Abstract
Neisseria gonorrhoeae has evolved a complex and novel network of oxidative stress responses, including defense mechanisms that are dependent on manganese (Mn). We performed systematic analyses at the transcriptomic and proteomic (1D SDS-PAGE and Isotope-Coded Affinity Tag [ICAT]) levels to investigate the global expression changes that take place in a high Mn environment, which results in a Mn-dependent oxidative stress resistance phenotype. These studies revealed that 97 proteins are regulated at the post-transcriptional level under conditions of increased Mn concentration, including proteins involved in virulence (eg. Pilin, a key adhesin), oxidative stress defence (eg. superoxide dismutase), cellular metabolism, protein synthesis, RNA processing and cell division. Mn regulation of inorganic pyrophosphatase (Ppa) indicated the potential involvement of phosphate metabolism in the Mn-dependent oxidative stress defense. A detailed analysis of the role of Ppa and polyphosphate kinase (Ppk) in the gonococcal oxidative stress response revealed that ppk and ppa mutant strains showed increased resistance to oxidative stress. Investigation of these mutants grown with high Mn suggests that phosphate and pyrophosphate are involved in Mn-dependent oxidative stress resistance.
Keywords: manganese, microarray, Neisseria gonorrhoeae, oxidative stress, pili, proteomics
1. Introduction
Neisseria gonorrhoeae, the causative agent of the sexually transmitted infection, gonorrhea, is a facultative aerobe and a host-adapted pathogen that is frequently associated with inflamed urogenital tissues and activated polymorphonuclear leukocytes (PMNs) [1, 2]. Hence, N. gonorrhoeae is routinely exposed to substantial amounts of superoxide anion (O2.−), hydrogen peroxide (H2O2) and other reactive oxygen species (ROS), as well as reactive nitrogen species (RNS) [reviewed in 3, 4]. Oxidative stress, resulting from the action of ROS and RNS, causes damage to DNA, proteins and lipids [5-7]. The observation that N. gonorrhoeae can be isolated from PMN-laden purulent exudates, and can survive in PMNs [8] indicates that this bacterium has highly efficient defence systems to respond to oxidative stress, as previously reviewed [9].
Previous studies have shown that accumulation of manganese (Mn), via the ATP binding cassette (ABC)-type Mn transporter MntABC, protects N. gonorrhoeae from O2.− and H2O2 killing by a mechanism that is independent of superoxide dismutase (SOD) [10] and catalase [11], respectively. The increased resistance seen to oxidative challenge was Mn-specific; no increased resistance was seen when N. gonorrhoeae was grown with media supplemented with Co(II), Mg(II) or Zn(II) [10]. MntABC expression in N. gonorrhoeae is regulated by PerR, a transcriptional repressor from the Fur family [12]. Both mntC and perR mutants have reduced intracellular survival in a human cervical epithelial cell model [12]. Streptococcus pneumoniae has a similar Mn transport system, PsaBCA, which also plays a role in resistance to O2.− and H2O2, as well as in systemic virulence [13, 14].
Mn is now recognised as a key ion in the regulation of metabolism and stress responses and can play a variety of roles in cellular processes in many bacteria. As a consequence, this ion has a maior effect on virulence in several bacterial pathogens [reviewed in 15, 16]. Mn concentrations vary up to 1000 fold between different sites in the human body [16-18], providing a potential signal for N. gonorrhoeae to adapt to microenvironments within the host. Indeed, Mn regulates multiple genes in S. pneumoniae via the regulator PsaR, with Mn concentrations signalling expression of virulence factors within different host sites [19, 20]. Mn availability also affects the expression of Streptococcus mutans virulence genes differentially during planktonic or biofilm culture [21]. To investigate the precise nature of the oxidative stress resistant phenotype observed in N. gonorrhoeae grown with a Mn(II) supplement [10], we have used DNA microarray analysis and a shotgun proteomic approach that involved one dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (1D SDS-PAGE) coupled with one dimensional liquid chromatography – tandem mass spectrometry (1D LC -MS/MS) as well as isotope coded affinity tag (ICAT) studies coupled with MS/MS. The results of these studies provide new insights into the effect of Mn on the proteome of N. gonorrhoeae and the role of this ion in the oxidative stress response.
2. Experimental Procedures
2.1 Bacterial strains and culture conditions
N. gonorrhoeae strain 1291 was supplied by Dr. Michael Apicella (University of Iowa, USA). Bacteria were grown on brain heart infusion (BHI) agar or broth (Accumedia) supplemented with 10% (v/v) levinthal's base [22] and 1% (v/v) isovitalex (Becton Dickinson) at 37 °C in 5% CO2. N. gonorrhoeae was grown on BHI agar from freezer stocks for about 22 hr and approximately ten colonies were passed in supplemented BHI broth. After 18 hr, cell density was measured and diluted to optical density at 600 nm (OD600) ~0.5. Then, 500 l of this culture was inoculated into 5 ml of fresh BHI broth ±40 M manganese sulfate (MnSO4) and grown in a flask on a shaking incubator for approximately 5 hr to mid-log phase (OD600 ~ 0.5).
E.coli DH5α was cultured at 37 °C in Luria-Bertani (LB) broth or on LB-1.5% bacteriological agar (Difco) plates [23]. Ampicillin (Amp) and kanamycin (Kan) were used at a final concentration of 100 μg/ml and 50 μg/ml, respectively.
2.2 Subcellular fractionation
N. gonorrhoeae was grown as described above to mid-log phase. Cultures were harvested by centrifugati on at 6,000 rpm for 10 min at 4 °C. The cell pellet was washed with phosphate buffered saline (PBS) twice and heat killed at 56 °C for at least 30 min. Cells were sonicated for 30 sec at setting 4 in a microson ultrasonic cell disrupter (Misonix Incorporated, New York, USA) followed by cooling on ice for 30 sec; this was repeated three times. Cells were centrifuged at 5,000 rpm for 10 min to remove debris. The supernatant represented the whole-cell extract. A portion of the whole-cell extract was subjected to further sonication and centrifuged at 100,000 g for 60 min; the supernatant contained the cytoplasmic proteins. The pellet was resuspended in 10 mM Tris pH 8.0, 1% sarcosyl (N-lauroylsarcosine sodium salt) and sonicated as described above. This extract was centrifuged at 100,000 g for 60 min; the supernatant contained the cytoplasmic membrane proteins. The pellet containing the outer membrane was resuspended in 100 μl H20.
2.3 SDS-PAGE
The protein concentration was measured using the Bradford method [24]. An appropriate amount of total cell protein was loaded onto a SDS-PAGE gel with a 5% (v/v) acrylamide stacking gel and a 10, 12.5 or 18% (v/v) resolving gel according to Laemmli [25]. Low Molecular Weight Protein Standard (Amersham) or Protein Molecular Weight Marker (Fermentas) was used for protein standards. After electrophoresis, the gel was fixed by 10% (v/v) methanol and 7% (v/v) glacial acetic acid for 30 min and then stained by SYPRO® Ruby Protein Gel stain (Sigma) in the dark overnight. The gel was then destained by 10% (v/v) methanol and 7% (v/v) glacial acetic acid for 30 min and washed with distilled water for 10 min.
2.4 N-terminal protein sequence analysis
For the analysis of N-terminal amino acid sequences, proteins were subjected to SDS-PAGE using the Tris-Tricine system described by Schagger and von Jagow [26]. After electrophoresis, proteins were blotted onto Polyscreen polyvinylidene difluoride (PVDF) Transfer Membrane (NEN Life Science Products, Boston, USA) using CAPS buffer (10 mM 3-[cylcohexylamino]-1-propanesulfonic acid, pH 11) by the method of Towbin et al. [27]. The PVDF membrane was soaked in MilliQ water for 10 min with shaking and stained with 0.1% (w/v) Coomassie Blue R250, 50% (v/v) methanol, 10% (v/v) acetic acid for 5 min. The membrane was destained by 50% (v/v) methanol, 10% (v/v) acetic acid and rinsed in MilliQ water. The desired protein band was cut out and the N-terminal sequence of the protein was determined using a PE Biosystems 492cLC protein sequencer. “NGOxxxx” Gene ID refers to the annotation of the N. gonorrhoeae FA1090 genome strain (http://cmr.jcvi.org/tigr-scripts/CMR/GenomePage.cgi?org=ntng03).
2.5 Immunoblotting
Overnight cultures of N. gonorrhoeae were harvested from agar plates into an appropriate volume of PBS and were heat killed at 56 °C for 1 hr. Then, cell suspensions were boiled for 5 min and their protein concentrations were measured by absorbance at 280 nm. 500 μg of total cell protein was loaded onto an SDS-PAGE gel with a 5% (v/v) acrylamide stacking gel and a 12% (v/v) resolving gel [25]. The protein standard used was BenchMark™ Prestained Protein Ladder (Invitrogen). Usually, two gels were run simultaneously, one for Coomassie staining and the other for immunoblotting. Following electrophoresis, polyacrylamide gels were transferred onto nitrocellulose membrane (Bio-Rad) for 1 hr at 15 V according to Towbin et al. [27]. The immunological detection was performed by blocking in 5% (w/v) skim milk, followed by incubation with primary antibody (1/100 dilution of pilin specific SM1 [28]) for 1 hr, then secondary antibody (1/7,500 dilution of anti-mouse alkaline phosphatase conjugate; Sigma) for 1 hr and then detection of bound antibody as previously described [29].
2.6 In-gel tryptic digestion
The protein bands on 1D gels were manually excised from the gel and cut into pieces. The gel pieces were reduced with 50 mM 1,4-dithioerythreitol (DTE) (Fluka) in 25 mM ammonium bicarbonate, pH 8.5, at 37 °C for 1 hr, and subsequently alkylated with 100 mM iodoacetamide (IAA) (Fluka) in 25 mM ammonium bicarbonate, pH 8.5, at room temperature for 1 hr. The pieces were then washed twice with 50% acetonitrile (ACN) in 25 mM ammonium bicarbonate, pH 8.5 for 15 min each, dehydrated with 100% ACN for 5 min, dried and then rehydrated with a total of 22.5 ng of sequencing grade modified trypsin (Promega, Madison, WI, USA) in 25 mM ammonium bicarbonate, pH 8.5, at 37 °C for 16 hr. Following digestion, tryptic peptides were extracted twice with 50% ACN containing 5% formic acid for 15 min each with moderate sonication. The extracted solutions were pooled and evaporated to dryness under a vacuum, then the samples were analysed by the nanoLC-MS/MS system (Micromass/Waters).
2.7 ICAT
Triplicate cultures (biological replicates) of N. gonorrhoeae strain 1291 wild type were grown to exponential phase (OD600 ~ 0.5) in the presence and absence of 40 M MnSO4 (as described above). 500 g of total protein extracts were quantified by Cleavable ICAT Reagent Kit for Protein Labelling (Applied Biosystem, Foster City, CA, USA). This was performed according to the manufacturer's instructions. The sample was analysed by the nanoLC-MS/MS system and the data was analysed by the Micromass ProteinLynx™ Global Server (PGS) 2.0 data processing software (Micromass/Waters).
2.8 1D LC-nanoESI-MS/MS analysis for protein identification
Direct 1D LC-nano electrospray ionization (ESI)-MS/MS analyses were performed on an integrated nanoLC-MS/MS system (Mircomass) comprising a 3-pumping Micromass/Waters CapLC™ system with an autosampler, a stream select module configured for precolumn plus analytical capillary column, and a Micromass Q-Tof Ultima™ API mass spectrometer fitted with nano-LC sprayer, operated under MassLynx™ 4.0 control. Injected samples were first trapped and desalted isocratically on a C18 pre-column (5μm, 150μm ID. × 15 mm; produced in Facilities for Proteomics Research, Academia Sinica, Taiwan) for 2 min with 0.1% formic acid delivered by the auxiliary pump at 15 l/min after which the peptides were eluted from the precolumn and separated on an analytical C18 capillary column (5μm, 75 μm ID. × 25 mm) connected inline to the mass spectrometer, at 300 nl/min using a 50 and 240 min gradient of 5% to 80% ACN in 0.1% formic acid for in-gel digestion and ICAT samples, respectively.
The online nanoESI-MS survey scan and data dependent acquisition of collision-induced dissociation (CID) MS/MS were fully automated and synchronized with the nanoLC runs under the full software control of MassLynx™ 4.0. Prior to online analysis, the nanoLC sprayer and Z-spray source parameters were tuned and optimized with a 50 fmol/ l solution of glufibrinopeptide B in 50% ACN/0.1% formic acid, directly infused at 300 nl/min. Argon maintained at ~ 4.0×10−5 mbar was used as the collision gas for CID MS/MS. Calibration was performed using the product ions generated from fragmentation of the doubly charged molecular ion of glufibrinopeptide B at m/z 785.8. For routine protein identification analysis, the 1 sec survey scans were acquired over the mass range m/z 400 –2000 and a maximum of 3 concurrent MS/MS acquisitions would be triggered for 2+, 3+ and 4+ charged precursors detected at an intensity above the predefined threshold (20 counts/s). MS/MS acquisition is completed and switched back to survey scan when each of the precursor intensity falls below a predefined threshold (3 counts/s) or after a maximum of 6 sec acquisition.
After data acquisition, the individual MS/MS spectra acquired for each of the precursors within a single LC run were combined, smoothed, deisotoped (fast option, inclusive of simple transformation of multiply charged peaks) and centroided using the Micromass ProteinLynx™ Global Server (PGS) 2.0 data processing software and output as a single Mascot-searchable peak list (.pkl) file.
2.9 Protein identification and quantification
The peak list files from 1D LC-nanoESI-MS/MS were used to query the National Center for Biotechnology Information (NCBI) database using the Mascot 2.0 program (Matrix Science Ltd) with the following parameters: peptide mass tolerance, 50 ppm; MS/MS ion mass tolerance, 0.25 Da; allow up to one missed cleavage; variable modifications considered were methionine oxidation and cysteine carboxyamidomethylation. Only significant hits as defined by Mascot probability analysis were considered initially. In addition, a minimum total score of 20 comprising at least a peptide match of ion score more than 20 was arbitrarily set as the threshold for acceptance. In the case of data from 1D SDS-PAGE where the same protein may be found in more than one band, the total score for a particular protein hit comprises the best scoring peptide matches from all bands where the same protein hit was found.
2.10 Microarray analysis
Triplicate cultures (biological replicates) of N. gonorrhoeae strain 1291 wild type were grown to exponential phase (OD600 ~ 0.5) in the presence and absence of 40 M MnSO4 (as described above). Approximately 100 g of total RNA was prepared from each sample using the RNeasy Maxi Kit according to the manufacturer's instructions (Qiagen). The triplicate samples were pooled and the integrity and concentration of RNA was determined via micro-fluidic analysis on a bio-analyser (Agilent Technologies).
All microarray analysis was performed on N. gonorrhoeae/meningitidis genome arrays (The J. Craig Venter Institute (JCVI), formerly TIGR; http://pfgrc.jcvi.org/index.php/microarray/available_microarrays.html). Each microarray consists of 6,389 70mer oligonucleotides representing open reading frames (ORFs) from N. gonorrhoeae strains FA1090 and ATCC 700825 (reference strain), and N. meningitidis strains Z2491 (serogroup A) and MC58 (serogroup B). Methods and analysis were performed as previously described [30]. 5 g of each total RNA sample was labelled using random hexamers and direct incorporation of fluorescent Cy3- or Cy5-labelled nucleotides. The hybridisations were performed in triplicate and incorporated a dye-swap to account for dye bias. After 16 hr of hybridisation, the arrays were washed and scanned on an Agilent G2565BA microarray scanner at a 5 micron resolution. The resulting images of the hybridisations were analysed using Imagene 5.5 (BioDiscovery Inc.) and the mean foreground, mean background and spot/signal quality were determined.
All primary data was imported into an in-house installation of the comprehensive microarray relational database, BASE (http://kidney.scgap.org/base) (login: Wu 2006, password: Manganese; see experiment Wu 2006, N. gonorrhoeae vs manganese). After print-tip intensity independent Lowess normalisation, differential expression was defined using a robust statistical method rather than simple fold change. All genes were ranked using the B statistic method where both fold change and variance of signals in replicates is used to determine the likelihood that genes are truly differentially expressed. A threshold in the B statistic of 0.0 was adopted as genes with a B score >0 have a >50% probability of being truly differentially expressed [31]. The ranked B-scores for all genes in each experiment are also maintained in BASE.
2.11 Quantitative real-time PCR
Total RNA was isolated using the RNeasy kit (Qiagen) as described above. The equivalent of 1 g of the total RNA preparation was treated with RQ1 RNase-free DNase (Promega). RNA was reverse transcribed using random primers and the TaqMan® RT-PCR kit (PE Applied Biosystems) as recommended by the manufacturer. Primers were designed using Primer Express 1.0 software (ABI Prism; PE Biosystems). All quantitative Real-Time PCR (qRT-PCR) reactions were performed in triplicate in a 25 l mixture containing cDNA (5 l of 1/5 dilution), 1X SYBR Green buffer (PE Applied Biosystems) and approximately 2 M of each primer (see Error! Reference source not found. for primer sequences). 16S rRNA was used as the standard control in each quantitative PCR. Amplification and detection of specific products were performed with the ABI Prism 7700 sequence detection system (PE Applied Biosystems) with the following cycle profile: 95 °C for 10 min, followed by 45 cycles of 95 °C for 15 sec and 60 °C for 1 min. Data was analysed with ABI Prism 7700 v1.7 analysis software. Relative gene expression between the N. gonorrhoeae wild type strain grown in the presence and absence of Mn was determined using the 2ΔΔCT relative quantification method. P values for statistical significance were determined using Students t-tests.
2.12 Cloning and mutagenesis of the ppk and ppa genes of N. gonorrhoeae
The “NGO” gene designations used below were obtained from the annotated N. gonorrhoeae strain FA1090 genome (http://cmr.jcvi.org/tigr-scripts/CMR/GenomePage.cgi?org=ntng03). Knockout constructs of the ppk (NGO0003) and ppa (NGO0223) genes were constructed via the insertion of a kanamycin-resistance cassette (pUC4Kan: Amersham Biosciences) into suitable, unique, restriction sites within the coding region of each gene, as described by Tseng et al. [10]. The ppk gene was amplified from N. gonorrhoeae strain 1291 chromosomal DNA using the primers, Ppk-for and Ppk-rev (see Error! Reference source not found, for the primer sequences) and cloned into pGEM®-T Easy (Promega). The central 801 bp portion of the ppk gene was then deleted by digestion with StuI and XcmI and replaced with a HincII DNA fragment containing the kanamycin-resistance cassette from plasmid pUC4kan. The ppa gene, plus the region 400 bp up- and downstream of this gene, was amplified using the primers, ppa-KO-F and ppa-KO-R, and cloned into pGEM®-T Easy. The kanamycin-resistance cassette was cloned into the PflMI restriction endonuclease site located within ppa. Mutant strains were generated by digesting pGEMppk::kan with NotI and pGEMppa::kan with AlUI followed by transforming these linearised fragments into N. gonorrhoeae strain 1291. Recombinant strains were selected by growth on BHI agar containing kanamycin (100 g/ml) as described by Jennings et al. [32]. Previous work has demonstrated that the pUC4kan kanamycin cassette has no promoter nor terminator that is active in Neisseria and will neither affect transcription nor have a polar effect on expression of adjacent genes [32, 33].
2.13 SOD assay
Cell free extracts of N. gonorrhoeae strains were prepared by resuspending an equal amount of cells (as determined by equal optical density of samples at 600 nm) in PBS, followed by three cycles of freezing and thawing. Cell debris was removed by centrifugation at 13,000 rpm for 20 min, and the supernatant was collected. Protein concentration of samples, approximated by absorbance at 280 nm, was used to normalise samples. The SOD assay was performed as described by Crapo et al. [34]. A 500 μl mixture was prepared containing 50 μl of 0.1 mM cytochrome c (Sigma), 250 μl of 0.1 mM xanthine and 190 μl of potassium buffer (+/− the sample) and the reaction was initiated by adding 10 μl of 175 mU/ml xanthine oxidase. The rate of increase in absorbance at 550 nm was recorded. A unit is defined as the quantity of SOD required to produce 50% inhibition of the rate of reduction of cytochrome c under the specified conditions. P values for statistical significance were determined using Students t-tests.
2.14 Pyrophosphatase assay
Samples were prepared as described above for the SOD assay. The pyrophosphatase assay is based on the method described by Heinonen and Lahti [35]. The assay mixture was prepared containing 500 l of 50 mM Tris-HCl, pH 8 and 1 mM MgCl2, 20 l 1.7 mM Na-pyrophosphate, and 50 l of cell free extract. The reaction was incubated at 37 °C for 30 min then stopped with 100 μl of 100 mM citric acid. 120 l of the assay mix was then mixed with 1 ml of detection solution (1 volume of 10 mM ammonium-molybdate, 1 volume of 5 N sulfuric acid, 2 volumes of acetone). The amount of phosphomolybdate was determined by measuring the absorbance at 420 nm. P values for statistical significance were determined using Students t-tests.
2.15 Oxidative stress killing assays
Paraquat (PQ) [36], xanthine/xanthine oxidase (X/XO) [37] and H2O2 [38] killing assays were performed using the established methods described by Tseng et al. [10]. Briefly, cells from agar plates were harvested, resuspended in PBS, and 105 to 107 cells were added to a solution of BHI broth to a final volume of 100 l. The killing assay was started by the addition of a final concentration of either 10 mM PQ (Sigma), 4.3 mM xanthine and 300mU/ml xanthine oxidase (Sigma), or 40 mM H2O2 (Riedel-de Haen). Cultures were incubated at 37 °C/5%CO2. At various time points, samples were taken, serially diluted, plated onto BHI agar and incubated at 37 °C in 5% CO2to determine colony forming units (CFU). Experiments were done in triplicate and repeated on at least three occasions, with representative results shown. P-values were performed using student's t-test. Differences seen between strains and growth conditions were considered significant if the P-value was ≤ 0.05. Safety considerations: Paraquat is very toxic by inhalation, ingestion and if absorbed through skin.
2.16 Assay for the survival of N. gonorrhoeae in primary human cervical epithelial cells
Primary human cervical epithelial (pex) cells were procured and maintained as described previously [39]. Confluent cell monolayers were challenged with the wild type or mutant gonococci at a multiplicity of infection of 100. Association, invasion, and survival assays were performed as we have described previously using a modified gentamicin-survival assay [30]. Association is defined as the number of extra- and intracellular bacteria associated with pex cells at 90 min post-infection following extensive rinsing of the cell monolayer. Invasion is defined as the number of gonococci that survive gentamicin treatment (100 g ml−1; 30 min) subsequent to a 90 min challenge of pex cells. The ability of gonococci to survive within pex cells was determined by rinsing and then re-incubating (37 °C, 5% CO2) the infected pex cell monolayers in antibiotic-free medium for an additional 1 h (i.e., survival 1h) or 2 h (i.e., survival 2h) time period following gentamicin treatment. At each time point, viable gonococci were enumerated by counting CFUs obtained from plating serial dilutions of the pex cell lysates. Percent association, invasion, or survival was determined as a function of the original inoculum and the mean number of CFUs calculated for each condition assayed. P-values were determined using a Kruskal-Wallis non-parametric analysis of variance.
3 Results
3.1 The effect of Mn on the transcriptome and proteome of N. gonorrhoeae Microarray analysis of Mn-dependent regulation
To examine the effect of Mn on transcription in N. gonorrhoeae, gene expression in N. gonorrhoeae strain 1291 was investigated using N. gonorrhoeae / N. meningitidis genome microarrays (JCVI). Total RNA was isolated from wild type strain cultures that had been grown to exponential phase in the presence or absence of added Mn (±40 M MnSO4). N. gonorrhoeae strain 1291 grows at an equal rate in media ± 40 M MnSO4 (data not shown). Growth in the presence of higher Mn did not result in a significant change in the expression level of any gene within N. gonorrhoeae, as determined using a cut-off of 1.5 fold change in expression and a threshold in the B statistic of > 0 (See Experimental procedures). Results from the microarray analysis were confirmed using quantitative real time (qRT)-PCR on a selection of genes (see footnotes of Tables 2 and 3 for details). This result suggested that the Mn-dependent resistance to oxidative stress that is seen in N. gonorrhoeae may be mediated by post-transcriptional mechanisms.
Table 2.
1D SDS-PAGE analyses comparing the cell fractions of N. gonorrhoeae strain 1291 grown in the absence and presence of 40 M Mn(II)
| Gene Numbera | Protein Name | Gene Name | Classb | Theoretical Mr (Da) |
|---|---|---|---|---|
| Proteins with increased expression in the presence of Mn | ||||
| Soluble fractions: | ||||
| NGO0335 | polyribonucleotide nucleoti dyltransferase/Phosphorylase | pnp | A | 76448 |
| NGO0199 | transcription termination factor rho | rho | A | 47308 |
| NGO1974 | elongation factor EF-Tu (elongation factor TS) | tSf / EF-Tu | B | 30345 |
| NGO1843 | translation elongation factor EF-G | fusA | B | 77167 |
| NGO1824 | 30S ribosomal protein S5 | rpsE | B | 18231 |
| NGO2025 | 30S ribosomal protein S9 | rpsI | B | 14363 |
| NGO1838 | 50S ribosomal protein L3 | rpIC | B | 22663 |
| NGO1837 | 50S ribosomal protein L4 | rpID | B | 23288 |
| NGO0584 | 50S ribosomal protein L9 | rpII | B | 15687 |
| NGO1853 | 50S ribosomal protein L10 | rpIJ | B | 17589 |
| NGO1855 | 50S ribosomal protein L11 | rpIK | B | 14942 |
| NGO0298 | 50S ribosomal protein L20 | rpIT | B | 13664 |
| NGO0710 | A/G-specific adenine glycosylase | mutY | E | 39542 |
| NGO0398 | adenylosuccinate synthetase | purA | F | 45970 |
| NGO0353 | uracil phosphoribosyltransferase | upp | F | 22863 |
| NGO1241 | histidinol-phosphate aminotransferase | hisC | G | 39245 |
| NGO1358 | glutamate dehydrogenase | gdhA | G | 48462 |
| NGO0040 | glutamate 1-semialdehyde 2,1-aminotransferase | hemL/ gsa | H | 44999 |
| NGO1931 | glyceraldehyde 3-phosphate dehydrogenase C | gapC | I | 35748 |
| NGO1082 | isocitrate dehydrogenase | idh | I | 79943 |
| NGO0921 | succinate dehydrogenase flavoprotein subunit | dhsA/sdhA | I | 64441 |
| NGO0687 | ferredoxin--NADP reductase | fenR | I | 29317 |
| NGO0829 | chaperone protein | hscA | J | 66413 |
| NGO2095 | heat shock protein, 60 kD subunit (GroEL) | groEL | J | 57342 |
| NGO0450 | iron-superoxide dismutase* ~ | sodB | K | 17395 |
| NGO0794 | bacterioferritin A | bfrA | L | 17962 |
| NGO0832 | oxidoreductase, short chain reductase family | N/A | M | 25965 |
| NGO1709 | conserved hypothetical protein | N/A | N | 21913 |
| NGO1583 | conserved hypothetical protein | N/A | N | 40892 |
| NGO0361 | conserved hypothetical protein (possible hemY) | N/A | N | 45187 |
| NGO0571 | conserved hypothetical protein (possible MP) | N/A | N | 65933 |
| NGO0156 | conserved hypothetica, possible lipid A synthesis | N/A | N | 30975 |
| NGO1043 | conserved hypothetical protein | N/A | N | 11395 |
| NGO1635 | hypothetical protein | N/A | P | 17060 |
| Membrane fractions: | ||||
| NGO0096 | PilO / pilus assembly protein | pilO | O | 23330 |
| NGO1673 | type IV pilus assembly protein (PilF) | pilF | O | 61882 |
| NGO1806 | UDP-N-acetylglucosamine acyltransferase | lpxA | O | 28157 |
| NGO1067 /NGO1972 | MafA adhesin - Neisseria specific | mafA | O | 34738 |
| NGO0994 | H.8 OMP (azurin-like protein, Laz) | azu / laz/ H.8 | O | 18516 |
| NGO1812 | Neisseria-specific major OMP porin P.IB | pIB | O | 37174 |
| NGO1780 | probable outer membrane lipoprotein | omlA | O | 13904 |
| NG1577 | OMP PIII, Omp3 (OMP class 4, RmpM) | omp3 | O | 25525 |
| NGO2147 | ATP synthase delta chain | atpH | I | 19482 |
| NGO1363 | multidrug efflux pump channel protein | mtrE | Q | 50401 |
| NGO1765 | probable glycosyltransferase | pglA | B | 41995 |
| NGO2057 | Integral MP | N/A | M | 30694 |
| NGO1800 | conserved hypothetical protein (possible integral MP) | N/A | N | 48088 |
| Proteins with decreased expression in the presence of Mn | ||||
| Soluble fractions: | ||||
| NGO0002 | DNA polymerase III, beta subunit | dnaN | E | 40857 |
| NGO0259 | ribonuclease III | rnc | A | 26934 |
| NGO1858 | translation elongation factor Tu, TufA | tufA | B | 42926 |
| NGO1832 | 30S ribosomal protein S3 | rpsC | B | 25811 |
| NGO1844 | 30S ribosomal protein S7 | rpsG | B | 17629 |
| NGO1826 | 30S ribosomal protein S8 | rpsH | B | 14108 |
| NGO1854 | 50S ribosomal protein L1 | rplA | B | 24107 |
| NGO1829 | 50S ribosomal protein L14 | rplN | B | 13387 |
| NGO1823 | 50S ribosomal protein L15 | rplO | B | 14938 |
| NGO18311 | 50S ribosomal protein L16 | rl16 | B | 14776 |
| NGO18241 | 50S ribosomal protein L18 | rl18 | B | 12781 |
| NGO0171 | 50S ribosomal protein L19 | rl19 | B | 13751 |
| NGO1676 | 50S ribosomal protein L21 | rplU | B | 11431 |
| NGO1828 | 50S ribosomal protein L24 | rplX | B | 11591 |
| NGO0442 | 50S ribosomal protein L25 | rplY | B | 20939 |
| NGO1454 | lysyl-tRNA synthetase (LysRS) | lysS | B | 57370 |
| NGO0799 | inosine-5′-monophosphate dehydrogenase | imdH | F | 52429 |
| NGO1667 | 2,3,4,5-tetrahydropyridine-2-carboxylate N-succinyltransferase, DapD | dapD | G | 29291 |
| NGO1238 | ATP phosphoribosyltransferase | hisG | G | 26232 |
| NGO1134 | GTP cyclohydrolase II | gch2 | H | 22098 |
| NGO1310 | guanylate kinase / GMP kinase | Gmk/kguA | F | 23412 |
| NGO1668 | glucose-6-phosphate isomerase | pgi | I | 60308 |
| NGO0918 | citrate synthase | cisY/gltA | I | 48110 |
| NGO0912 | succinyl-CoA synthetase, alpha subunit | sucD | I | 30536 |
| NGO1743 | NADH dehydrogenase I chain I | nuol | I | 18790 |
| NGO0116 | protein-export protein subunit SecB | secB | C | 16317 |
| NGO2141 | chromosome segregation protein SpoOJ (ParB family) | spooJ/parB | D | 31518 |
| NGO1815 | septum site-determining protein | minD | D | 29581 |
| NGO1422 | heat shock protein/ HSP-70 cofactor; nucleotide exchange factor | grpE | J | 21335 |
| NGO1901 | heat shock protein HSP-40/chaperone DnaJ | dnaJ | J | 40590 |
| NGO1378 | transport protein, ExbB | exbB | L | 25836 |
| NGO0425 | RdgC homolog / phosphatase | rdgC | Q | 23134 |
| NGO0561 | conserved hypothetical protein | N/A | N | 57081 |
| NGO0905 | conserved hypothetical protein | N/A | N | 25780 |
| NGO1280 | conserved hypothetical protein | N/A | N | 53783 |
| NGO1709 | conserved hypothetical protein | N/A | N | 21913 |
| NGO1655 | Neisseria-specific protein, uncharacterized | N/A | N | 28555 |
| NGO0387 | conserved hypothetical protein | N/A | N | 28733 |
| NGO1656 | possible cell-binding factor (possible protein export) | N/A | M | 31514 |
| NGO1873 | conserved hypothetical protein | N/A | N | 45436 |
| Membrane fractions: | ||||
| NGO2061 | pilin (fimbrial protein) | pilE | O | 10386 |
| NGO0055 | pilus-associated protein, PilC1 | pilC1 | O | 115036 |
| NGO0094 | pilus secretion protein; OMP-molecular complex, type II secretion pathway D protein | pilQ | O | 77943 |
| NGO0070 | OMP opacity protein B, opaB | P.II / opaB | O | 29630 |
| NGO1076 | OMP opacity protein (Opa protein) | opaH /opaK | O | 28339 |
| NGO0915 | dihydrolipoamide dehydrogenase E3 component | dldH | I | 50068 |
| NGO2150 | ATP synthase beta chain (ATPase beta-subunit) | atpD | I | 50413 |
| NGO1495 | transferrin binding protein A | tbp-1/ tbpA | L | 101940 |
| NGO2059 | peptide methionine sulfoxide reductase, PilB | msrAB /pilB | Q | 58047 |
| NGO2118 | conserved hypothetical protein (possible MSD element in ABC transport system) | N/A | N | 19526 |
| NGO0284 | Neisseria-specific protein, uncharacterized | N/A | R | 15725 |
"NGO" gene designations were obtained from the annotated N. gonorrhoeae strain FA1090 genome (http://cmr.jcvi.org/tigr-scripts/CMR/GenomePage.cgi?org=ntng03).
Functional classifications as defined by the JCVI website (http://cmr.jcvi.org/tigrscripts/CMR/shared/RoleList.cgi) are included solely as a reference-point for this large group of proteins. A: Transcription; B: Translation; C: Cellular processes; Protein and peptide secretion; D: Cellular processes; Cell division; E: Replication; DNA replication, restriction, modification, recombination, and repair; F: Purines, pyrimidines, nucleosides, and nucleotides; G: Amino acid biosynthesis; H: Biosynthesis of cofactors, prosthetic groups, and carriers; I: Energy metabolism; J: Cellular processes; Chaperones; K: Cellular processes; Detoxification; L: Transport and binding proteins; M: Unassigned; N: Unknown; O: Cell envelope; P: Hypothetical; Q: Other categories; R: Neisseria -specific protein
Using a biochemical assay for SOD activity it was found that cells grown on Mn had approximately 2.5 fold higher SOD activity than cells grown in the absence of added Mn (P=0.01 using a student's t-test).
qRT-PCR was used to confirm microarray results. The ratio of transcription of these genes, wild type:wild type plus Mn, was below 1.5 fold (P >0.4).
N/A: Not available. OMP: outer membrane protein. MP: membrane protein.
Table 3.
Differentially expressed proteins in N. gonorrhoeae strain 1291 grown in the absence and presence of 40 M Mn from ICAT. The proteins listed are either down- or up- regulated by Mn
| Gene Numbera | Protein Name | Gene Name | Classb | Light/High area Ratioc |
|---|---|---|---|---|
| Increased expression in the presence of Mn | ||||
| Soluble fractions: | ||||
| NGO0794 | Bacteriofemtin § | bfrA | L | 0.5 |
| Reduced expression in the presence of Mn | ||||
| Soluble fractions: | ||||
| NGO1845 | 30S ribosomal protein S12 | rplS | B | 2.1±0.56 |
| NGO1835 | 50S ribosomal protein L2 | rplB | B | 1.57 |
| NGO1858 | translation elongation factor Tu, TufA § | tufA | B | 2.1±0.44 |
| NGO1227 | Cytosol leucyl aminopeptidase, LAP (aminopeptidase A) | ampA/pepA /lap | B | 1.9 |
| NGO0564 | dihydrolipoamide S-acetyltransferase complex, E2 component of pyruvate dehydrogenase | aceF | I | 1.810.45 |
| NGO0249 | acetyl-CoA carboxylase, beta subunit | accD | S | 2.75 |
| NGO0200 | phosphoenolpyruvate (PEP) synthase ~ | ppsA | T | 2.2±1.05 |
| NGO0223 | inorganic pyrophosphatase* ~ | ppa | T | 4.7±2.82 |
| NGO1521 | acetate kinase | ackA | T | 4.1±2.84 |
| NGO0926 | peroxiredoxin 2 family protein/glutaredoxin | prx | K | 4.0±1.26 |
| NGO2095 | heat shock protein , 60 kD subunit | groEL | J | 3.6±2.33 |
| NGO1046 | endopeptidase Clp ATP-binding chain B / heat shock protein F84.1 | clpB | Q | 4.2±3.67 |
| NGO0186 | zinc binding alcohol dehydrogenase | aid | M | 2.9±1.83 |
| Membrane fractions: | ||||
| NGO0346 | pilus retraction, twitching motility protein | pilT | O | 1.91±1.30 |
| NGO0562 | pyruvate E3 component, lipoamide dehydrogenase / glycine cleavage L protein (OMP P64k or PM 6) § | ipdA/dldH | T | 3.06±2.15 |
| NGO0177 | two-component system transcriptional response regulator OmpR | ompR/cpxR | U | 3.20 |
"NGO" gene designations were obtained from the annotated N. gonorrhoeae strain FA1090 genome (http://cmr.jcvi.org/tigr-scripts/CMR/GenomePage.cgi?org=ntng03).
See the functional classification in Table 2.
The cutoff of the ICAT experiment is 1.5-fold. The ratio is the mean of three independent ICAT experiments ± standard deviation.
The proteins were also seen to have different expression in response to Mn by 1D SDS-PAGE.
Using a biochemical assay for Ppa activity it was found that cells grown on Mn had approximately 1.9 fold less activity than cells grown in the absence of added Mn (P= 0.019 using a student's t-test).
qRT-PCR was used to confirm microarray results. The ratio of transcription of these genes, wild type:wild type plus Mn, was below 1.5 fold (P>0.4).
1D SDS-PAGE analysis of the effect of Mn on protein expression in N. gonorrhoeae
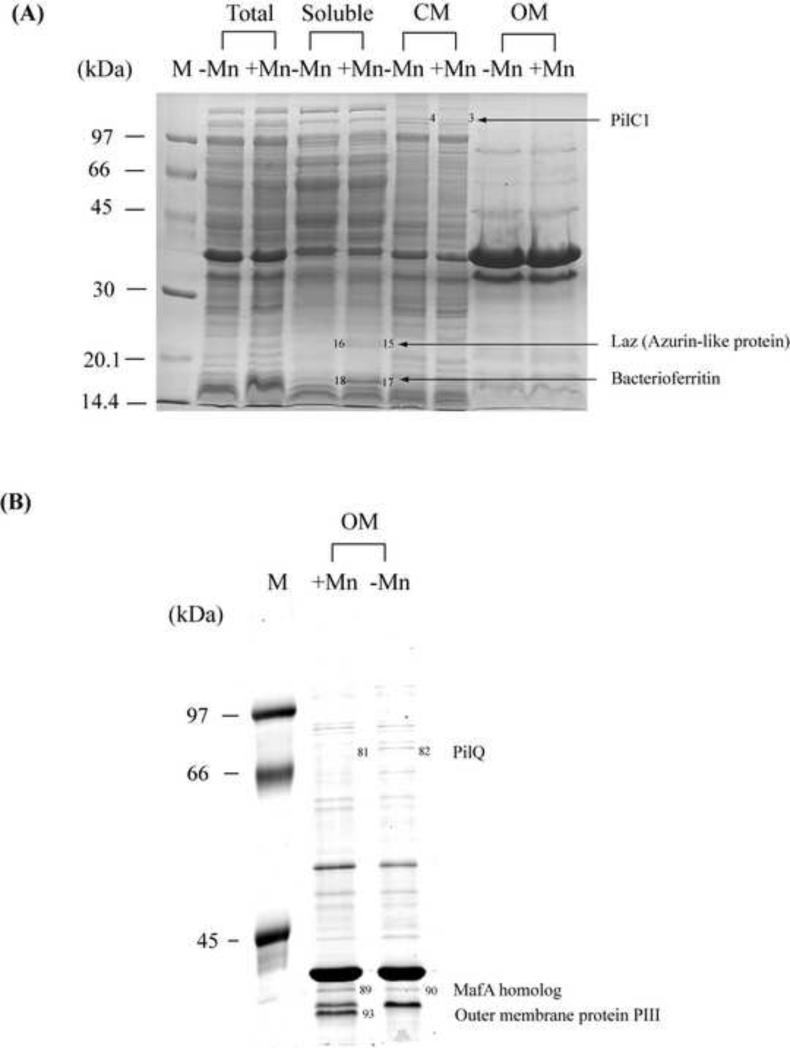
To investigate this possibility of Mn-dependent post-transcriptional regulation, we conducted a detailed analysis of the effect of Mn on the proteome of N. gonorrhoeae. A shotgun proteomic approach was used which involved two separate methods to identify Mn-regulated proteins, (1) 1D SDS-PAGE coupled with 1D LC-MS/MS and (2) ICAT studies coupled with MS/MS. This shotgun approach was used rather than 2D-gel based proteome profiling as the development of methods and instrumentation for automated data-dependent ESI MS/MS, in conjunction with nanoLC and database searching, has significantly increased the sensitivity and speed for the identification of gel-separated proteins [40, 41]. For 1D SDS-PAGE analysis, cells grown to exponential phase in the presence or absence of added Mn (under the same conditions used for the transcriptomic studies described above) were fractionated into different cell compartments and visualised on 1D gels with varying percentage polyacrylamide and size in order to reduce the complexity of the whole-cell sample. A total of 97 proteins were identified by LC-ESI-MS/MS as having a different level of expression between samples grown in the presence and absence of Mn (Error! Reference source not found.; representative gels are shown in Figure 1). 1D SDS-PAGE is a semi-quantitative method, therefore Error! Reference source not found. only includes proteins that have obviously altered expression (i.e. present versus absent) in the presence of low or high Mn and does not include quantitative information, unlike the ICAT results, which quantitatively identify differentially regulated proteins. In the presence of Mn, 47 proteins showed an increased level of expression. These proteins were found both in the soluble fraction and the membrane fractions and had diverse roles in metabolism, biosynthesis of cellular compounds, protein synthesis, stress defences and virulence (Error! Reference source not found.). The levels of expression of bacterioferritin (Bfr) [42], azurin (Laz) [43] (Figure 1A) and iron-superoxide dismutase (SodB) [44] were higher with increased Mn. These proteins are all associated with defense against oxidative stress. However, decreased levels of MsrAB, an outer membrane methionine sulfoxide reductase involved in protection from O2.− and H2O2 [45], were seen. Several proteins showing increased expression in the presence of Mn in the membrane fractions were potential virulence factors, including the major gonococcal outer membrane protein (OMP) Porin IB, and the adhesin MafA (Figure 1B).
Figure 1.
Representative 1D SDS-PAGE analyses comparing the soluble fractions of N. gonorrhoeae strain 1291 grown in the absence (−) and presence (+) of 40 M Mn(II) on (A) 1D 12.5% 7 cm, (B) 10% 18 cm gels. Total: total cell lysates; soluble: cytoplasmic and periplasmic proteins. CM: cytoplasmic membrane proteins; OM: outer membrane proteins. The numbers and protein names indicate examples of proteins showing differences in expression level between the control and Mn-treated sample.
A total of 50 proteins showed decreased expression in the presence of Mn (Error! Reference source not found.). Again, Mn-regulated proteins in the soluble fraction can be classified into several functional categories including metabolism, biosynthesis, protein synthesis, RNA processing, cell defence, cell division and transport. Several proteins in the membrane fraction that are decreased in the presence of Mn are involved in pilus biogenesis (Error! Reference source not found., Figure 1 and Figure 2), including PilE (structural subunit protein pilin), PilC1 (involved in pilus assembly and biogenesis) and PilQ (involved in translocation of pili to the cell surface) [46]. However, it is interesting to note that PilO, which functions in competence for transformation [47], was increased in the presence of Mn (Error! Reference source not found.). Other decreased OMPs include virulence factors, such as transferrin-binding protein A [48] and Opa [49, 50]. The cytoplasmic stress protein DnaJ [51] was also decreased in the presence of Mn. This stress protein was present in the outer membrane (OM) preparation indicating contamination of this fraction from the cytoplasmic fraction. Ribosomal proteins decreased in the presence of Mn were also present in the cytoplasmic membrane fractions instead of the cytoplasmic fraction. Cell fractions were primarily used to enable easier separation and identification of proteins, so cross fraction contamination was not considered to be a significant issue. However, these ribosomal proteins are often highly abundant and may obscure other membrane proteins in SDS-PAGE analysis [52]. These 1D SDS-PAGE results indicate that Mn is an important global regulator in N. gonorrhoeae and led to further quantitative investigation.
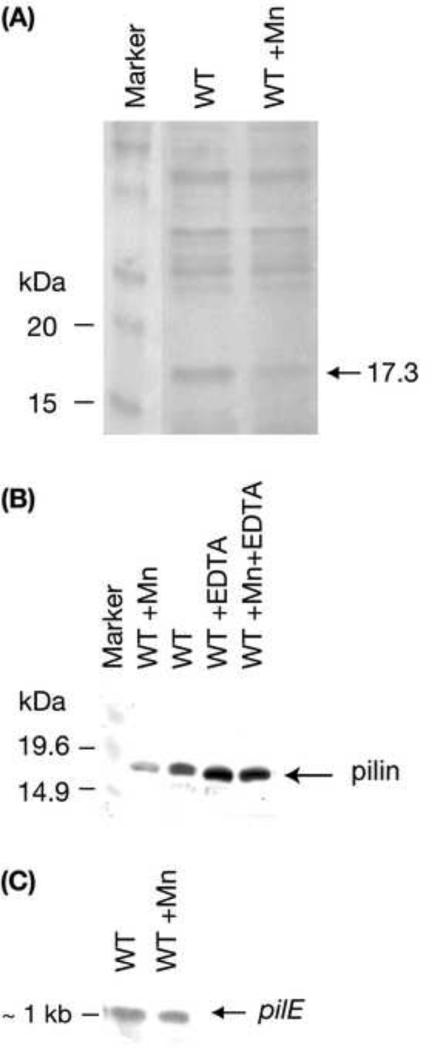
Figure 2.
(A) Coomassie stained SDS-PAGE, (B) Western blot and (C) Northern blot of Mn(II) regulated proteins in N. gonorrhoeae. Panel A, N. gonorrhoeae wild type (WT) cells were grown on BHI agar ±40 M MnSO4. Identical loadings of total cell protein were run on a 12% SDS-PAGE gel and Coomassie stained. A band of 17.3 kDa (calc.) is indicated that displayed a four-fold decrease in the presence of 40 M Mn (determined by densitometry in several independent experiments). Panel B, Western blot analysis of whole cells of N. gonorrhoeae strain 1291. Samples were run on a 12% SDS-PAGE gel, transferred to nitrocellulose and probed with the pilin specific probe, SM1. Panel C, Northern blot analysis of RNA isolated from N. gonorrhoeae strain 1291 WT grown on BHI agar ± 40 μM MnSO4, probed with a pilE specific probe (amplified by the PilEsignalF and PilEsignalR primers, see Table 1).
ICAT analysis of Mn-dependent regulation in N. gonorrhoeae
For quantitative proteomics, a combination of ICAT and MS-MS was employed. ICAT is currently one of the most widely adopted isotopic labeling approaches [53, 54]. The ICAT proteomic analysis revealed that the level of bacterioferritin was increased by greater than 1.5 fold by Mn. On the other hand, 16 proteins were decreased by greater than 1.5 fold in the presence of added Mn, including PilT (involved in pili retraction), peroxiredoxin (Prx; involved in peroxide reduction), OMP P64k (Mr=64 kDa), OmpR (Mr=25 kDa) and pyrophosphatase (Ppa), (Error! Reference source not found.). OMP P64k is present in the majority of the meningococcal strains [55], elicits bactericidal antibodies in animal models and has been considered as a potential vaccine candidate [56]. Of particular interest is Ppa, which hydrolyses pyrophosphate (PPi) to orthophosphate (Pi), since PPi can stabilise Mn(III) ions [57].
3.2 Post-transcriptional Mn-dependent regulation of pilin, superoxide dismutase and pyrophosphatase
The proteomic and genomic analyses described above show that the level of several proteins in N. gonorrhoeae is affected by the concentration of Mn present in the growth media, but that transcription of the genes encoding these proteins is unaltered under the same conditions. To further investigate this finding and to confirm that Mn-dependent regulation of protein levels is post-transcriptional, three of the Mn-regulated proteins, Pilin, Ppa and SOD, were further examined.
Pilin expression is down-regulated by Mn
Our preliminary investigations of the Mn-dependent response of N. gonorrhoeae involved an examination of protein expression in wild type strain 1291 cells grown on medium ± 40 μM Mn(II) by analysing polypeptide profiles in whole cell lysates after 1D SDS-PAGE and Coomassie staining. The most striking observation was a decrease in the level of a 17.3 kDa protein in the presence of high Mn(II) (4-fold by densitometry, Figure 2A). N-terminal amino acid sequencing of this polypeptide was performed and it was determined to be identical to the N-terminal sequence of the pili subunit protein, pilin, which is encoded by pilE(NGO2061). Pili are long polymeric proteins that protrude from the bacterial surface and have a crucial role in both bacterial colonization and adherence to host cells [58, 59].
We further investigated pilin expression by western blot analysis using a pilin-specific monoclonal antibody, SM1, in the wild type strain 1291 grown under different conditions. The western blot shows that N. gonorrhoeae wild type has decreased pilin production when grown on Mn-supplemented medium (Figure 2B), which is in agreement with the Coomassie stained SDS-PAGE results described above (Figure 2A). Addition of EDTA to growth media caused induction of pilin production, presumably by chelating Mn(II) and preventing Mn(II)-dependent suppression of expression (Figure 2B).
The level of pilE transcription in northern blot analysis was similar between N. gonorrhoeae 1291 grown with and without Mn(II) supplementation (Figure 2C), indicating that Mn(II) regulation of pilin expression is post-transcriptional.
SOD activity is increased in the presence of Mn
SOD is a major component of the oxidative defence response of the majority of organisms and catalyses the disproportionation of superoxide to hydrogen peroxide and water [60, 61]. 1D SDS-PAGE analysis revealed that addition of Mn to growth media resulted in an increased level of SodB (Error! Reference source not found.). Using a biochemical assay for SOD activity it was found that cells grown on Mn had an average of 2.5 fold higher SOD activity than cells grown in the absence of added Mn (P-value = 0.01; data not shown). Expression of the sodB gene was not significantly altered between cultures grown in the presence versus the absence of Mn (qRT-PCR; 1.21 fold, P-value = 0.53), indicating that the difference in activity is a result of Mn-dependent post-transcriptional regulation.
Pyrophosphatase activity is reduced in the presence of Mn
Soluble inorganic pyrophosphatases hydrolyse inorganic pyrophosphate (PPi) into two molecules of orthophosphate (Pi), thus making it possible for many biosynthetic reactions to proceed [62]. ICAT analysis revealed that addition of Mn to growth media resulted in a 4.7 ± 2.82 fold decrease in the level of Ppa (Error! Reference source not found.). Using a biochemical assay for Ppa activity it was found that cells grown on Mn had approximately 2 fold less activity than cells grown in the absence of added Mn (P= 0.019; data not shown). In addition to the decreased Ppa protein level seen in the ICAT result, growth in the presence of high Mn levels could result in this decrease in Ppa activity via displacement of Mg by Mn at the active site. The majority of Ppa proteins have higher activity when cofactored with Mg than Mn [63], however some Ppa proteins have a unique requirement for Mn [64]. No significant difference was seen in expression of the ppa gene between cultures grown in the presence versus absence of Mn (qRT-PCR: 1.29 fold, P-value = 0.47), indicating that the difference in activity is a result of Mn-dependent post-transcriptional regulation.
3.3 Ppa and Ppk of N. gonorrhoeae
Interestingly, both ICAT and enzymatic assay demonstrated that in the presence of Mn(II), the level of expression of pyrophosphatase (Ppa) decreased. These results are consistent with previous studies that have outlined the relationships between Mn, PPi, polyphosphate (polyP) and oxidative stress. In lactic acid bacteria, high concentrations of Mn(II) (about 30 mM) accumulate within the cytoplasm, chelated by polyP, providing a SOD-independent mechanism of resistance to oxidative stress [65, 66]. PolyP, a polymer of large numbers (>100) of orthophosphate (Pi), can chelate Mn(II) and is linked to the various functions in several pathogens [67]. PolyP is synthesised from Pi by polyphosphate kinase (Ppk), and hydrolysed to Pi by exopolyphosphatase (Ppx). PPi can also stabilize Mn(III) ions [57], preventing spontaneous dismutation to Mn(II) and Mn(IV). PPi is hydrolysed to Pi by pyrophosphatase (Ppa). It is established that the pathogenic Neisseria can accumulate up to 10% of their phosphate as polyP. In E. coli, ppk mutants that lack Ppk activity and are unable to synthesize polyP from Pi, are sensitive to oxidative, osmotic and heat stresses and have decreased survival in stationary phase [68]. Some of the properties of a N. gonorrhoeae mutant lacking the ppk gene have been described [69], although the affect of Ppk on resistance to oxidative stress was not reported.
To determine whether PPi or polyP or are involved in the oxidative stress response, in particular the Mn-dependent oxidative stress response of N. gonorrhoeae, mutant strains lacking the genes encoding ppk or ppa were generated. In E. coli and Vibrio cholerae, the ppk and ppx genes are adjacent and form an operon [70]. However, in N. gonorrhoeae ppk (NGO0003) is flanked by the dnaN gene at its 3’ end and by two genes encoding hypothetical proteins at the 5’ end. Thus, it appears that ppk is probably monocistronic. The ppa gene (NGO0223) is flanked by an uncharacterised gene at its 3’ end and the ntpA (nucleoside triphosphate pyrophosphohydrolase) gene at its 5’ end. Kornberg and coworkers have shown that a number of bacteria contain a second ppk gene that encodes an enzyme (Ppk2) with distinct biochemical properties, including preference for GTP over ATP as a substrate [71]. In their search of incomplete microbial genomes these researchers established that N. gonorrhoeae possessed a single ppk gene encoding the enzyme that is the homologue of PPK1. Our more recent search of the annotated N. gonorrhoeae genome (http://cmr.jcvi.org/tigr-scripts/CMR/GenomePage.cgi?org=ntng03) confirmed the presence of a single ppk gene (NGO0003).
Tinsley and Gotschlich [69] have shown that a ppk mutant of N. gonorrhoeae was defective in growth on a defined medium. Using the richer growth medium, BHI broth, we observed no significant differences in growth rate and growth yield between wild type and the ppk mutant (data not shown). As a consequence, we were able to comparatively analyse differences between these strains with confidence that our data were not the result of differences in growth rate. However, the ppa strain did have a slightly increased lag phase with respect to the wild type strain (data not shown).
Oxidative stress response of N. gonorrhoeae ppk and ppa mutant strains
To determine whether Ppk and Ppa contribute to the defence of gonococci against oxidative stress, we compared the survival capability of wild type strain 1291 and ppk and ppa mutant cells after exposure to H2O2 as well as to two generators of O2.−: PQ, which generates O2.− inside the cell, and X/XO, which generates O2.− and H2O2 external to the cell, as described previously [10].
As mentioned above, we have previously observed that accumulation of Mn(II) by N. gonorrhoeae cells correlates with resistance to oxidative killing [10], and Figure 3 and 4 show that there was a significant increase in survival of wild type cells grown in the presence of Mn compared to cells grown without Mn supplementation in all assays.
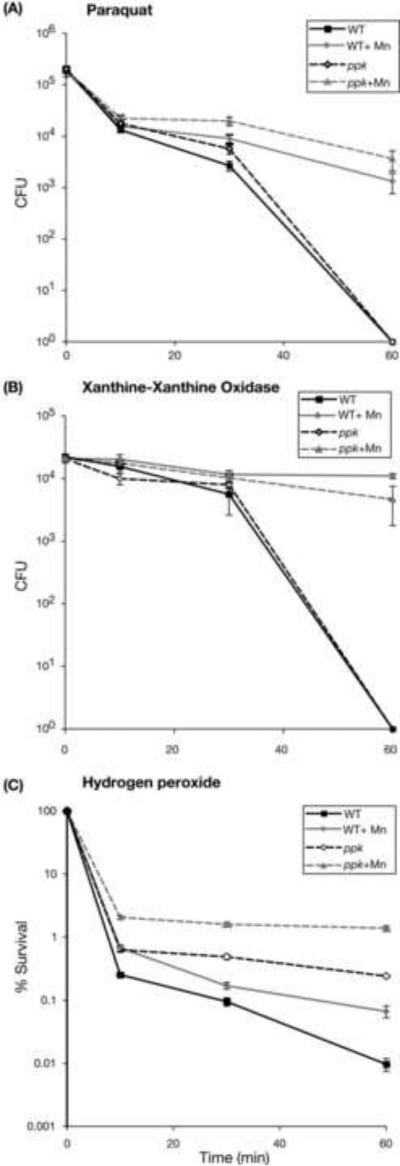
Figure 3.
(A) Paraquat, (B) xanthine-xanthine oxidase and (C) hydrogen peroxide oxidative stress killing assays of N. gonorrhoeae strain 1291 (wild type) and the ppk mutant strains grown on BHI agar or BHI plus 100 M Mn (Mn). Experiments were performed in triplicate. Error bars indicate ±1 standard deviation of the mean. P values were determined using Students t-tests. Mn protected the wild type cells in all assays; PQ (on average approximately 2000-fold higher survival at 60 min), X/XO (approximately 6000-fold at 60 min) and H2O2 (approximately 5-fold at 60 min; P <0.05 at the final time point for all assays). The ppk mutant was slightly more resistant than the wild type to PQ with increased survival seen in the absence of Mn (2.1-fold at 30 min, P=0.016; equal at 60 min, P=0.37) and the presence of Mn (2.2-fold at 30 min, P=0.011; equal at 60 min, P=0.069). The ppk mutant was significantly more resistant to H2O2 than was the wild type with increased survival seen in both the absence (25-fold at 60 min, P=0.00005) and the presence of Mn (21-fold at 60 min, P=0.0004). Mn protected the ppk mutant in all assays; PQ (>3000-fold at 60 min, P=0.014), X/XO (>4000-fold at 60 min, P=0.041) and H2O2 (6-fold at 60 min, P=0.001).
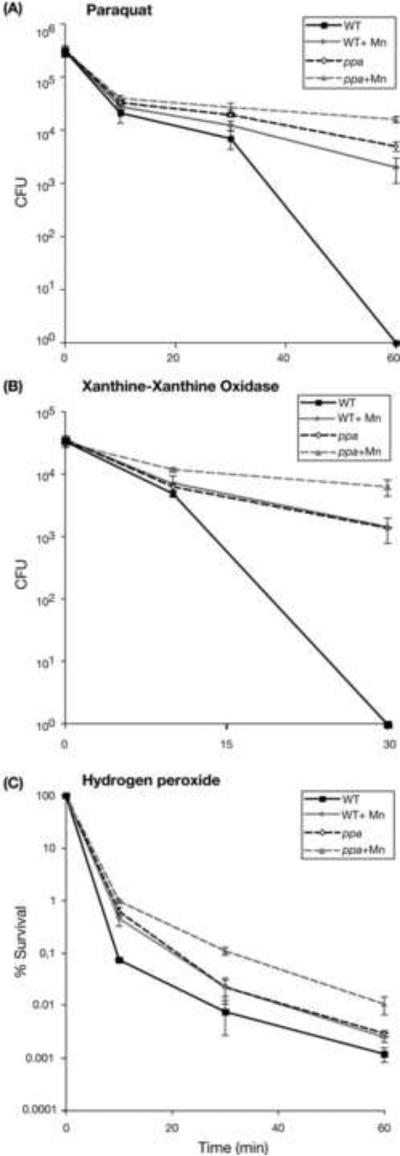
Figure 4.
(A) Paraquat, (B) xanthine-xanthine oxidase and (C) hydrogen peroxide oxidative stress killing assays of N. gonorrhoeae strain 1291 (wild type) and the ppa mutant strain. Experiments were performed in triplicate. Error bars indicate ±1 standard deviation of the mean. P values were determined using Students t-tests. The ppa mutant was more resistant than was the wild type strain to killing by PQ in the absence of Mn (>5000-fold at 60 min, P=0.001) and in the presence of Mn (8-fold at 60 min, P=0.004). Increased resistance was also seen in the ppa mutant in the X/XO assay in the absence of Mn (>1300-fold at 60 min, P=0.016) and presence of Mn (5-fold increase in survival at 60 min, P=0.011), and the H2O2 assay in the absence of Mn (2.4-fold at 60 min, P=0.0043) and presence of Mn (4.2-fold at 30 min, P=0.008; >5000-fold increase at 60 min, P=0.05). Growth of the ppa mutant in the presence of Mn did not provide protection (with respect to the ppa mutant on unsuplemented media) in the PQ assay (3-fold at 60 min, P= 0.001) or the X/XO assay (4.5-fold at 60 min, P=0.011) and the difference seen in the H2O2 assay is not significant as judged by a P-value > 0.5 (5-fold at 30 min, P=0.008; 4-fold at 60 min, P=0.055).
The ppk mutant was slightly more resistant than the wild type to PQ (Figure 3A) with increased survival seen in the absence and the presence of Mn. The ppk mutant behaved like the wild type in the X/XO assay (Figure 3B) but was significantly more resistant to H2O2 (Figure 3C) than was the wild type with increased survival seen in both the absence and the presence of Mn. The ppk mutant grown on Mn also exhibited enhanced survival compared to the ppk mutant cells grown on medium without Mn-supplementation in all assays (Figure 3A-C).
The ppa mutant was more resistant than was the wild type strain to killing by PQ (Figure 4A) with an increase in survival seen in both the absence and presence of Mn. Increased resistance was also seen in the ppa mutant in the X/XO assay (Figure 4B) and H2O2 assay (Figure 4C) in both the absence and presence of Mn. However, growth of the ppa mutant in the presence of Mn did not provide protection against ROS to the extent typically seen for the wild type in the PQ assay or the X/XO assay and the difference seen in the H2O2 assay is not significant as judged by a P-value > 0.5 (Figure 4A-C). This decreased Mn-mediated protection against ROS in ppa suggests that the protection of cells against oxidative stress by Mn may involve Mn-PPi complexes.
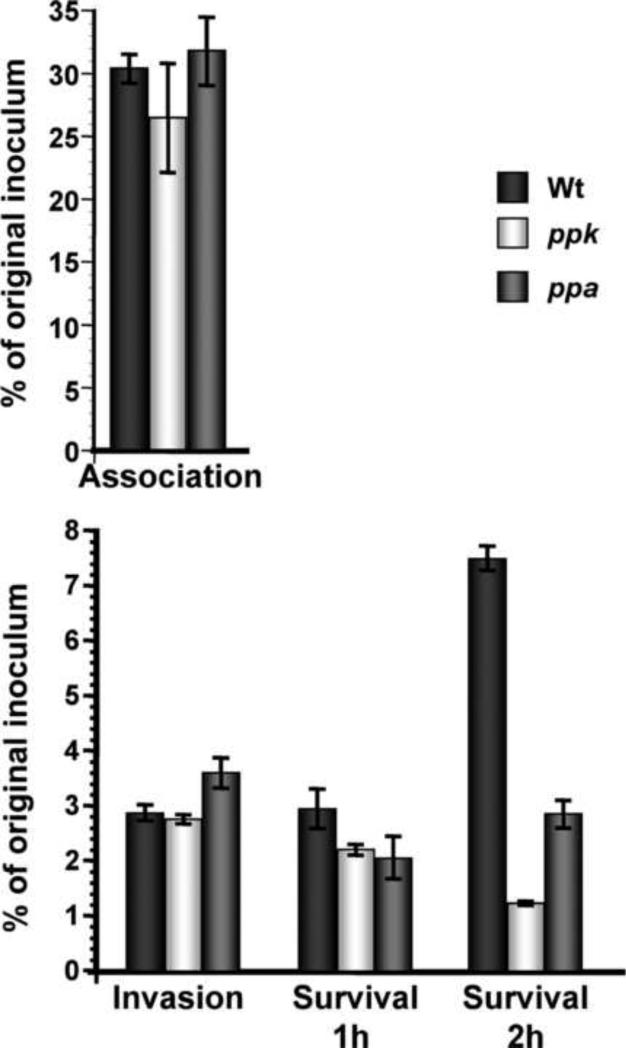
Survival of ppk and ppa mutant gonococci in primary cervical cells
N. gonorrhoeae adherence to and invasion of epithelial cells is a complex process that is mediated by several factors [72, 73]. We evaluated the ability of wild type and ppk and ppa mutant gonococci to associate with, to invade, and to survive within pex cells, using a modified gentamicin-survival assay as we have described previously [12, 30]. Figure 5 demonstrates that the wild type gonococci and the ppk and ppa mutants exhibited a similar degree of association with pex cells (P-values ≥ 0.67, as determined using a Kruskal-Wallis non-parametric analysis of variance). However, both of these mutant gonococcus strains exhibited a distinct phenotype in their ability to invade and to survive within pex cells upon comparison to the wild type bacteria. Consistent with our previous studies, the wild type gonococci were able to invade and survive one-hour post gentamicin treatment of pex. At two hours following gentamicin treatment the number of viable wild type gonococci had more than doubled, presumably indicating growth within pex cells.
Figure 5.
Gonococcal association with and intracellular survival within primary human cervical epithelial (pex) cells. The histogram shows the mean percent association, invasion and survival as a function of the original inoculum of the N. gonorrhoeae wild type strain (WT), and the ppk and ppa mutant strains. Data, determined from the number of colony forming units formed upon plating of the cervical cell lysates, were obtained from three experiments performed in triplicate. Y-error bars show +/− 1 variance. P-values were determined using a Kruskal-Wallis non-parametric analysis of variance.
Although the ppk mutant gonococci were not significantly impaired in their ability to invade pex cells when compared to the wild type (96% invasion relative to the wild type, P=0.67), they had decreased survival within pex cells relative to the wild type at the one-hour post gentamicin treatment time point (75%, P-value= 0.0001). At the two-hours post gentamicin treatment the number of viable ppk gonococci was further decreased both with respect to the previous time point (55%) and the wild type strain (16%, P-value= 0.0001), suggesting a role for polyP synthesis in the intracellular survival of gonococci.
The ppa mutant displayed an increased invasive phenotype relative to the wild type strain (126%, P=0.0001) but showed decreased intracellular survival relative to the wild type by one-hour post gentamicin treatment (69%, P=0.0001). However, by two-hours post gentamicin treatment the number of viable ppa mutant gonococci had increased with respect to the previous time point (141%), but still had decreased survival with respect to the wild type (38%, P-value= 0.0001).
4. Discussion
Over the last decade Mn has emerged as a trace element of significance in bacterial physiology and virulence, where it can function as a regulator of gene expression and as a modulator of metabolism [15, 74]. It has also been reported that Mn levels vary from nM to μM concentrations within the human host [17, 18], indicating that Mn could be an important environmental signal for human pathogens. Our previous studies have confirmed a key role of Mn in defence against ROS [10]; however, the mechanism has not been fully resolved. In order to investigate the regulatory and ROS defence roles of Mn in N. gonorrhoeae, we used a combined transcriptomic and proteomic approach.
4.1 Mn-dependent protection against oxidative stress
Our proteomic study showed that several oxidative stress defences proteins of N. gonorrhoeae were present at higher levels under high Mn conditions. These proteins included, SodB, bacterioferritin and Laz (Tables 2 and 3). SodB is a Fe-dependent enzyme that detoxifies superoxide, while bacterioferritin is an iron storage protein that can also contribute towards protection against oxidative stress [42]. These data indicate that a higher level of Mn may enable higher levels of iron acquisition, which otherwise would be disadvantageous to the cell due to Fenton chemistry [75], and this results in higher levels of these two iron-dependent proteins. Similarly, the Laz protein is a copper-binding protein found in the outer membrane and although its precise function is not known, it is involved in protection of gonococcus against peroxide killing [43]. Again, the increased level of this protein may be the result of increased copper acquisition which is facilitated by higher levels of Mn. MsrAB, a methionine sulfoxide reductase that is involved in protection from hydrogen peroxide and superoxide [45, 76], was also decreased (Table 2). This may reflect a decreased requirement for this outer membrane protein under high Mn conditions where other oxidative stress defenses become more prominent.
Mn(II) and Mn(III) can scavenge ROS non-enzymatically [77 , 78] and N. gonorrhoeae, like many lactic acid bacteria [65, 66, 77, 79, 80], may use accumulated intracellular Mn as a defence against ROS independently of SodB [10] or catalase [11]. Mn complexes, including Mn(II)-PPi and Mn(II)-polyP, have been shown to be potential non-enzymatic antioxidants [81]. Ppa had reduced expression under conditions on high Mn ( Error! Reference source not found.), which would result in increased levels of PPi that would be available to form Mn(II)-PPi complexes for antioxidant activity. PPi is generated in cells either as a metabolic byproduct of numerous biochemical and biosynthetic reactions [82] or directly by pyrophosphohydrolysis of the phosphodiesterase I bond in purine and pyrimidine nucleoside triphosphates by members of the phosphodiesterase nucleotide pyrophosphatase (PDNP) family [83]. It is established that the pathogenic Neisseria can accumulate up to 10% of their phosphate as polyP. Although most of this polyP is intracellular, there is evidence that polyP is also loosely associated with the extracellular surface where it forms a capsule-like structure around the gonococcus [84]. To investigate the role of PPi and polyP in oxidative stress, oxidative killing assays were performed using ppk and ppa mutant strains. The ppk and ppa mutations cause cells to be more resistant to oxidative killing. The level of Mn-mediated protection against ROS seen in the ppa mutant strain was drastically reduced with respect to the wild type strain (Figure 4). These data suggest that the protection of cells against oxidative stress by Mn may be due to Mn(II)-PPi complex rather than Mn(II)-polyP complex formation. However, since some Mn(II)-dependent protection was maintained in the ppa mutant strain, the Mn(II)-dependent effect is unlikely to completely depend on PPi. Also, since Mn provides protection in the ppk mutant against ROS to a similar extent as that seen in the wild type strain, it can be concluded that Mn is not directly dependent upon polyP for its antioxidant action. Therefore, unlike the situation in L. plantarum [66] Mn(II)-polyP complexes do not have a direct role in ROS quenching within N. gonorrhoeae.
The role of polyP and PPi in invasion of and survival in primary cervical epithelial cells was also investigated. Whereas the ppk and ppa mutations did not affect the association of gonococci with pex cells, we did observe increased invasion by the ppa mutant. Both mutant strains had decreased survival in the pex cells relative to the wild type. These data show that polyP and pyrophosphate metabolisms in N. gonorrhoeae have a significant influence on invasion of and survival within cervical epithelial cells. This may be linked to defense against with oxidative stress and varying Mn levels during gonococcal adherence to and invasion of target cells, as outlined in the section above.
4.2 Mn regulation of pili and other virulence factors
Mn is involved in virulence in Salmonella enterica serovar Typhimurium [15, 74] and S. pneumoniae [19, 85] and regulates the expression of several virulence factors in N. gonorrhoeae (Tables 2 and 3). It has also been reported that Mn levels vary from nM to M concentrations within the human host [17, 18]. Thus, differences in Mn concentration between host microenvironments may provide a signal for expression of certain virulence factors at sites of gonococcal infection. Indeed, it has recently been shown that S. pneumoniae uses Mn as a signal for the expression of virulence factors within different host sites, and that the disruption of this Mn-dependent regulation reduces virulence in an animal model [19, 20]. Pilin (PilE) levels of N. gonorrhoeae, as well as several pili associated proteins (PilC1, PilT, PilQ), were reduced in the presence of increased Mn concentrations (Table 2 and 3 Error! Reference source not found.). PilC1 is involved in pilus assembly, PilQ is involved in translocation of pili to the cell surface and PilT is required for pilus retraction [46]. Pili of pathogenic Neisseria spp. are typical of a family of adhesins, type IV fimbriae, found in a wide range of Gram-negative pathogens. These long polymeric proteins protrude from the bacterial surface and have a crucial role in both colonization of the host and adhesion to host cells [58, 59] and only piliated bacteria are recovered from gonorrhoea patients [86]. Piliation increases the ability of gonococci to adhere to numerous cell types including human amniotic cells [87], sperm [88, 89], erythrocytes [90, 91], buccal epithelial cells, neutrophils [91], vaginal epithelial cells [92] and non-ciliated cells in fallopian-tube organ cultures [93]. Pili are also required for CR3-mediated endocytosis of primary cervical epithelial (pex) cells [72]. Although pili are critical in mediating adhesion to host cells, it is also generally believed that these adhesins are not expressed upon the invasion of a host cell [73, 94]. Environmental factors causing pilus release are not known. In light of the role pili plays in gonococcal virulence, Mn-dependent regulation of pilin suggests that Mn may play an important role in pathogenicity of N. gonorrhoeae and our data show that this effect is exerted at the post-transcriptional level.
Mn is also linked with regulation of other known and potential virulence factors of N. gonorrhoeae, including Porin and the adhesin MafA (increased expression in the presence of Mn) as well as Opa, OMP P64K, OmpR and transferrin binding protein A (decreased expression in the presence of Mn) (Table 2 and 3). Although development of a vaccine against N. gonorrhoeae has been hampered by the variability of antigens between gonococcal strains, research has focused on pilin, porin and transferrin binding proteins [95, 96]. Understanding of Mn-dependent regulation of these proteins may aid in future vaccine development.
4.3 Mn regulation of carbon metabolism
Many of the Mn-dependent changes in protein levels observed in this study relate to enzymes involved in intermediary carbon metabolism. Mn has previously been linked to cell metabolism, with higher Mn levels being correlated with slow growing or stationary phase bacterial cells [15]. Kehres and Maguire [15] reviewed several Mn regulated enzymes that are involved in intermediary carbon metabolism and described a possible network of co-regulated enzymes involved in phosphoenolpyruvate (PEP) and pyruvate metabolism. N. gonorrhoeaemetabolises glucose via the Entner-Douderoff (ED) pathway and the citric acid cycle (CAC) [97, 98], and the ED pathway results in the formation of glyceraldehyde 3-phosphate (GAP) and pyruvate as primary C3 products [97, 98].
Changes to the levels of proteins involved in carbon metabolism may be related to the effect of Mn on the redox environment of the cell that is described above, e.g. the sensitivity of GAP dehydrogenase (GAPDH) to inactivation by oxidative stress [99]. Conversely, it would be expected that under conditions of low Mn levels, GAPDH would be lowered and the activity of the enzymes involved in pyruvate and PEP production, via this branch of the ED pathway, would also be lowered. Restriction of production of pyruvate from GAP would mean that the pyruvate produced directly from the ED Pathway would take on greater importance for production of carbon intermediates. PEP production is critical and it is notable that under low Mn conditions there is a higher level of PEP synthase (Error! Reference source not found.). Another consequence of a decreased GAPDH activity, which would be observed under conditions of low Mn, would be an elevation of the level of this intermediate, GAP. C3 sugars (i.e. GAP) cannot cyclise to prevent further oxidation of a carbon atom to form dicarbonyls which are highly reactive and toxic species [100, 101]. Thus, it is critical to remove accumulating GAP and it is postulated that this function is taken by the zinc-dependent alcohol dehydrogenase which would use NADH to produce glycerol-3-phosphate. Perhaps, this also explains why acetyl-CoA carboxylase is co-ordinately regulated ( Error! Reference source not found.) so that production of fatty acyl-CoA intermediates can be used along with glycerol-3-phophate in phospholipid synthesis. It is notable that acetate kinase levels also increase under conditions of low Mn (Table 3) and this provides for increased production of acetyl-P which in turn can lead to formation of acetyl-CoA.
4.4 Mechanism of Mn-dependent post-transcriptional regulation
While the mechanism of Mn-dependent post-transcriptional regulation in N. gonorrhoeae has not been determined in this study, there are several ways Mn could regulate the levels of the proteins listed in Tables 2 and 3. Changes in the Mn concentration could result in: (1) Modification of activity of the Mn-dependent ppGpp hydrolase (SpoT), which is involved in controlling the level of ppGpp in response to changes in the environment, which in turn globally coordinates mRNA and stable RNA synthesis; (2) binding of Mn instead of Mg to compounds which can alter the catalytic activity of enzymes and tertiary structure of tRNA; (3) binding of Mn to small RNAs involved in translational regulation, leading to RNA structural changes; and (4) decreased protein turnover due to the role of Mn in protection from ROS [reviewed in 15]. There are also up and down-regulation of ribosomal proteins by Mn (Table 2), which might be involved in the change of mRNA synthesis according to the environment changes sensed by Mn. Or this might be due to the inefficient cell separation, because it has been reported that even after lysis in PBS buffer a lot of soluble and membrane-associated proteins remain in the membrane fraction (for example, the ribosomal proteins L1/L2/L3/L4/L5 or the elongation factor Tu) [52]. These proteins are often highly abundant and may therefore obscure membrane proteins in the SDS-PAGE. We note that Mn caused some changes in the level of chaperone proteins (GroEL) and proteases (ClpX) associated with protein turnover. Mn homeostasis and Mn-dependent regulation appears to involve several factors, and more work is necessary to determine the mechanism of Mn regulation in N. gonorrhoeae and the role of Mn in pathogenesis in this organism.
Supplementary Material
Table 1.
Primers used in this study for PCR and qRT-PCR
| Primer | Sequence (5′ - 3′) |
|---|---|
| PilEsignalF | ATGAATACCCTTCAAAAAGGCTTTACCC |
| PilEsignalR | AGTCTTGGTAGGCGGGAAGGGCGAC |
| ppa_RTfor | TTACCGAACAACCTCTGGCAAC |
| ppa_RTrev | GCAGGTACGCAGACGATTTTG |
| ppsa_RTfor | GGCAAATCGGTAACCAACGTC |
| ppsa_RTrev | TTTCGATGGTCAGCGCGTA |
| sodB_RTfor | TCCAAGAAGCGTTCAATGCC |
| sodB_RTrev | GGCGTTGGAAGTGGAAATCA |
| Ppk_for | CGTCATGCCGCCTGAAACCGGCGCA |
| Ppk_rev | GCGGGTCAGCCTCGGAGCAAATC |
| ppa-KO-F | CCAGCGCGTTTTCGACAAAGG |
| ppa-KO-R | ATCCTGCTCATCGAACGCAC |
Acknowledgement
We thank Dr. K. H. Khoo for his valuable consultation in the field of proteomics. This work was supported by Program Grant 284214 from the National Health and Medical Research Council of Australia. The Core Facilities for Proteomics Research, Institute of Biological Chemistry, Academia Sinica, is supported by a National Science Council grant (NSC 93-3112-B-001-010-Y) and Academia Sinica. Hsing-Ju Wu was supported by the fellowship of Distinguished Postdoctoral Scholar, Institute of Biological Chemistry, Academia Sinica, Taiwan, and also by a CJ Martin NHMRC Career Development award. SMG is a recipient of a NHMRC Career Development award and a senior research affiliate of the ARC Special Research Centre for Functional and Applied Genomics. The authors would like to thank NIH and TIGR for the provision of the Neisseria arrays. We acknowledge the Gonococcal Genome Sequencing Project supported by USPHS/NIH grant #AI38399, and B.A. Roe, L. Song, S. P. Lin, X. Yuan, S. Clifton, Tom Ducey, Lisa Lewis and D.W. Dyer at the University of Oklahoma. The authors further thank the Cooperative Human Tissue Network (Columbus, Ohio, USA) for providing cervical tissue specimens and the Columbus Children's Research Institute for funding to JLE.
Abbreviations
- ABC
ATP binding cassette
- ABTS
2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- BHI
brain heart infusion
- CAC
citric acid cycle
- ED
Entner-Douderoff
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IAA
iodoacetamide
- Mn
manganese
- NCBI
National Center for Biotechnology Information
- OM
outer membrane
- PEP
phosphoenolpyruvate
- PMN
polymorphonuclear leukocyte
- PolyP
polyphosphate
- Ppa
inorganic pyrophosphatase
- RNS
reactive nitrogen species
- SOD
superoxide dismutase
- TCEP
Tris(carboxyethyl) phosphine
- TIGR
The Institute for Genomic Research
References
- 1.Archibald FS, Duong MN. Superoxide dismutase and oxygen toxicity defenses in the genus Neisseria. Infect Immun. 1986;51:631–41. doi: 10.1128/iai.51.2.631-641.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apicella MA, Ketterer M, Lee FK, Zhou D, Rice PA, Blake MS. The pathogenesis of gonococcal urethritis in men: confocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae. J Infect Dis. 1996;173:636–46. doi: 10.1093/infdis/173.3.636. [DOI] [PubMed] [Google Scholar]
- 3.Burg ND, Pillinger MH. The neutrophil: function and regulation in innate and humoral immunity. Clin Immun. 2001;99:7–17. doi: 10.1006/clim.2001.5007. [DOI] [PubMed] [Google Scholar]
- 4.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–17. [PubMed] [Google Scholar]
- 5.Nunoshiba T, DeRojas-Walker T, Tannenbaum SR, Demple B. Roles of nitric oxide in inducible resistance of Escherichia coli to activated murine macrophages. Infect Immun. 1995;63:794–8. doi: 10.1128/iai.63.3.794-798.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imlay JA. Pathways of oxidative damage. Ann Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 7.Storz G, Imlay JA. Oxidative stress. Curr Opin Microbiol. 1999;2:188–94. doi: 10.1016/s1369-5274(99)80033-2. [DOI] [PubMed] [Google Scholar]
- 8.Simons MP, Nauseef WM, Apicella MA. Interactions of Neisseria gonorrhoeae with adherent polymorphonuclear leukocytes. Infect Immun. 2005;73:1971–7. doi: 10.1128/IAI.73.4.1971-1977.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seib KL, Wu HJ, Kidd SP, Apicella MA, Jennings MP, McEwan AG. Defenses against oxidative stress in Neisseria gonorrhoeae: a system tailored for a challenging environment. Microbiol Mol Biol Rev. 2006;70:344–61. doi: 10.1128/MMBR.00044-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tseng HJ, Srikhanta Y, McEwan AG, Jennings MP. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol Microbiol. 2001;40:1175–86. doi: 10.1046/j.1365-2958.2001.02460.x. [DOI] [PubMed] [Google Scholar]
- 11.Seib KL, Tseng HJ, McEwan AG, Apicella MA, Jennings MP. Defenses against Oxidative Stress in Neisseria gonorrhoeae and Neisseria meningitidis: Distinctive Systems for Different Lifestyles. J Infect Dis. 2004;190:136–47. doi: 10.1086/421299. [DOI] [PubMed] [Google Scholar]
- 12.Wu HJ, Seib KL, Srikhanta YN, Kidd SP, Edwards JL, Maguire TL, Grimmond SM, Apicella MA, McEwan AG, Jennings MP. PerR controls Mn-dependent resistance to oxidative stress in Neisseria gonorrhoeae. Mol Microbiol. 2006;60:401–16. doi: 10.1111/j.1365-2958.2006.05079.x. [DOI] [PubMed] [Google Scholar]
- 13.Johnston JW, Myers LE, Ochs MM, Benjamin WH, Jr., Briles DE, Hollingshead SK. Lipoprotein PsaA in virulence of Streptococcus pneumoniae: surface accessibility and role in protection from superoxide. Infect Immun. 2004;72:5858–67. doi: 10.1128/IAI.72.10.5858-5867.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McAllister LJ, Tseng HJ, Ogunniyi AD, Jennings MP, McEwan AG, Paton JC. Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol Microbiol. 2004;53:889–901. doi: 10.1111/j.1365-2958.2004.04164.x. [DOI] [PubMed] [Google Scholar]
- 15.Kehres DG, Maguire ME. Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol Rev. 2003;27:263–90. doi: 10.1016/S0168-6445(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 16.Jakubovics NS, Jenkinson HF. Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiol. 2001;147:1709–18. doi: 10.1099/00221287-147-7-1709. [DOI] [PubMed] [Google Scholar]
- 17.Chicharro JL, Serrano V, Urena R, Gutierrez AM, Carvajal A, Fernandez-Hernando P, Lucia A. Trace elements and electrolytes in human resting mixed saliva after exercise. Br J Sports Med. 1999;33:204–7. doi: 10.1136/bjsm.33.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheuhammer AM, Cherian MG. Binding of manganese in human and rat plasma. Biochim Biophys Acta. 1985;840:163–9. doi: 10.1016/0304-4165(85)90115-1. [DOI] [PubMed] [Google Scholar]
- 19.Johnston JW, Briles DE, Myers LE, Hollingshead SK. Mn2+-dependent regulation of multiple genes in Streptococcus pneumoniae through PsaR and the resultant impact on virulence. Infect Immun. 2006;74:1171–80. doi: 10.1128/IAI.74.2.1171-1180.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosch JW, Gao G, Ridout G, Wang YD, Tuomanen EI. Role of the manganese efflux system mntE for signalling and pathogenesis in Streptococcus pneumoniae. Mol Microbiol. 2009;72:12–25. doi: 10.1111/j.1365-2958.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arirachakaran P, Benjavongkulchai E, Luengpailin S, Ajdic D, Banas JA. Manganese affects Streptococcus mutans virulence gene expression. Caries Res. 2007;41:503–11. doi: 10.1159/000110883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander HE. In: Bacterial and mycotic infection in man. Dubos RJ, Hirsch JG, editors. Pitman Medical Publishing; London: 1965. pp. 724–41. [Google Scholar]
- 23.Sambrook J, Maniatis T, Fritsch EF. Molecular cloning : a laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y.: 1989. [Google Scholar]
- 24.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–79. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 27.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Virji M, Heckels JE, Potts WJ, Hart CA, Saunders JR. Identification of epitopes recognized by monoclonal antibodies SM1 and SM2 which react with all pili of Neisseria gonorrhoeae but which differentiate between two structural classes of pili expressed by Neisseria meningitidis and the distribution of their encoding sequences in the genomes of Neisseria spp. J Gen Microbiol. 1989;135(Pt 12):3239–51. doi: 10.1099/00221287-135-12-3239. [DOI] [PubMed] [Google Scholar]
- 29.Gallagher S. In: Current Protocols in Molecular Biology. Jannsen K, editor. John Wiley and Sons Inc; 1992. pp. 10.8.1–.8.6. [Google Scholar]
- 30.Seib KL, Wu HJ, Srikhanta YN, Edwards JL, Falsetta ML, Hamilton AJ, Maguire TL, Grimmond SM, Apicella MA, McEwan AG, Jennings MP. Characterization of the OxyR regulon of Neisseria gonorrhoeae. Mol Microbiol. 2007;63:54–68. doi: 10.1111/j.1365-2958.2006.05478.x. [DOI] [PubMed] [Google Scholar]
- 31.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- 32.Jennings MP, Hood DW, Peak IR, Virji M, Moxon ER. Molecular analysis of a locus for the biosynthesis and phase-variable expression of the lacto-N-neotetraose terminal lipopolysaccharide structure in Neisseria meningitidis. Mol Microbiol. 1995;18:729–40. doi: 10.1111/j.1365-2958.1995.mmi_18040729.x. [DOI] [PubMed] [Google Scholar]
- 33.van der Ley P, Kramer M, Martin A, Richards JC, Poolman JT. Analysis of the icsBA locus required for biosynthesis of the inner core region from Neisseria meningitidis lipopolysaccharide. FEMS Microbiol Lett. 1997;146:247–53. doi: 10.1111/j.1574-6968.1997.tb10201.x. [DOI] [PubMed] [Google Scholar]
- 34.Crapo JD, McCord JM, Fridovich I. Preparation and assay of superoxide dismutases. Methods Enzym. 1978;53:382–93. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- 35.Heinonen JK, Lahti RJ. A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal Biochem. 1981;113:313–7. doi: 10.1016/0003-2697(81)90082-8. [DOI] [PubMed] [Google Scholar]
- 36.Hassett DJ, Bean K, Biswas G, Cohen MS. The role of hydroxyl radical in chromosomal and plasmid damage in Neisseria gonorrhoeae in vivo. Free Radic Res Commun. 1989;7:83–7. doi: 10.3109/10715768909087927. [DOI] [PubMed] [Google Scholar]
- 37.Wilks KE, Dunn KL, Farrant JL, Reddin KM, Gorringe AR, Langford PR, Kroll JS. Periplasmic superoxide dismutase in meningococcal pathogenicity. Infect Immun. 1998;66:213–7. doi: 10.1128/iai.66.1.213-217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson SR, Steiner BM, Cruce DD, Perkins GH, Arko RJ. Characterization of a catalase-deficient strain of Neisseria gonorrhoeae: evidence for the significance of catalase in the biology of N. gonorrhoeae. Infect Immun. 1993;61:1232–8. doi: 10.1128/iai.61.4.1232-1238.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards JL, Shao JQ, Ault KA, Apicella MA. Neisseria gonorrhoeae elicits membrane ruffling and cytoskeletal rearrangements upon infection of primary human endocervical and ectocervical cells. Infect Immun. 2000;68:5354–63. doi: 10.1128/iai.68.9.5354-5363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Link AJ, Eng J, Schieltz DM, Carmack E, Mize GJ, Morris DR, Garvik BM, Yates JR., 3rd Direct analysis of protein complexes using mass spectrometry. Nat Biotechnol. 1999;17:676–82. doi: 10.1038/10890. [DOI] [PubMed] [Google Scholar]
- 41.Opiteck GJ, Lewis KC, Jorgenson JW, Anderegg RJ. Comprehensive on-line LC/LC/MS of proteins. Anal Chem. 1997;69:1518–24. doi: 10.1021/ac961155l. [DOI] [PubMed] [Google Scholar]
- 42.Chen CY, Morse SA. Neisseria gonorrhoeae bacterioferritin: structural heterogeneity, involvement in iron storage and protection against oxidative stress. Microbiol. 1999;145:2967–75. doi: 10.1099/00221287-145-10-2967. [DOI] [PubMed] [Google Scholar]
- 43.Wu HJ, Seib KL, Edwards JL, Apicella MA, McEwan AG, Jennings MP. Azurin of pathogenic Neisseria spp. is involved in defense against hydrogen peroxide and survival within cervical epithelial cells. Infect Immun. 2005;73:8444–8. doi: 10.1128/IAI.73.12.8444-8448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carlioz A, Ludwig ML, Stallings WC, Fee JA, Steinman HM, Touati D. Iron superoxide dismutase. Nucleotide sequence of the gene from Escherichia coli K12 and correlations with crystal structures. J Biol Chem. 1988;263:1555–62. [PubMed] [Google Scholar]
- 45.Skaar EP, Tobiason DM, Quick J, Judd RC, Weissbach H, Etienne F, Brot N, Seifert HS. The outer membrane localization of the Neisseria gonorrhoeae MsrA/B is involved in survival against reactive oxygen species. Proc Natl Acad Sci U S A. 2002;99:10108–13. doi: 10.1073/pnas.152334799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morand PC, Rudel T. In: Handbook of Meningicoccal Disease Infection Biology, Vaccination, Clinical Management. Frosch M, Maiden MCJ, editors. Wiley-VCH Verlag GmbH & Co.; Weinheim: 2006. pp. 235–54. [Google Scholar]
- 47.Drake SL, Sandstedt SA, Koomey M. PilP, a pilus biogenesis lipoprotein in Neisseria gonorrhoeae, affects expression of PilQ as a high-molecular-mass multimer. Mol Microbiol. 1997;23:657–68. doi: 10.1046/j.1365-2958.1997.2511618.x. [DOI] [PubMed] [Google Scholar]
- 48.Palmer HM, Powell NB, Ala'Aldeen DA, Wilton J, Borriello SP. Neisseria meningitidis transferrin-binding protein 1 expressed in Escherichia coli is surface exposed and binds human transferrin. FEMS Microbiol Lett. 1993;110:139–45. doi: 10.1111/j.1574-6968.1993.tb06310.x. [DOI] [PubMed] [Google Scholar]
- 49.Swanson J. Studies on gonococcus infection. XII. Colony color and opacity varienats of gonococci. Infect Immun. 1978;19:320–31. doi: 10.1128/iai.19.1.320-331.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swanson J. Studies on gonococcus infection. XIV. Cell wall protein differences among color/opacity colony variants of Neisseria gonorrhoeae. Infect Immun. 1978;21:292–302. doi: 10.1128/iai.21.1.292-302.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grudniak AM, Kuc M, Wolska KI. Role of Escherichia coli DnaK and DnaJ chaperones in spontaneous and induced mutagenesis and their effect on UmuC stability. FEMS Microbiol Lett. 2005;242:361–6. doi: 10.1016/j.femsle.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 52.Schluesener D, Fischer F, Kruip J, Rogner M, Poetsch A. Mapping the membrane proteome of Corynebacterium glutamicum. Proteomics. 2005;5:1317–30. doi: 10.1002/pmic.200400993. [DOI] [PubMed] [Google Scholar]
- 53.Bouyssie D, Gonzalez de Peredo A, Mouton E, Albigot R, Roussel L, Ortega N, Cayrol C, Burlet-Schiltz O, Girard JP, Monsarrat B. Mascot file parsing and quantification (MFPaQ), a new software to parse, validate, and quantify proteomics data generated by ICAT and SILAC mass spectrometric analyses: application to the proteomics study of membrane proteins from primary human endothelial cells. Mol Cell Proteomics. 2007;6:1621–37. doi: 10.1074/mcp.T600069-MCP200. [DOI] [PubMed] [Google Scholar]
- 54.Cannataro M, Cuda G, Gaspari M, Greco S, Tradigo G, Veltri P. The EIPeptiDi tool: enhancing peptide discovery in ICAT-based LC MS/MS experiments. BMC Bioinformatics. 2007;8:255. doi: 10.1186/1471-2105-8-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silva R, Selman M, Guillen G, Herrera L, Fernandez JR, Novoa LI, Morales J, Morera V, Gonzalez S, Tamargo B, De Valle J, Caballero E, Alvarez A, Coizeau E, Cruz S, Musacchio A. 1992 [Google Scholar]
- 56.Silva R, Menendez T, Alonso LM, Iglesias E, Musacchio A, Leal MJ, Alvarez A, Coizeau E, Martin A, Herrera L, Guillen G. Characterisation of the lpdA gene from Neisseria meningitidis by polymerase chain reaction, restriction fragment length polymorphism and sequencing. FEMS Microbiol Lett. 1999;174:191–9. doi: 10.1111/j.1574-6968.1999.tb13568.x. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen M, Quemard A, Broussy S, Bernadou J, Meunier B. Mn(III) pyrophosphate as an efficient tool for studying the mode of action of isoniazid on the InhA protein of Mycobacterium tuberculosis. Antimicrobial Agents Chemo. 2002;46:2137–44. doi: 10.1128/AAC.46.7.2137-2144.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGee ZA, Stephens DS. Common pathways of invasion of mucosal barriers by Neisseria gonorrhoeae and Neisseria meningitidis. Sur Synth Path Res. 1984;3:1–10. [PubMed] [Google Scholar]
- 59.Virji M, Kayhty H, Ferguson DJ, Alexandrescu C, Heckels JE, Moxon ER. The role of pili in the interactions of pathogenic Neisseria with cultured human endothelial cells. Mol Microbiol. 1991;5:1831–41. doi: 10.1111/j.1365-2958.1991.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 60.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244:6049–55. [PubMed] [Google Scholar]
- 61.Fridovich I. Superoxide radical and superoxide dismutases. Ann Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 62.Samygina VR, Popov AN, Rodina EV, Vorobyeva NN, Lamzin VS, Polyakov KM, Kurilova SA, Nazarova TI, Avaeva SM. The structures of Escherichia coli inorganic pyrophosphatase complexed with Ca(2+) or CaPP(i) at atomic resolution and their mechanistic implications. J Mol Biol. 2001;314:633–45. doi: 10.1006/jmbi.2001.5149. [DOI] [PubMed] [Google Scholar]
- 63.Cooperman BS, Baykov AA, Lahti R. Evolutionary conservation of the active site of soluble inorganic pyrophosphatase. Trends Biochem Sci. 1992;17:262–6. doi: 10.1016/0968-0004(92)90406-y. [DOI] [PubMed] [Google Scholar]
- 64.Parfenyev AN, Salminen A, Halonen P, Hachimori A, Baykov AA, Lahti R. Quaternary structure and metal ion requirement of family II pyrophosphatases from Bacillus subtilis, Streptococcus gordonii, and Streptococcus mutans. J Biol Chem. 2001;276:24511–8. doi: 10.1074/jbc.M101829200. [DOI] [PubMed] [Google Scholar]
- 65.Archibald FS, Fridovich I. Manganese and defenses against oxygen toxicity in Lactobacillus plantarum. J Bacteriol. 1981;145:442–51. doi: 10.1128/jb.145.1.442-451.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Archibald FS, Fridovich I. Investigations of the state of the manganese in Lactobacillus plantarum. Arch Biochem Biophys. 1982;215:589–96. doi: 10.1016/0003-9861(82)90120-5. [DOI] [PubMed] [Google Scholar]
- 67.Kornberg A, Rao NN, Ault-Riche D. Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem. 1999;68:89–125. doi: 10.1146/annurev.biochem.68.1.89. [DOI] [PubMed] [Google Scholar]
- 68.Rao NN, Kornberg A. Inorganic polyphosphate supports resistance and survival of stationary-phase Escherichia coli. J Bacteriol. 1996;178:1394–400. doi: 10.1128/jb.178.5.1394-1400.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tinsley CR, Gotschlich EC. Cloning and characterization of the meningococcal polyphosphate kinase gene: production of polyphosphate synthesis mutants. Infect Immun. 1995;63:1624–30. doi: 10.1128/iai.63.5.1624-1630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tzeng CM, Kornberg A. Polyphosphate kinase is highly conserved in many bacterial pathogens. Mol Microbiol. 1998;29:381–2. doi: 10.1046/j.1365-2958.1998.00887.x. [DOI] [PubMed] [Google Scholar]
- 71.Zhang H, Ishige K, Kornberg A. A polyphosphate kinase (PPK2) widely conserved in bacteria. Proc Natl Acad Sci U S A. 2002;99:16678–83. doi: 10.1073/pnas.262655199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Edwards JL, Brown EJ, Uk-Nham S, Cannon JG, Blake MS, Apicella MA. A co-operative interaction between Neisseria gonorrhoeae and complement receptor 3 mediates infection of primary cervical epithelial cells. Cell Microbiol. 2002;4:571–84. doi: 10.1046/j.1462-5822.2002.t01-1-00215.x. [DOI] [PubMed] [Google Scholar]
- 73.Merz AJ, So M. Interactions of pathogenic neisseriae with epithelial cell membranes. Annu Rev Cell Dev Biol. 2000;16:423–57. doi: 10.1146/annurev.cellbio.16.1.423. [DOI] [PubMed] [Google Scholar]
- 74.Papp-Wallace KM, Maguire ME. Manganese transport and the role of manganese in virulence. Annu Rev Microbiol. 2006;60:187–209. doi: 10.1146/annurev.micro.60.080805.142149. [DOI] [PubMed] [Google Scholar]
- 75.Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–2. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 76.Lowther WT, Weissbach H, Etienne F, Brot N, Matthews BW. The mirrored methionine sulfoxide reductases of Neisseria gonorrhoeae pilB. Nat Struct Biol. 2002;9:348–52. doi: 10.1038/nsb783. [DOI] [PubMed] [Google Scholar]
- 77.Archibald FS, Fridovich I. The scavenging of superoxide radical by manganous complexes: in vitro. Arch Biochem Biophys. 1982;214:452–63. doi: 10.1016/0003-9861(82)90049-2. [DOI] [PubMed] [Google Scholar]
- 78.Coassin M, Ursini F, Bindoli A. Antioxidant effect of manganese. Arch Biochem Biophys. 1992;299:330–3. doi: 10.1016/0003-9861(92)90282-2. [DOI] [PubMed] [Google Scholar]
- 79.Archibald FS, Duong MN. Manganese acquisition by Lactobacillus plantarum. J Bacteriol. 1984;158:1–8. doi: 10.1128/jb.158.1.1-8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Archibald FS, Fridovich I. Manganese, superoxide dismutase, and oxygen tolerance in some lactic acid bacteria. J Bacteriol. 1981;146:928–36. doi: 10.1128/jb.146.3.928-936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheton PL, Archibald FS. Manganese complexes and the generation and scavenging of hydroxyl free radicals. Free Radic Biol Med. 1988;5:325–33. doi: 10.1016/0891-5849(88)90104-9. [DOI] [PubMed] [Google Scholar]
- 82.Rachow JW, Ryan LM. Inorganic pyrophosphate metabolism in arthritis. Rheum Dis Clin North Am. 1988;14:289–302. [PubMed] [Google Scholar]
- 83.Johnson K, Vaingankar S, Chen Y, Moffa A, Goldring MB, Sano K, Jin-Hua P, Sali A, Goding J, Terkeltaub R. Differential mechanisms of inorganic pyrophosphate production by plasma cell membrane glycoprotein-1 and B10 in chondrocytes. Arthritis and rheumatism. 1999;42:1986–97. doi: 10.1002/1529-0131(199909)42:9<1986::AID-ANR26>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 84.Noegel A, Gotschlich EC. Isolation of a high molecular weight polyphosphate from Neisseria gonorrhoeae. J Exp Med. 1983;157:2049–60. doi: 10.1084/jem.157.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Berry AM, Paton JC. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect Immun. 1996;64:5255–62. doi: 10.1128/iai.64.12.5255-5262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Swanson J, Robbins K, Barrera O, Corwin D, Boslego J, Ciak J, Blake M, Koomey JM. Gonococcal pilin variants in experimental gonorrhea. J Exp Med. 1987;165:1344–57. doi: 10.1084/jem.165.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973;137:571–89. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.James-Holmquest AN, Swanson J, Buchanan TM, Wende RD, Williams RP. Differential attachment by piliated and nonpiliated Neisseria gonorrhoeae to human sperm. Infect Immun. 1974;9:897–902. doi: 10.1128/iai.9.5.897-902.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.James AN, Knox JM, Williams RP. Attachment of gonococci to sperm. Influence of physical and chemical factors. Br J Vener Dis. 1976;52:128–35. doi: 10.1136/sti.52.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Buchanan TM, Pearce WA. Pili as a mediator of the attachment of gonococci to human erythrocytes. Infect Immun. 1976;13:1483–9. doi: 10.1128/iai.13.5.1483-1489.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Punsalang AP, Jr., Sawyer WD. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973;8:255–63. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mardh PA, Westtom L. Adherence of bacterial to vaginal epithelial cells. Infect Immun. 1976;13:661–6. doi: 10.1128/iai.13.3.661-666.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McGee ZA, Johnson AP, Taylor-Robinson D. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J Infect Dis. 1981;143:413–22. doi: 10.1093/infdis/143.3.413. [DOI] [PubMed] [Google Scholar]
- 94.Nassif X. Interactions between encapsulated Neisseria meningitidis and host cells. Int Microbiol. 1999;2:133–6. [PubMed] [Google Scholar]
- 95.Stanberry LR, Rosenthal SL. Progress in vaccines for sexually transmitted diseases. Infect Dis Clin North Am. 2005;19:477–90. doi: 10.1016/j.idc.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 96.Mietzner TA, Thomas CE, Hobbs MM, Cohen MS. In: New Generation Vaccines. Levine MM, Kaper JB, Rappouli R, Liu MA, Good MF, editors. Marcel Dekker, Inc.; New York: 2004. pp. 755–73. [Google Scholar]
- 97.Morse SA, Stein S, Hines J. Glucose metabolism in Neisseria gonorrhoeae. J Bacteriol. 1974;120:702–14. doi: 10.1128/jb.120.2.702-714.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hebeler BH, Morse SA. Physiology and metabolism of pathogenic neisseria: tricarboxylic acid cycle activity in Neisseria gonorrhoeae. J Bacteriol. 1976;128:192–201. doi: 10.1128/jb.128.1.192-201.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lind C, Gerdes R, Schuppe-Koistinen I, Cotgreave IA. Studies on the mechanism of oxidative modification of human glyceraldehyde-3-phosphate dehydrogenase by glutathione: catalysis by glutaredoxin. Biochem Biophys Res Commun. 1998;247:481–6. doi: 10.1006/bbrc.1998.8695. [DOI] [PubMed] [Google Scholar]
- 100.Papoulis A, al-Abed Y, Bucala R. Identification of N2-(1-carboxyethyl)guanine (CEG) as a guanine advanced glycosylation end product. Biochemistry. 1995;34:648–55. doi: 10.1021/bi00002a032. [DOI] [PubMed] [Google Scholar]
- 101.Lo TW, Westwood ME, McLellan AC, Selwood T, Thornalley PJ. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J Biol Chem. 1994;269:32299–305. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.