Damage to the adult mammalian central nervous system (CNS) often results in persistent neurological deficits with limited recovery of functions. The past decade has seen increasing research efforts in neural regeneration research with the ultimate goal of achieving functional recovery. Many studies have focused on prevention of further neural damage and restoration of functional connections that are compromised after injury or pathological damage. Compared to the peripheral nervous system, the failure of the adult CNS to regenerate is largely attributed to two basic aspects: inhibitory environmental influences and decreased growth capabilities of adult CNS neurons. Since early demonstration of successful growth of injured CNS axons into grafted peripheral nerve (David and Aguayo, 1981), multiple CNS axonal growth inhibitory factors have been identified and are mainly associated with degenerating CNS myelin (such as Nogo, MAG, OMgp) and with glial scar (such as chondroitin sulfate proteoglycans, CSPGs) (Yiu and He, 2006). However, blockade of these extracellular inhibitory signals alone is often insufficient for the majority of injured axons to achieve long-distance regeneration, as intrinsic regenerative capacity of mature CNS neurons is also a critical determinant for axon re-growth(Sun et al., 2011). Combinatory strategies that enhance neuronal growth and in the meantime overcome environmental inhibitory cues appear to confer better axonal regeneration and neural repair (Wang et al., 2012).
While animal models are instrumental and indispensable to our understanding of CNS responses to injury and investigation of intervention strategies and functional regeneration, in vitro models have been designed to address specific and unique questions due to their accessibilities to experimental manipulations and relatively low cost. For instance, early findings from in vitro culture experiments formed the basis for the concept of CNS myelin-associated inhibitory molecules such as Nogo (Schwab and Thoenen, 1985; Chen et al., 2000; GrandPre et al., 2000). Attempts to identify compounds that overcome the inhibition of CNS myelin and CSPGs on neurite outgrowth in culture have revealed key neuronal signaling components that mediate the inhibitory effects of myelin and CSPGs (Sivasankaran et al., 2004). However, conventional culture system often has to use neuronal cultures at low cell density and for only a short period of time due to technical difficulties in monitoring and quantifying axonal growth. To develop effective CNS regenerative strategies, fast and reliable assessment of axonal growth would be critical not only to identify and select potentially interesting candidate molecules that promote axon extension over inhibitory molecules, but also to rule out poor ones. Equally important are considerations that neurons are highly polarized cells and that damaged axon could be extending far away from the cell body and encountering drastically different microenvironment than that of the soma. As such, signaling events elicited by extrinsic factors are most likely spatially regulated and may have different functional outputs. Over the years, compartmentalized neuronal culture systems have been developed. Now the emergence of microfluidics technology offers many advantages and versatilities for axonal growth and regeneration studies (Figure 1).
Figure 1.
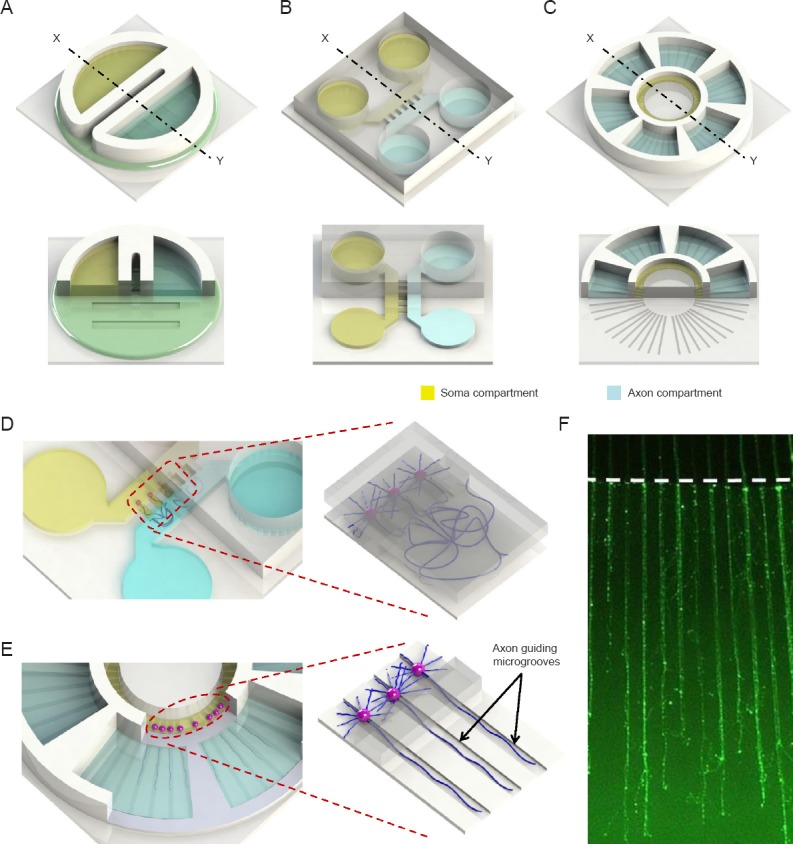
Schematic illustrations of compartmentalized neuron culture platforms for axon isolation.
(A) Conventional Campenot chamber. (B) The first generation of microfluidic-based compartmentalized neuron culture platform by Taylor et al. (2005). (C) Axon growth/regeneration quantification microchip capable of guiding axonal growth by Park et al. (2014). (D, E) Close-up of (B) and (C), respectively, showing isolation scheme of the platform. (F) Isolated/guided axons inside the axon compartment of (E) at 11 days in vitro (axons were visualized with Calcein-AM). White dotted lines indicate the axon compartment boundary.
Microfluidics: definition, application and advantages in axonal growth/regeneration studies
Microfluidics utilizes microfabrication techniques to produce microdevices with accurately patterned features in the range of 0.1 μm–1 mm to precisely manipulate small volumes of fluids. Traditionally, silicon and glass were major materials for microfabrication; however, polydimethylsiloxane polymer-based microfluidic devices are the most widely used due to their advantages in biocompatibility, low cost, optical transparency, practical scalability, gas permeability and easy fabrication. In the field of neuroscience, microfluidic devices have been increasingly used to achieve spatial-temporal control of cellular microenvironments such as those of the axon and soma (for review see (Millet and Gillette, 2012; Harink et al., 2013; Park et al., 2013)) to investigate axon elongation, local signaling events (Hengst et al., 2009; Taylor et al., 2009) as well as interactions with other cells such as oligodendroglia (Park et al., 2012), astrocytes (Li et al., 2012) and microglia (Hosmane et al., 2012).
We recently presented a microchip system that is capable of isolating CNS axons from neuronal cell bodies for quick and easy quantitative axonal growth analysis (Park et al., 2014) (Figure 1C, E, F). Similar to the earlier compartmentalized microfluidic device (Taylor et al., 2005) (Figure 1B, D), the microchip utilizes height difference of microchannels to isolate axons from neuronal somata. The shallow microgroove (3 μm) blocks the passage of cell bodies while allowing axonal growth along the microchannel into the axon compartment. The hydrostatic pressure produced by different volumes filled in each chamber then provides fluidic isolation of the two compartments. Rather than random growth in the axonal compartment, directed axonal extension is readily achieved by physical barrier with microgrooves pre-patterned on the bottom of the substrate connecting the central soma compartment and the surrounding axon compartments (Figure 1C, E). Each of the six satellite axon compartments is connected to the central chamber. The unique feature of this design is that the microgrooves not only isolate axons but also continue to physically guide axons to grow in straight lines, thereby allows easy experimental manipulation and automated quantitative analysis of axon growth using an image processing algorithm. Another advantage of the microdevice is that axons can be fully established and isolated without the use of nerve growth factor and prior to any experimental manipulations such as transections and treatments. Using this device and cortical neurons, we tested the effect of several extracellular matrix components and unexpectedly found an opposing effect of CSPGs on axonal growth depending on whether the neuronal cell body or the distal axon is exposed to CSPGs (Park et al., 2014). The device is a first step for further development of microfluidic systems with high throughput capacities that could be exploited to investigate key signaling components and identify potential compounds that promote axon growth and regeneration.
Current status and future direction
To achieve functional recovery, growing axons have to navigate through complex microenvironment, guided by gradients of attractive and repulsive molecules, to reach and innervate their targets. Microfluidics can be readily adapted to generate controllable distributions of guidance molecules and has thus emerged as a promising new approach to provide new insights into the mechanism of axon guidance (Dupin et al., 2013).
Glia play fundamental roles in injury and tissue remodeling and repair. In vitro myelination has been shown successfully using microfluidic neuron-oligodendrocyte co-culture systems (Park et al., 2012; Yang et al., 2012). Considering myelinating and non-myelinating roles of oligodendrocytes as well as diverse functions of astrocyte and microglia such as regulation of synaptic properties, control of the peri-axonal ion microenvironment and immune-modulation, understanding neuron-glia interactions during normal development as well as after injury is an important aspect of neural regenerative research. Moreover, to better understand how neuron grows and regenerates functionally in the nervous system, integrated “tissue-on-chip” model systems that better mimic the complex and systemic environments of in vivo situations could provide in vitro platforms for preclinical testing of potential therapeutic interventions. In this regard, a few 3D microfluidic neural culture devices have been developed and trans-differentiation of human adipose-derived stem cells into neurons has been achieved (Choi et al., 2011; Kunze et al., 2011).
In summary, understanding the molecular mechanisms governing neural degeneration and regeneration is a foundation for the development of potential protective and regenerative strategies for many neurological disorders and injuries. Microfluidics-based devices have emerged as new culture platforms for neurobiology research due to their excellent spatial and temporal control capacities, easy assembly, reproducibility, flexibility, amenability in imaging and biochemical analyses as well as high-throughput potentials, and are likely to play an increasingly important role in establishing physiologically relevant culture/tissue models, and help to answer some important and fundamental questions such as how growing axons convert external cues into directed movement and innervate targets and how microenvironments reminiscent those of injured CNS affect those decisions.
Footnotes
Conflicts of interest: None declared.
Funding: This work was supported by grants from NIH National Institute of Mental Health MH085267 and National Institute of Neurological Disorders and Stroke NS060017.
References
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- Choi J, Kim S, Jung J, Lim Y, Kang K, Park S, Kang S. Wnt5a-mediating neurogenesis of human adipose tissue-derived stem cells in a 3D microfluidic cell culture system. Biomaterials. 2011;32:7013–7022. doi: 10.1016/j.biomaterials.2011.05.090. [DOI] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Dupin I, Dahan M, Studer V. Investigating axonal guidance with microdevice-based approaches. J Neurosci. 2013;33:17647–17655. doi: 10.1523/JNEUROSCI.3277-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Harink B, Le Gac S, Truckenmuller R, van Blitterswijk C, Habibovic P. Regeneration-on-a-chip? The perspectives on use of microfluidics in regenerative medicine. Lab Chip. 2013;13:3512–3528. doi: 10.1039/c3lc50293g. [DOI] [PubMed] [Google Scholar]
- Hengst U, Deglincerti A, Kim HJ, Jeon NL, Jaffrey SR. Axonal elongation triggered by stimulus-induced local translation of a polarity complex protein. Nat Cell Biol. 2009;11:1024–1030. doi: 10.1038/ncb1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmane S, Tegenge MA, Rajbhandari L, Uapinyoying P, Kumar NG, Thakor N, Venkatesan A. Toll/interleukin-1 receptor domain-containing adapter inducing interferon-â mediates microglial phagocytosis of degenerating axons. J Neurosci. 2012;32:7745–7757. doi: 10.1523/JNEUROSCI.0203-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze A, Giugliano M, Valero A, Renaud P. Micropatterning neural cell cultures in 3D with a multi-layered scaffold. Biomaterials. 2011;32:2088–2098. doi: 10.1016/j.biomaterials.2010.11.047. [DOI] [PubMed] [Google Scholar]
- Li L, Ren L, Liu W, Wang JC, Wang Y, Tu Q, Xu J, Liu R, Zhang Y, Yuan MS, Li T, Wang J. Spatiotemporally controlled and multifactor involved assay of neuronal compartment regeneration after chemical injury in an integrated microfluidics. Anal Chem. 2012;84:6444–6453. doi: 10.1021/ac3013708. [DOI] [PubMed] [Google Scholar]
- Millet LJ, Gillette MU. New perspectives on neuronal development via microfluidic environments. Trends Neurosci. 2012;35:752–761. doi: 10.1016/j.tins.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Koito H, Li J, Han A. Multi-compartment neuron-glia co-culture platform for localized CNS axon-glia interaction study. Lab Chip. 2012;12:3296–3304. doi: 10.1039/c2lc40303j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kim S, Park SI, Choe Y, Li J, Han A. A microchip for quantitative analysis of CNS axon growth under localized biomolecular treatments. J Neurosci Methods. 2014;221:166–174. doi: 10.1016/j.jneumeth.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Kim HJ, Kang MW, Jeon NL. Advances in microfluidics-based experimental methods for neuroscience research. Lab on a Chip. 2013;13:509–521. doi: 10.1039/c2lc41081h. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Thoenen H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J Neurosci. 1985;5:2415–2423. doi: 10.1523/JNEUROSCI.05-09-02415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasankaran R, Pei J, Wang KC, Zhang YP, Shields CB, Xu XM, He Z. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci. 2004;7:261–268. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G, Yankner BA, He Z. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480:372–375. doi: 10.1038/nature10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat Methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Berchtold NC, Perreau VM, Tu CH, Li Jeon N, Cotman CW. Axonal mRNA in uninjured and regenerating cortical mammalian axons. J Neurosci. 2009;29:4697–4707. doi: 10.1523/JNEUROSCI.6130-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hasan O, Arzeno A, Benowitz LI, Cafferty WB, Strittmatter SM. Axonal regeneration induced by blockade of glial inhibitors coupled with activation of intrinsic neuronal growth pathways. Exp Neurol. 2012;237:55–69. doi: 10.1016/j.expneurol.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang I, Gary D, Malone M, Dria S, Houdayer T, Belegu V, McDonald J, Thakor N. Axon myelination and electrical stimulation in a microfluidic, compartmentalized cell culture platform. Neuromolecular Med. 2012;14:112–118. doi: 10.1007/s12017-012-8170-5. [DOI] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]


