Abstract
Previous data suggest that apolipoprotein (apo) CIII may inhibit both triglyceride hydrolysis by lipoprotein lipase (LPL) and apo E-mediated uptake of triglyceride-rich lipoproteins by the liver. We studied apo B metabolism in very low density (VLDL), intermediate density (IDL), and low density lipoproteins (LDL) in two sisters with apo CIII-apo AI deficiency. The subjects had reduced levels of VLDL triglyceride, normal LDL cholesterol, and near absence of high density lipoprotein (HDL) cholesterol. Compartmental analysis of the kinetics of apo B metabolism after injection of 125I-VLDL and 131I-LDL revealed fractional catabolic rates (FCR) for VLDL apo B that were six to seven times faster than normal. Simultaneous injection of [3H]glycerol demonstrated rapid catabolism of VLDL triglyceride. VLDL apo B was rapidly and efficiently converted to IDL and LDL. The FCR for LDL apo B was normal. In vitro experiments indicated that, although sera from the apo CIII-apo-AI deficient patients were able to normally activate purified LPL, increasing volumes of these sera did not result in the progressive inhibition of LPL activity demonstrable with normal sera. Addition of purified apo CIII to the deficient sera resulted in 20-50% reductions in maximal LPL activity compared with levels of activity attained with the same volumes of the native, deficient sera. These in vitro studies, together with the in vivo results, indicate that in normal subjects apo CIII can inhibit the catabolism of triglyceride-rich lipoproteins by lipoprotein lipase.
Full text
PDF

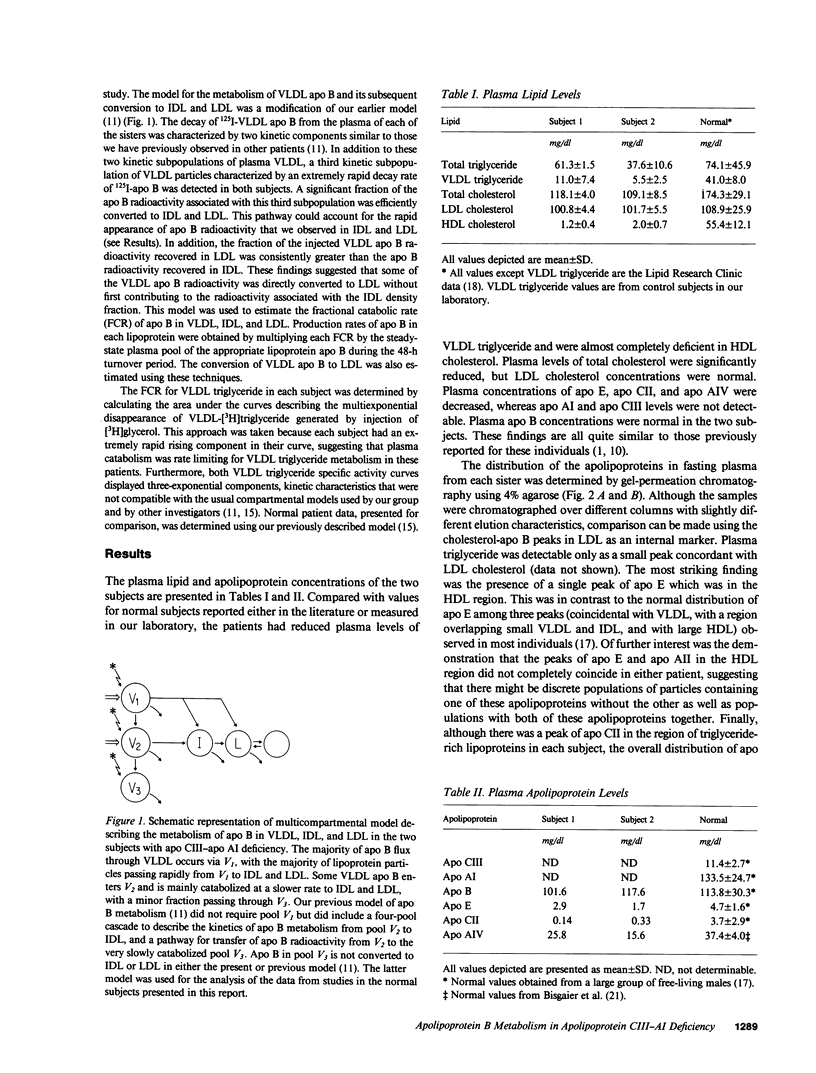
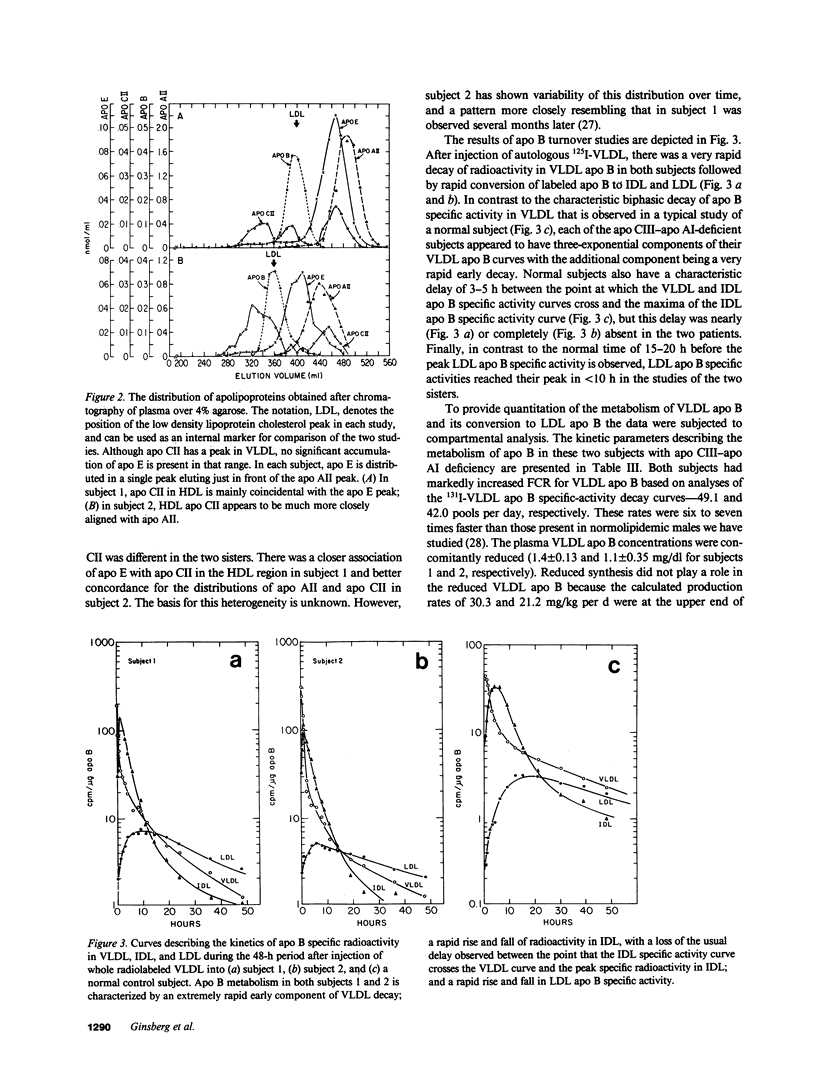
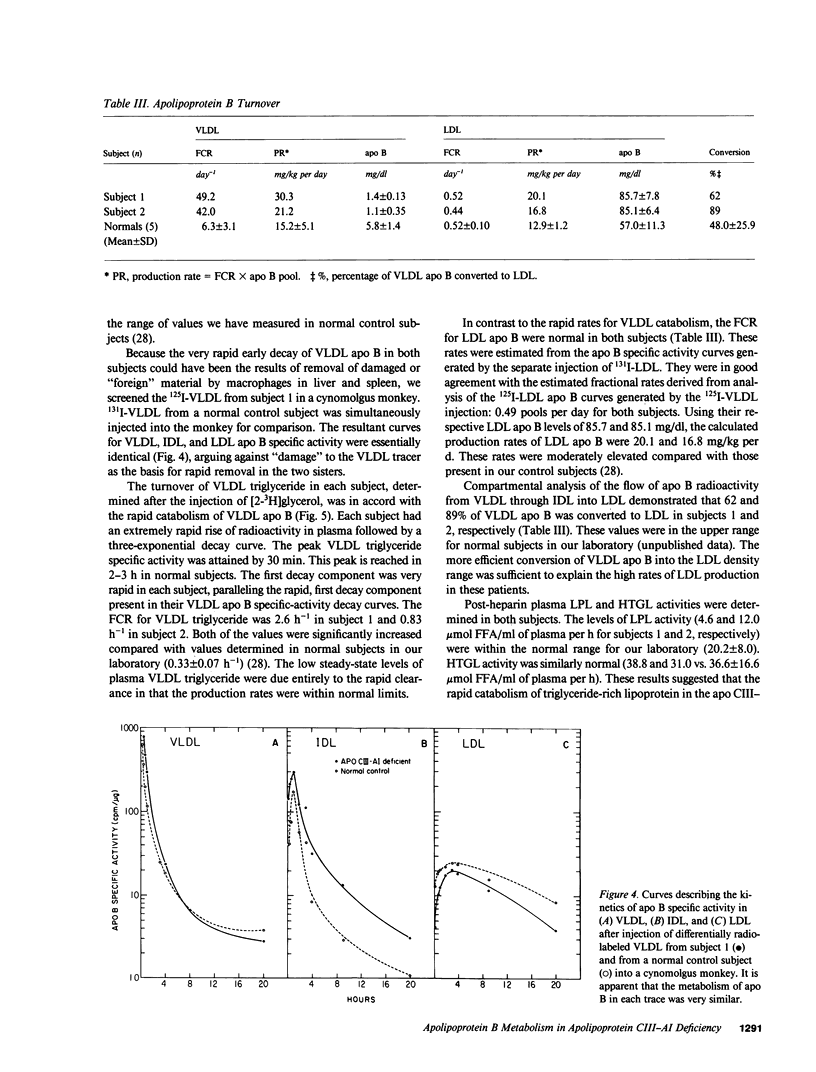
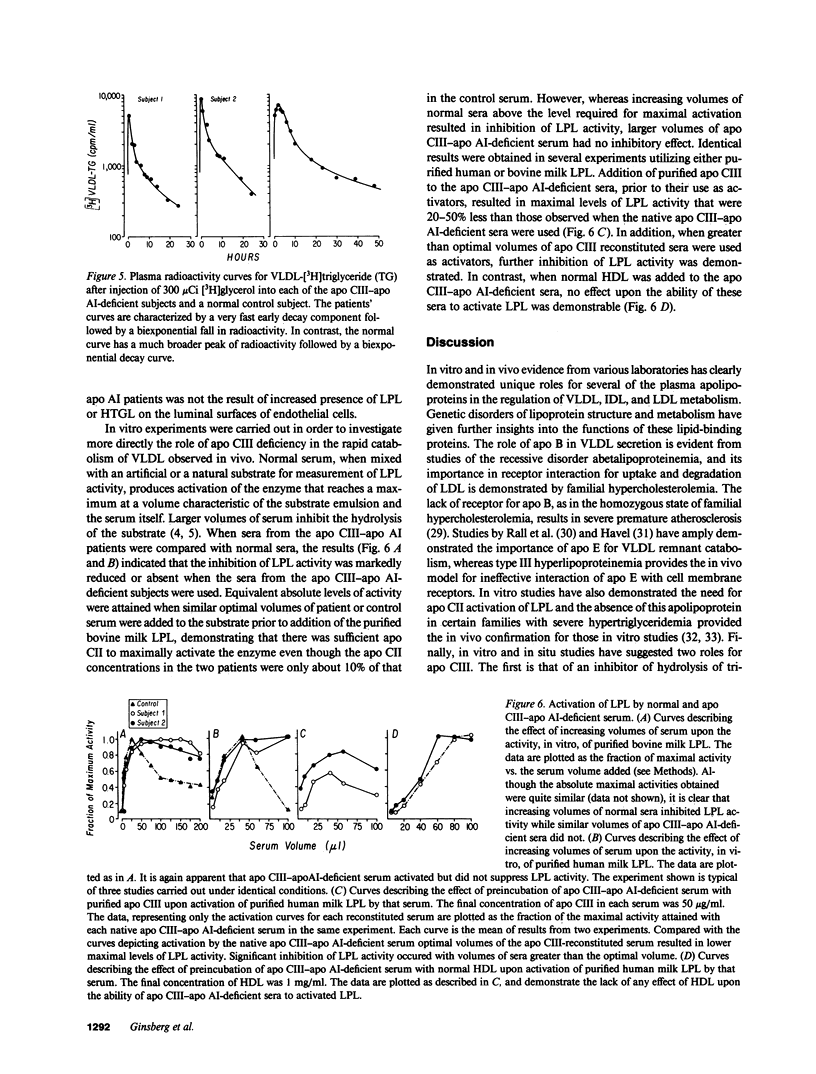



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belfrage P., Vaughan M. Simple liquid-liquid partition system for isolation of labeled oleic acid from mixtures with glycerides. J Lipid Res. 1969 May;10(3):341–344. [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Bisgaier C. L., Sachdev O. P., Megna L., Glickman R. M. Distribution of apolipoprotein A-IV in human plasma. J Lipid Res. 1985 Jan;26(1):11–25. [PubMed] [Google Scholar]
- Breckenridge W. C., Alaupovic P., Cox D. W., Little J. A. Apolipoprotein and lipoprotein concentrations in familial apolipoprotein C-II deficiency. Atherosclerosis. 1982 Aug;44(2):223–235. doi: 10.1016/0021-9150(82)90116-2. [DOI] [PubMed] [Google Scholar]
- Brown W. V., Baginsky M. L. Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochem Biophys Res Commun. 1972 Jan 31;46(2):375–382. doi: 10.1016/s0006-291x(72)80149-9. [DOI] [PubMed] [Google Scholar]
- Finley P. R., Schifman R. B., Williams R. J., Lichti D. A. Cholesterol in high-density lipoprotein: use of Mg2+/dextran sulfate in its enzymic measurement. Clin Chem. 1978 Jun;24(6):931–933. [PubMed] [Google Scholar]
- Forte T. M., Nichols A. V., Krauss R. M., Norum R. A. Familial apolipoprotein AI and apolipoprotein CIII deficiency. Subclass distribution, composition, and morphology of lipoproteins in a disorder associated with premature atherosclerosis. J Clin Invest. 1984 Nov;74(5):1601–1613. doi: 10.1172/JCI111576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funke H., Boyles J., Weisgraber K. H., Ludwig E. H., Hui D. Y., Mahley R. W. Uptake of apolipoprotein E-containing high density lipoproteins by hepatic parenchymal cells. Arteriosclerosis. 1984 Sep-Oct;4(5):452–461. doi: 10.1161/01.atv.4.5.452. [DOI] [PubMed] [Google Scholar]
- Gibson J. C., Rubinstein A., Brown W. V., Ginsberg H. N., Greten H., Norum R., Kayden H. Apo E-containing lipoproteins in low or high density lipoprotein deficiency. Arteriosclerosis. 1985 Jul-Aug;5(4):371–380. doi: 10.1161/01.atv.5.4.371. [DOI] [PubMed] [Google Scholar]
- Gibson J. C., Rubinstein A., Bukberg P. R., Brown W. V. Apolipoprotein E-enriched lipoprotein subclasses in normolipidemic subjects. J Lipid Res. 1983 Jul;24(7):886–898. [PubMed] [Google Scholar]
- Ginsberg H. N., Le N. A., Gibson J. C. Regulation of the production and catabolism of plasma low density lipoproteins in hypertriglyceridemic subjects. Effect of weight loss. J Clin Invest. 1985 Feb;75(2):614–623. doi: 10.1172/JCI111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H., Le N. A., Mays C., Gibson J., Brown W. V. Lipoprotein metabolism in nonresponders to increased dietary cholesterol. Arteriosclerosis. 1981 Nov-Dec;1(6):463–470. doi: 10.1161/01.atv.1.6.463. [DOI] [PubMed] [Google Scholar]
- Goldberg I. J., Le N. A., Paterniti J. R., Jr, Ginsberg H. N., Lindgren F. T., Brown W. V. Lipoprotein metabolism during acute inhibition of hepatic triglyceride lipase in the cynomolgus monkey. J Clin Invest. 1982 Dec;70(6):1184–1192. doi: 10.1172/JCI110717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R. B., Karlin J. B., Juhn D. J., Scanu A. M., Edelstein C., Rubenstein A. H. Characterization and measurement of human apolipoprotein A-II by radioimmunoassay. J Lipid Res. 1980 Sep;21(7):902–912. [PubMed] [Google Scholar]
- Grundy S. M., Mok H. Y., Zech L., Steinberg D., Berman M. Transport of very low density lipoprotein triglycerides in varying degrees of obesity and hypertriglyceridemia. J Clin Invest. 1979 Jun;63(6):1274–1283. doi: 10.1172/JCI109422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J. Familial dysbetalipoproteinemia. New aspects of pathogenesis and diagnosis. Med Clin North Am. 1982 Mar;66(2):441–454. doi: 10.1016/s0025-7125(16)31429-8. [DOI] [PubMed] [Google Scholar]
- Havel R. J., Shore V. G., Shore B., Bier D. M. Role of specific glycopeptides of human serum lipoproteins in the activation of lipoprotein lipase. Circ Res. 1970 Oct;27(4):595–600. doi: 10.1161/01.res.27.4.595. [DOI] [PubMed] [Google Scholar]
- Huttunen J. K., Ehnholm C., Kekki M., Nikkilä E. A. Post-heparin plasma lipoprotein lipase and hepatic lipase in normal subjects and in patients with hypertriglyceridaemia: correlations to sex, age and various parameters of triglyceride metabolism. Clin Sci Mol Med. 1976 Apr;50(4):249–260. doi: 10.1042/cs0500249. [DOI] [PubMed] [Google Scholar]
- Janus E. D., Nicoll A., Wootton R., Turner P. R., Magill P. J., Lewis B. Quantitative studies of very low density lipoprotein: conversion to low density lipoprotein in normal controls and primary hyperlipidaemic states and the role of direct secretion of low density lipoprotein in heterozygous familial hypercholesterolaemia. Eur J Clin Invest. 1980 Apr;10(2 Pt 1):149–159. doi: 10.1111/j.1365-2362.1980.tb02075.x. [DOI] [PubMed] [Google Scholar]
- Karathanasis S. K., McPherson J., Zannis V. I., Breslow J. L. Linkage of human apolipoproteins A-I and C-III genes. 1983 Jul 28-Aug 3Nature. 304(5924):371–373. doi: 10.1038/304371a0. [DOI] [PubMed] [Google Scholar]
- Karathanasis S. K., Norum R. A., Zannis V. I., Breslow J. L. An inherited polymorphism in the human apolipoprotein A-I gene locus related to the development of atherosclerosis. Nature. 1983 Feb 24;301(5902):718–720. doi: 10.1038/301718a0. [DOI] [PubMed] [Google Scholar]
- Kesaniemi Y. A., Grundy S. M. Significance of low density lipoprotein production in the regulations of plasma cholesterol level in man. J Clin Invest. 1982 Jul;70(1):13–22. doi: 10.1172/JCI110585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen P. K., Ehnolm C. Effect of serum and C-apoproteins from very low density lipoproteins on human postheparin plasma hepatic lipase. FEBS Lett. 1976 Jun 15;65(3):354–357. doi: 10.1016/0014-5793(76)80145-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le N. A., Melish J. S., Roach B. C., Ginsberg H. N., Brown W. V. Direct measurement of apoprotein B specific activity in 125I-labeled lipoproteins. J Lipid Res. 1978 Jul;19(5):578–584. [PubMed] [Google Scholar]
- Matsuoka N., Shirai K., Johnson J. D., Kashyap M. L., Srivastava L. S., Yamamura T., Yamamoto A., Saito Y., Kumagai A., Jackson R. L. Effects of apolipoprotein C-II (apoC-II) on the lipolysis of very low density lipoproteins from apoC-II deficient patients. Metabolism. 1981 Aug;30(8):818–824. doi: 10.1016/0026-0495(81)90029-9. [DOI] [PubMed] [Google Scholar]
- Nestel P. J., Connor W. E., Reardon M. F., Connor S., Wong S., Boston R. Suppression by diets rich in fish oil of very low density lipoprotein production in man. J Clin Invest. 1984 Jul;74(1):82–89. doi: 10.1172/JCI111422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norum R. A., Lakier J. B., Goldstein S., Angel A., Goldberg R. B., Block W. D., Noffze D. K., Dolphin P. J., Edelglass J., Bogorad D. D. Familial deficiency of apolipoproteins A-I and C-III and precocious coronary-artery disease. N Engl J Med. 1982 Jun 24;306(25):1513–1519. doi: 10.1056/NEJM198206243062503. [DOI] [PubMed] [Google Scholar]
- Pittman R. C., Khoo J. C., Steinberg D. Cholesterol esterase in rat adipose tissue and its activation by cyclic adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1975 Jun 25;250(12):4505–4511. [PubMed] [Google Scholar]
- Quarfordt S. H., Michalopoulos G., Schirmer B. The effect of human C apolipoproteins on the in vitro hepatic metabolism of triglyceride emulsions in the rat. J Biol Chem. 1982 Dec 25;257(24):14642–14647. [PubMed] [Google Scholar]
- Rall S. C., Jr, Weisgraber K. H., Innerarity T. L., Mahley R. W. Identical structural and receptor binding defects in apolipoprotein E2 in hypo-, normo-, and hypercholesterolemic dysbetalipoproteinemia. J Clin Invest. 1983 Apr;71(4):1023–1031. doi: 10.1172/JCI110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon M. F., Sakai H., Steiner G. Roles of lipoprotein lipase and hepatic triglyceride lipase in the catabolism in vivo of triglyceride-rich lipoproteins. Arteriosclerosis. 1982 Sep-Oct;2(5):396–402. doi: 10.1161/01.atv.2.5.396. [DOI] [PubMed] [Google Scholar]
- Shelburne F., Hanks J., Meyers W., Quarfordt S. Effect of apoproteins on hepatic uptake of triglyceride emulsions in the rat. J Clin Invest. 1980 Mar;65(3):652–658. doi: 10.1172/JCI109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sniderman A., Shapiro S., Marpole D., Skinner B., Teng B., Kwiterovich P. O., Jr Association of coronary atherosclerosis with hyperapobetalipoproteinemia [increased protein but normal cholesterol levels in human plasma low density (beta) lipoproteins]. Proc Natl Acad Sci U S A. 1980 Jan;77(1):604–608. doi: 10.1073/pnas.77.1.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windler E., Chao Y., Havel R. J. Regulation of the hepatic uptake of triglyceride-rich lipoproteins in the rat. Opposing effects of homologous apolipoprotein E and individual C apoproteins. J Biol Chem. 1980 Sep 10;255(17):8303–8307. [PubMed] [Google Scholar]
- Windler E., Havel R. J. Inhibitory effects of C apolipoproteins from rats and humans on the uptake of triglyceride-rich lipoproteins and their remnants by the perfused rat liver. J Lipid Res. 1985 May;26(5):556–565. [PubMed] [Google Scholar]


