Abstract
Background:
Growing antimicrobial resistance and limited therapeutic options to treat carbapenem-resistant bacteremia prompted us to evaluate the clinical outcomes associated with healthcare-associated bacteremia.
Methods:
This was a retrospective observational study of carbapenem-resistant Gram-negative bacteremia performed at a tertiary care facility in Chennai, India between May 2011 and May 2012.
Results:
In our study, patients had mean 11.76 days of intensive care unit (ICU) care and mean time to onset of bacteremia was 6.4 days after admission. The commonest organism was Klebsiella pneumoniae (44%). Patients with combination treatment had lower mortality (44.8%) compared with colistin monotherapy (66.6%); (P = 0.35).
Conclusion:
Carbapenem resistant bacteremia is a late onset infection in patients with antibiotic exposure in the ICU and carries a 30 days mortality of 60%; K. pneumoniae is the most common organism at our center. Two drug combinations appear to carry a lower mortality compared with monotherapy.
Keywords: Bacteremia in Indian intensive care unit, carbapenem resistant Gram-negative bacteremia, carbapenem resistant Klebsiella pneumoniae, multidrug-resistant bacteremia
Introduction
Carbapenem resistant Gram-negative bacteria (GNB) such as Enterobacteriaceae (CRE) (including Escherichia coli [CREC] and Klebsiella pneumoniae [CRKP]), Pseudomonas aeruginosa (CRPA) and Acinetobacter baumanni (CRAB) are important emerging causes of nosocomial blood stream infections and carry significant mortality. CRE has been recently classified as an “urgent threat” by the US Centers for Disease Control and Prevention (CDC), while the Antimicrobial Availability Task Force formed by the Infectious Diseases Society of America had identified CRPA and CRAB of particular importance,[1,2] because of growing antimicrobial resistance among these isolates and shrinking therapeutic options.[3] Indian single center studies overall report a prevalence of carbapenem resistance in up to 12-15% of Enterobacteriaceae and 40-60% in A. baumanii and P. aeruginosa.[4,5] However, there is little data from India about the clinical profile and treatment outcome of infections caused by these organisms.
Methods
This was a retrospective study performed at a 600 bed tertiary care facility in Chennai, India between May 2011 and May 2012. We identified all blood culture results that had yielded a growth of carbapenem-resistant GNB during the study period. We then reviewed the medical records of these patients.
Only the first bacteremic episode for each patient was included in the analysis. Blood cultures were performed using BacT/ALERT (Biomerieux) culture system, and species' identification was carried out with VITEK-GNI card (Biomerieux). Antibiotic susceptibility was performed using disc diffusion interpretive criteria as per revised Clinical and Laboratory Standards Institute performance standards.[6] Gram-negative bacteremia was defined as the isolation of Gram-negative bacilli in blood culture specimen in at least two bottles of two different culture sets with clinical features compatible with systemic inflammatory response syndrome.
Medical records were reviewed for demographic and clinical data. Clinical parameters analyzed included severity of illness as calculated by Acute Physiology and Chronic Health Evaluation (APACHE) II score on the day of bacteremia onset, presumed source of infection and presence of arterial and venous central catheters or hemodialysis catheters. The source of bacteremia was defined on the basis clinical and microbiologic evaluation using infections criteria proposed by CDC.[7] Presence of risk factors such diabetes, recent surgery, organ transplant and immunosuppression were recorded. Prior antibiotic usage, dose of colistin and requirement for renal replacement therapy (RRT) after treatment with colistin commenced were also noted. Survival at 30 days after bacteremia onset was recorded.
Results
The records of 50 patients with bacteremia caused by CREC, CRKP, CRAB, CRPA were identified and analyzed. The mean age of patients was 52.3 years and 32 (64%) were male. The most common co-morbid condition was diabetes mellitus (30%), followed by chronic kidney disease (22%). Five patients (10%) were postliver transplant, while three had other surgeries. The total mean duration of hospitalization was 24.68 days with a mean of 11.76 days of intensive care unit (ICU) care. The mean time to onset of bacteremia was 6.4 days after admission. Of the 50 patients, 96% had already received antibiotics, including beta-lactam/beta-lactamase inhibitors (32%) and carbapenems (40%) [Table 1].
Table 1.
Clinical data
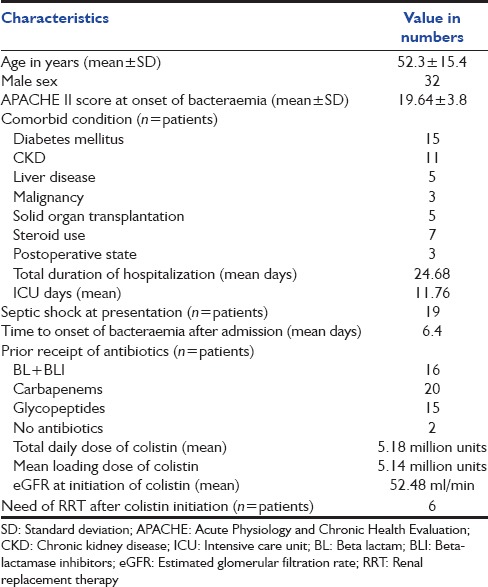
The mean (± standard deviation [SD]) APACHE II score at the onset of bacteremia was 19.64 ± 3.8. Thirty-eight percent (n = 19) patients had septic shock complicating bacteremia. Fourteen episodes (28%) of bacteremia were attributed to ventilator-associated pneumonia (VAP), 12 episodes (24%) to the central line related bloodstream infections (CLABSI), 10 episodes (20%) were due to intra-abdominal infections (IAI) and 9 episodes (18%) to urinary tract infections. The most common causative organism in our study was CRKP (44%) followed by CREC (26%), while CRAB and CRPA were isolated in 20% and 10% patients respectively [Table 2].
Table 2.
Observed outcome of patients

All patients received a loading dose of colistin with a mean of 5.14 million units intravenously followed by a mean 5.18 million units total daily dose. Mean creatinine clearance (calculated estimated glomerular filtration rate [eGFR]) at the initiation of colistin was 52.48 ml/min. Six patients (12%) required RRT after initiation of colistin for worsening renal functions; all recovered to normal renal function.
The overall survival at 30 days was 40% in our study with lowest survival rates for CLABSI (33.3%), with survival rates for VAP, IAI and urosepsis patients of 35.7%, 40% and 44.4% respectively. Overall 30 days survival for CRAB and CRKP bacteraemia were lowest (30% and 31.8% respectively), while survival rates for CRPA and CREC bacteraemia were 40% and 61.5% respectively.
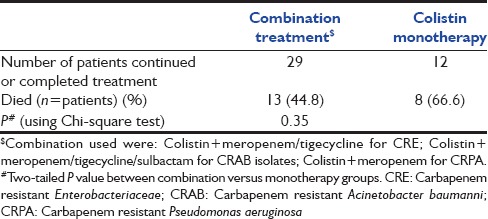
We also found that patients treated with combination therapy had lower mortality (44.8%) compared with colistin monotherapy (66.6%) although this was statistically not significant (P = 0.35) [Table 3].
Table 3.
Combination Vs Monotherapy: Treatment outcome
Discussion
Carbapenem resistance in Gram-negative organisms is increasingly encountered in healthcare-associated infections in India. Bacteremic episodes due to these organisms carry a high mortality as shown by previous studies from other countries.[8]
In our study, the most common carbapenem resistant Gram-negative isolate was CRKP (44%) followed by CREC (26%) with more than half of all episodes due to pneumonia or CLABSI, similar to two previous Indian studies presented as abstracts in scientific meetings.[9,10] Our patients had a high APACHE II score (19.64 ± 3.8 SD) and had a mean ICU stay of 11.76 days, with a mean time of onset of bacteremia after admission of 6.4 days. These results are in concordance with previous studies implicating these organisms as causes of healthcare-associated infections.[9]
Seriously ill-patients in ICUs for prolonged periods often require central lines, dialysis catheters, broad spectrum antibiotics, and mechanical ventilation, which make them susceptible to resistant hospital-acquired infections. Notably only 40% of our patients had received carbapenems, but virtually all had antibiotic exposure, suggesting that healthcare exposure and overall prior antibiotic exposure may be more important risk factors for developing carbapenem resistant infections rather than prior receipt of carbapenems.
Colistin has emerged as the cornerstone of therapy for carbapenem-resistant GNB, but the correct dose and frequency of dosing is a subject of on-going study. The mean colistin loading dose in our study was only 5.14 million units followed by mean total daily dose of 5.18 million units intravenously, even with a mean eGFR of 52.48 ml/min at colistin initiation. Use of low colistin doses may be because of a perceived concern about nephrotoxicity; our study period was also prior to evolution of new colistin dosing recommendations. In our study, six patients (12%) required new onset RRT due to worsening renal functions after colistin initiation but this was fully reversible with dosing adjustments, which was reassuring and similar to other studies.
The overall 30 days survival rate in our cohort was only 40%, but it was difficult to assess whether this was due to sepsis itself or due to underlying severe illness and multiple co-morbidities. Mortality in our patients was highest when CLABSI or VAP was the source of bacteremia and when CRAB and CRKP were the causative organisms. Better survival rates from the west have been reported, but similar results to ours have been observed in two previous Indian abstract presentations. Higher colistin doses are associated with higher microbiologic clearance and reduced mortality at 7 days although 28 days mortality did not differ. It is also possible that relatively low dose our patients received may have contributed to mortality. We also observed that patients treated with two drug combinations had a trend towards a higher survival rate than those treated with colistin monotherapy, as observed in other studies also. Small study numbers in the two groups and the fact that more severely ill patients may receive combination treatment, may have resulted in the absence of a statistically significant difference.
We acknowledge significant limitations in our study: Its retrospective nature, relatively small sample size and absence of a control group for comparison.
Conclusion
We have shown that carbapenem-resistant Gram-negative bacteremia is a serious healthcare-associated infection in critically ill-patients in Indian ICUs with co-morbidities and prior antibiotic exposure; K. pneumoniae is the most common organism at our center. Thirty days survival rate is only 40% and even lower for CLABSI or VAP or when due to A. baumanii or K. pneumoniae. Colistin is the cornerstone of therapy and may need to be studied at higher doses to optimize the outcome; nephrotoxicity requiring RRT was seen in 12% of patients only and was reversible in all. Combinations of other antibiotics with colistin may result in a lower mortality compared with monothrapy, however this needs needs to be explored through a randomized controlled study
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Untreatable: Report by CDC details today's drug-resistant health threats. [Last accessed on 2014 Mar 01]. Available from: http://www.cdc.gov/media/releases/2013/p0916-untreatable.html .
- 2.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 3.Deshpande P, Rodrigues C, Shetty A, Kapadia F, Hedge A, Soman R. New Delhi Metallo-beta lactamase (NDM-1) in Enterobacteriaceae: Treatment options with carbapenems compromised. J Assoc Physicians India. 2010;58:147–9. [PubMed] [Google Scholar]
- 4.Nair PK, Vaz MS. Prevalence of carbapenem resistant Enterobacteriaceae from a tertiary care hospital in Mumbai, India. J Microbiol Infect Dis. 2013;3:207–10. [Google Scholar]
- 5.Gupta E, Mohanty S, Sood S, Dhawan B, Das BK, Kapil A. Emerging resistance to carbapenems in a tertiary care hospital in North India. Indian J Med Res. 2006;124:95–8. [PubMed] [Google Scholar]
- 6.Performance standards for antimicrobial susceptibility testing; Twenty-first informational supplement; M100-S21. Wayne, PA: Clinical and Laboratory Standards Institute; 2011. Clinical and Laboratory Standards Institute. [Google Scholar]
- 7.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–40. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 8.Shorr AF. Review of studies of the impact on Gram-negative bacterial resistance on outcomes in the intensive care unit. Crit Care Med. 2009;37:1463–9. doi: 10.1097/CCM.0b013e31819ced02. [DOI] [PubMed] [Google Scholar]
- 9.Parthasarathy P. Risk factors and mortality in carbapenem resistant gram negative bacteremias: A retrospective analysis from a tertiary health care setting in India. Poster Abstract 1608, ID Week. 2013 [Google Scholar]
- 10.Ghafur A, Kannaian P, Tayade A. Clinical study of carbapenum-sensitive and carbapenem-resistant bacteraemia in neutropenic and non-neutropenic patients: The first series from India. Abstract P 1368, 23rd ECCMID. 2013 doi: 10.4103/0019-509X.175362. [DOI] [PubMed] [Google Scholar]


