumonia in patients with cholinesterase inhibitor poisoning. Respirology. 2010;15:961–8. doi: 10.1111/j.1440-1843.2010.01806.x. [DOI] [PubMed] [Google Scholar]
- 99.Tsao TC, Juang YC, Lan RS, Shieh WB, Lee CH. Respiratory failure of acute organophosphate and carbamate poisoning. Chest. 1990;98:631–6. doi: 10.1378/chest.98.3.631. [DOI] [PubMed] [Google Scholar]
- 100.Bledsoe FH, Seymour EQ. Acute pulmonary edema associated with parathion poisoning. Radiology. 1972;103:53–6. doi: 10.1148/103.1.53. [DOI] [PubMed] [Google Scholar]
- 101.Kass R, Kochar G, Lippman M. Adult respiratory distress syndrome from organophosphate poisoning. Am J Emerg Med. 1991;9:32–3. doi: 10.1016/0735-6757(91)90009-9. [DOI] [PubMed] [Google Scholar]
- 102.Gaspari RJ, Paydarfar D. Pathophysiology of respiratory failure following acute dichlorvos poisoning in a rodent model. Neurotoxicology. 2007;28:664–71. doi: 10.1016/j.neuro.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gaspari RJ, Paydarfar D. Respiratory recovery following organophosphate poisoning in a rat model is suppressed by isolated hypoxia at the point of apnea. Toxicology. 2012;302:242–7. doi: 10.1016/j.tox.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gaspari RJ, Paydarfar D. Respiratory failure induced by acute organophosphate poisoning in rats: Effects of vagotomy. Neurotoxicology. 2009;30:298–304. doi: 10.1016/j.neuro.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 105.Klein-Rodewald T, Seeger T, Dutschmann M, Worek F, Mörschel M. Central respiratory effects on motor nerve activities after organophosphate exposure in a working heart brainstem preparation of the rat. Toxicol Lett. 2011;206:94–9. doi: 10.1016/j.toxlet.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 106.Villa AF, Houze P, Monier C, Risède P, Sarhan H, Borron SW, et al. Toxic doses of paraoxon alter the respiratory pattern without causing respiratory failure in rats. Toxicology. 2007;232:37–49. doi: 10.1016/j.tox.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 107.Martinez-Chuecos J, del Carmen Jurado M, Paz Gimenez M, Martinez D, Menendez M. Experience with hemoperfusion for organophosphate poisoning. Crit Care Med. 1992;20:1538–43. doi: 10.1097/00003246-199211000-00010. [DOI] [PubMed] [Google Scholar]
- 108.Moses V, Mahendri NV, John G, Peter JV, Ganesh A. Early hypocaloric enteral nutritional supplementation in acute organophosphate poisoning - A prospective randomized trial. Clin Toxicol (Phila) 2009;47:419–24. doi: 10.1080/15563650902936664. [DOI] [PubMed] [Google Scholar]
- 109.Dressel TD, Goodale RL, Jr, Arneson MA, Borner JW. Pancreatitis as a complication of anticholinesterase insecticide intoxication. Ann Surg. 1979;189:199–204. doi: 10.1097/00000658-197902000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hamaguchi M, Namera A, Tsuda N, Uejima T, Maruyama K, Kanai T, et al. Severe acute pancreatitis caused by organophosphate poisoning. Chudoku Kenkyu. 2006;19:395–9. [PubMed] [Google Scholar]
- 111.Hsiao CT, Yang CC, Deng JF, Bullard MJ, Liaw SJ. Acute pancreatitis following organophosphate intoxication. J Toxicol Clin Toxicol. 1996;34:343–7. doi: 10.3109/15563659609013800. [DOI] [PubMed] [Google Scholar]
- 112.Sahin I, Onbasi K, Sahin H, Karakaya C, Ustun Y, Noyan T. The prevalence of pancreatitis in organophosphate poisonings. Hum Exp Toxicol. 2002;21:175–7. doi: 10.1191/0960327102ht234cr. [DOI] [PubMed] [Google Scholar]
- 113.Saadeh AM. Metabolic complications of organophosphate and carbamate poisoning. Trop Doct. 2001;31:149–52. doi: 10.1177/004947550103100311. [DOI] [PubMed] [Google Scholar]
- 114.Akyildiz BN, Kondolot M, Kurtoglu S, Akin L. Organophosphate intoxication presenting as diabetic keto-acidosis. Ann Trop Paediatr. 2009;29:155–8. doi: 10.1179/146532809X440789. [DOI] [PubMed] [Google Scholar]
- 115.Eddleston M, Eyer P, Worek F, Mohamed F, Senarathna L, von Meyer L, et al. Differences between organophosphorus insecticides in human self-poisoning: A prospective cohort study. Lancet. 2005;366:1452–9. doi: 10.1016/S0140-6736(05)67598-8. [DOI] [PubMed] [Google Scholar]
- 116.Peter JV, Jerobin J, Nair A, Bennett A. Is there a relationship between the WHO hazard classification of organophosphate pesticide and outcomes in suicidal human poisoning with commercial organophosphate formulations? Regul Toxicol Pharmacol. 2010;57:99–102. doi: 10.1016/j.yrtph.2010.01.004. [DOI] [PubMed] [Google Scholar]


