Abstract
Neuroprotection and neuroregeneration are two of the most promising disease-modifying therapies for the incurable and widespread Parkinson's disease. In Parkinson's disease, progressive degeneration of nigrostriatal dopaminergic neurons causes debilitating motor symptoms. Neurotrophic factors play important regulatory roles in the development, survival and maintenance of specific neuronal populations. These factors have the potential to slow down, halt or reverse the loss of nigrostriatal dopaminergic neurons in Parkinson's disease. Several neurotrophic factors have been investigated in this regard. This review article discusses the neurodevelopmental roles and therapeutic potential of three dopaminergic neurotrophic factors: glial cell line-derived neurotrophic factor, neurturin and growth/differentiation factor 5.
Keywords: Parkinson's disease, neuroprotection, neurotrophic factors, nervous system development, nigrostriatal dopaminergic neurons, glial cell line-derived neurotrophic factor, neurturin, growth/differentiation factor 5
Parkinson's disease (PD) is the second-most common neurodegenerative disease, affecting over 10 million people worldwide. The incidence of PD increases with age, and its prevalence is predicted to double by 2040 due to significant increases in life-expectancy. PD is a movement disorder caused by the progressive degeneration of the ventral midbrain (VM) dopaminergic (DA) neurons which project to the dorsal striatum, disrupting the neural circuitry responsible for regulating voluntary movement. Despite decades of research, PD remains incurable. Current treatments are symptomatic and centre on dopamine-replacement strategies, which are effective for a limited time; however, the neurodegeneration continues unabated (Toulouse and Sullivan, 2008). Presently, two of the most promising disease-modifying therapies are: 1) Neuroprotection by administering neurotrophic factor(s) to protect and regenerate the remaining nigrostriatal DA pathway (Figure 1) and 2) Neurorepair and Neuroregeneration by transplantating new DA neurons to replace those that have been lost. Following the recent commencement of clinical trials using neurotrophic factors, this article comments on their therapeutic potential in PD.
Figure 1.
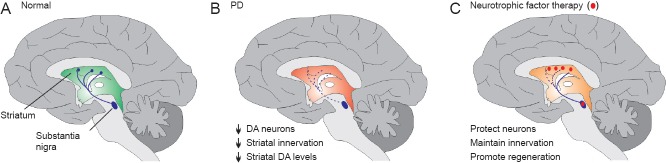
The potential of neurotrophic factors in PD.
(A) Cartoon of the intact nigrostriatal pathway showing ascending axons (blue) of DA neurons originating in the substantia nigra of the VM and innervating the striatum. (B) In PD, the progressive degeneration of these neurons leads to a loss of DA innervation of the striatum and consequently a reduction in striatal dopamine levels, which is one of the pathological hallmarks of PD. (C) Exogenous application of neurotrophic factors (red) to the striatum and/or VM has the potential to protect the remaining DA neurons from the degenerative process, maintain nigrostriatal innervation and promote regeneration of the damaged nigrostriatal pathway. DA: Dopaminergic; PD: Parkinson's disease; VM: ventral midbrain.
Neurotrophic factors are secreted proteins that play important roles in the development and maintenance of the nervous system, influencing the development of distinct neuronal populations, and maintaining the survival and neuritic arbors of mature neurons into adulthood. Given these properties, exogenous administration of neurotrophic factors to the PD midbrain or striatum could potentially slow down, halt or even reverse the loss of nigrostriatal DA neurons. The potential of several neurotrophic factors has been investigated in this regard, many of which have been members of the transforming growth factor β (TGF-β) superfamily (Hegarty et al., 2014a). This review article will discuss the neurodevelopmental roles and therapeutic potential of three neurotrophic factors that are members of the TGF-β superfamily: 1) glial cell line-derived neurotrophic factor (GDNF), 2) neurturin and 3) growth/differentiation factor (GDF)5.
GDNF is the neurotrophic factor that has attracted the most attention in terms of its potential as a neuroprotective/regenerative therapy for PD. GDNF was isolated for its ability to promote the survival of cultured VM DA neurons (Lin et al., 1993). Many subsequent studies replicated and built upon these findings in vitro (Clarkson et al., 1997; Eggert et al., 1999; Hegarty et al., 2014a). In addition, GDNF has been shown to promote the neurite growth of VM DA neurons in vitro (Lin et al., 1993; Hegarty et al., 2014a). Taken together with its expression in the developing and adult rat midbrain and striatum (Choi-Lundberg and Bohn, 1995; Gavin et al., 2013), these findings suggest a role for GDNF in the establishment and maintenance of the nigrostriatal DA pathway. Indeed, these in vitro findings have been supported in vivo. GDNF has consistently been shown to promote the survival of VM DA neurons in vivo, and to protect them in animal models of PD (Tomac et al., 1995; Granholm et al., 1997, 2000; Sullivan et al., 1998; Kholodilov et al., 2004; Hegarty et al., 2014a). Furthermore, the neurite growth-promoting effects of GDNF on VM DA neurons have also been demonstrated in vivo (Granholm et al., 2000; Kholodilov et al., 2004). In terms of clinical relevance, this large body of evidence led to the initiation of clinical trials of GDNF administration in PD patients.
The clinical application of neurotrophic factors is hampered by the fact that they do not cross the blood-brain barrier, and are rapidly degraded in vivo. The initial GDNF clinical trial–a randomised trial using intraventricular delivery of GDNF in PD patients–reported no significant benefits of treatment over placebo, likely because GDNF did not reach the striatum in sufficient amounts (Nutt et al., 2003). Promising results emerged from two further open-label trials, which directly infused GDNF into the putamen (Gill et al., 2003; Slevin et al., 2005). Both trials reported improvements in patients’ motor symptoms, without serious side-effects. These two open-label studies demonstrated the feasibility and sustainability of GDNF treatment by intraputaminal infusion, while also providing proof-of-principle for neuroprotective therapy. However, a subsequent placebo-controlled trial showed no significant motor improvements in PD patients (Nutt et al., 2003; Lang et al., 2006). The discrepancies between the open-label and placebo-controlled trials may have been due to variations in surgical methodologies, patient selection and the placebo effect. Furthermore, it is generally accepted that GDNF therapy requires further development in pre-clinical trials. Indeed, it remains unclear how GDNF mediates its DA neurotrophic effects, and why GDNF failed to exert neurotrophic effects in the α-synuclein model of PD (Decressac et al., 2011). The clinical failure of GDNF needs to be addressed and explained at a molecular and cellular level. In addition to this, the lack of neuroprotective action of GDNF against α-synuclein toxicity raises important concerns regarding the use of cellular models and neurotoxic animal models to represent true synucleinopathies. The utilisation of the α-synuclein model of PD in pre-clinical neuroprotective studies will be crucial for overcoming this challenge.
The employment of alternative methodologies, such as the use of viral vectors, may provide more long-term, targeted neurotrophic support for the nigrostriatal DA pathway in PD. Indeed, the gene therapy approach has been used in clinical trials on PD patients to deliver neurturin, a member of the GDNF family which is as potent as GDNF at promoting the survival of midbrain DA neurons in vitro and in animal models (Horger et al., 1998; Tseng et al., 1998; Hegarty et al., 2014a). Following promising results in animal studies using AAV2-mediated gene transfer, an open-label clinical trial using AAV2 vector to deliver neurturin was initiated in PD patients by Ceregene (Marks et al., 2008). This initial trial showed promising symptomatic improvements; however, a subsequent double-blind trial reported only modest benefits in neurturin-treated patients (Marks et al., 2010). This failure was thought to be due to insufficient retrograde transport of neurturin to the VM, from its striatal injection site, which may reflect the compromised integrity of the nigrostriatal projections. Indeed, delivery of AAV2-neurturin to both VM and striatum enhances neuroprotection in PD models (Herzog et al., 2013). Thus Ceregene have designed a new trial, in which AAV2-neurturin will be injected into both striatum and VM to maximise neurturin delivery throughout the degenerating nigrostriatal system. Following evaluation of the safety and feasibility of this approach (Bartus et al., 2013), patient recruitment for this double-blind trial has now been completed.
Another TGF-β superfamily DA neurotrophic factor, GDF5, is in pre-clinical development as a therapeutic for PD. GDF5 is expressed in the developing and adult rat VM and striatum (Storm et al., 1994; Krieglstein et al., 1995; O’Keeffe et al., 2004b; Gavin et al., 2013), and has neurotrophic and protective actions on VM DA neurons in vitro that are comparable to those of GDNF (Krieglstein et al., 1995; O’Keeffe et al., 2004a; Clayton and Sullivan, 2007; O’Sullivan et al., 2010). In addition, GDF5 has been shown to promote neurite growth of rat VM DA neurons in vitro (Figure 2) (O’Keeffe et al., 2004a; Clayton and Sullivan, 2007; Hegarty et al., 2014b). Furthermore, several in vivo studies have shown that GDF5 can protect and, more importantly, regenerate adult rat nigrostriatal DA neurons in animal models of PD. GDF5 injection into the striatum or VM significantly improved motor function, protected nigrostriatal DA neurons and preserved striatal DA levels, in 6-hydroxydopamine (OHDA)-lesioned rats (Sullivan et al., 1997; Hurley et al., 2004). Pre-incubation with GDF5 improved the survival and function of grafted VM DA neurons in 6-OHDA-lesioned rats, to the same extent as GDNF (Sullivan et al., 1998). Long-term GDF5 delivery by overexpression in fetal rat VM transplants (O’Sullivan et al., 2010), or by using a GDF5-overexpressing cell line (Costello et al., 2012), significantly improves motor behaviour in 6-OHDA-lesioned rats, and the survival and function of (co-)implanted VM transplants. Furthermore, GDF5 was shown to increase neurite outgrowth of host nigrostriatal DA innervations, as well as transplanted embryonic VM DA neurons, in 6-OHDA-lesioned adult rats (Hurley et al., 2004; O’Sullivan et al., 2010; Costello et al., 2012). Taken together with its midbrain and striatal expression patterns, the survival-promoting, protective, restorative and neurite growth-promoting effects of GDF5 in vitro and in vivo suggest that GDF5 acts as a neurotrophic factor during nigrostriatal pathway development. Furthermore, GDF5 has been shown to be as potent as GDNF in PD models and thus is an ideal candidate for neurotrophic therapy. However, given the disappointing performance of GDNF clinically, it is important that further preclinical investigations are carried out with GDF5. Most pertinently, the neuroprotective actions of GDF5 should be assessed in the α-synuclein model of PD; such investigations are ongoing, as are further studies characterising GDF5's mechanism of action. GDF5 preferentially signals via a heterocomplex of BMP receptors (BMPR) type Ib (BMPRIb) and type II (BMPRII) (Nishitoh et al., 1996), which activate the canonical Smad 1/5/8 signalling pathway. These BMPRs are expressed in the developing and adult rat VM and striatum, as well as on cultured rat VM DA neurons (Hegarty et al., 2014b). We recently demonstrated that the neurotrophic effects of GDF5 on cultured VM DA neurons are dependent on BMPRIb activation of the Smad signalling pathway (Hegarty et al., 2014b). Due to the technical complexities of employing neurotrophic factors clinically, perhaps a more efficient strategy would be to directly target their downstream molecular effectors. The results of the AAV2-neurturin trial will deem whether this is a necessary alternative approach for neurotrophic therapy. Indeed, optimisation of surgical procedures, delivery methods and patient selection is still required if the use of neurotrophic factors as a therapy for PD is to ultimately become a clinical reality.
Figure 2.
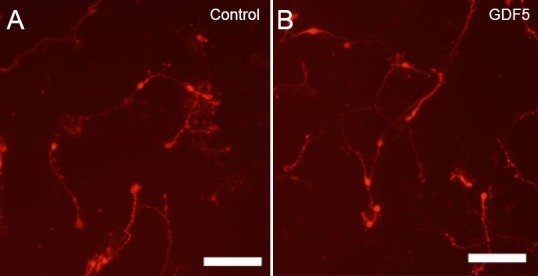
Neurotrophic factors promote the survival and growth of dopaminergic neurons in ventral midbrain.
(A, B) Representative examples of dopaminergic neurons identified by immunocytochemistry for tyrosine hydroxylase (red) in primary cultures of embryonic day 14 rat ventral midbrain. In the example shown, treatment with 10 ng/mL of growth/differentiation factor 5 (GDF5) for 4 days in vitro (B) promotes the survival and growth of DA neurons. For further details see Hegarty et al. (2014b).
Despite the limited success in clinical trials, neurotrophic therapy remains one of the most promising therapies for PD. It must be noted at this point that neuroprotective therapy for PD is not limited to the use of neurotrophic factors, and various compounds (such as antioxidants) and pharmacological tools (such as calcium-channel blockers) have shown promise in this regard. However, for the purpose of this review article, we have focused on developmentally-relevant dopaminergic neurotrophic factors. We suggest that neurotrophic therapy should not be considered mutually exclusive to what is arguably the most promising disease-modifying PD therapy, neurotransplantation. Indeed, DA neurotrophic factors may improve the survival and functional integration of transplanted VM DA neurons into the local neural circuitry, which is crucial for the success of this approach. Critically, the poor survival and growth of transplanted VM DA neurons is a major impediment to this therapeutic approach. Neurotrophic factor application should be seriously considered as an adjunct to neurotransplantation in PD, as these factors have the ability to promote the survival and growth of transplanted VM DA neurons. While the potential of developmentally-relevant DA neurotrophic factors as novel therapies for PD remains very encouraging, it is necessary to carefully refine the selection of specific neurotrophic factors, to employ appropriate preclinical models and to optimise clinical delivery methods, in order to harness the full potential of these factors.
Footnotes
Funding: Studies in the authors’ laboratories are supported by grants from the Irish Research Council (R13702 and R15897; SVH/AS/G’OK), the Health Research Board of Ireland (HRA/2009/127; GO’K/AS) and Science Foundation Ireland (10/RFP/NES2786; GO’K).
Conflicts of interest: None declared.
References
- Bartus RT, Baumann TL, Siffert J, Herzog CD, Alterman R, Boulis N, Turner DA, Stacy M, Lang AE, Lozano AM, Olanow CW. Safety/feasibility of targeting the substantia nigra with AAV2-neurturin in Parkinson patients. Neurology. 2013;80:1698–1701. doi: 10.1212/WNL.0b013e3182904faa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Bohn MC. Ontogeny and distribution of glial cell line-derived neurotrophic factor (GDNF) mRNA in rat. Brain Res Dev Brain Res. 1995;85:80–88. doi: 10.1016/0165-3806(94)00197-8. [DOI] [PubMed] [Google Scholar]
- Clarkson ED, Zawada WM, Freed CR. GDNF improves survival and reduces apoptosis in human embryonic dopaminergic neurons in vitro. Cell Tissue Res. 1997;289:207–210. doi: 10.1007/s004410050867. [DOI] [PubMed] [Google Scholar]
- Clayton KB, Sullivan AM. Differential effects of GDF5 on the medial and lateral rat ventral mesencephalon. Neurosci Lett. 2007;427:132–137. doi: 10.1016/j.neulet.2007.09.025. [DOI] [PubMed] [Google Scholar]
- Costello DJ, O’Keeffe GW, Hurley FM, Sullivan AM. Transplantation of novel human GDF-5-expressing CHO cells is neuroprotective in models of Parkinson's disease. J Cell Mol Med. 2012;16:2451–2460. doi: 10.1111/j.1582-4934.2012.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decressac M, Ulusoy A, Mattsson B, Georgievska B, Romero-Ramos M, Kirik D, Bjorklund A. GDNF fails to exert neuroprotection in a rat alpha-synuclein model of Parkinson's disease. Brain. 2011;134:2302–2311. doi: 10.1093/brain/awr149. [DOI] [PubMed] [Google Scholar]
- Eggert K, Schlegel J, Oertel W, Wurz C, Krieg JC, Vedder H. Glial cell line-derived neurotrophic factor protects dopaminergic neurons from 6-hydroxydopamine toxicity in vitro. Neurosci Lett. 1999;269:178–182. doi: 10.1016/s0304-3940(99)00443-7. [DOI] [PubMed] [Google Scholar]
- Gavin AM, Walsh S, Wyatt SL, O’ Keeffe GW, Sullivan AM. 6-Hydroxydopamine induces distinct alterations in GDF5 and GDNF mRNA expression in the rat nigrostriatal system in vivo. Neurosci Lett. 2013;561:176–181. doi: 10.1016/j.neulet.2013.12.046. [DOI] [PubMed] [Google Scholar]
- Gill SS, Patel NK, Hotton GR, O’sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Mott JL, Bowenkamp K, Eken S, Henry S, Hoffer BJ, Lapchak PA, Palmer MR, van Horne C, Gerhardt GA. Glial cell line-derived neurotrophic factor improves survival of ventral mesencephalic grafts to the 6-hydroxydopamine lesioned striatum. Exp Brain Res. 1997;116:29–38. doi: 10.1007/pl00005741. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Reyland M, Albeck D, Sanders L, Gerhardt G, Hoernig G, Shen L, Westphal H, Hoffer B. Glial cell line-derived neurotrophic factor is essential for postnatal survival of midbrain dopamine neurons. J Neurosci. 2000;20:3182–3190. doi: 10.1523/JNEUROSCI.20-09-03182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty SV, Sullivan AM, O’Keeffe GW. Roles for the TGFbeta Superfamily in the Development and Survival of Midbrain Dopaminergic Neurons. Mol Neurobiol. 2014a;50:559–573. doi: 10.1007/s12035-014-8639-3. [DOI] [PubMed] [Google Scholar]
- Hegarty SV, Collins LM, Gavin AM, Roche SL, Wyatt SL, Sullivan AM, O’Keeffe GW. Canonical BMP-Smad signalling promotes neurite growth in rat midbrain dopaminergic neurons. Neuromolecular Med. 2014b;16:473–489. doi: 10.1007/s12017-014-8299-5. [DOI] [PubMed] [Google Scholar]
- Herzog CD, Brown L, Kruegel BR, Wilson A, Tansey MG, Gage FH, Johnson EM, Jr, Bartus RT. Enhanced neurotrophic distribution, cell signaling and neuroprotection following substantia nigral versus striatal delivery of AAV2-NRTN (CERE-120) Neurobiol Dis. 2013;58:38–48. doi: 10.1016/j.nbd.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Horger BA, Nishimura MC, Armanini MP, Wang LC, Poulsen KT, Rosenblad C, Kirik D, Moffat B, Simmons L, Johnson E, Jr, Milbrandt J, Rosenthal A, Bjorklund A, Vandlen RA, Hynes MA, Phillips HS. Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J Neurosci. 1998;18:4929–4937. doi: 10.1523/JNEUROSCI.18-13-04929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley FM, Costello DJ, Sullivan AM. Neuroprotective effects of delayed administration of growth/differentiation factor-5 in the partial lesion model of Parkinson's disease. Exp Neurol. 2004;185:281–289. doi: 10.1016/j.expneurol.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Kholodilov N, Yarygina O, Oo TF, Zhang H, Sulzer D, Dauer W, Burke RE. Regulation of the development of mesencephalic dopaminergic systems by the selective expression of glial cell line-derived neurotrophic factor in their targets. J Neurosci. 2004;24:3136–3146. doi: 10.1523/JNEUROSCI.4506-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieglstein K, Suter-Crazzolara C, Hotten G, Pohl J, Unsicker K. Trophic and protective effects of growth/differentiation factor 5, a member of the transforming growth factor-beta superfamily, on midbrain dopaminergic neurons. J Neurosci Res. 1995;42:724–732. doi: 10.1002/jnr.490420516. [DOI] [PubMed] [Google Scholar]
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, Taylor R, Cahn-Weiner DA, Stoessl AJ, Olanow CW, Bartus RT. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N, Vitek J, Stacy M, Turner D, Verhagen L, Bakay R, Watts R, Guthrie B, Jankovic J, Simpson R, Tagliati M, Alterman R, Stern M, Baltuch G, Starr PA. Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- Nishitoh H, Ichijo H, Kimura M, Matsumoto T, Makishima F, Yamaguchi A, Yamashita H, Enomoto S, Miyazono K. Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J Biol Chem. 1996;271:21345–21352. doi: 10.1074/jbc.271.35.21345. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr, Lozano AM, Penn RD, Simpson RK, Jr, Stacy M, Wooten GF. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- O’Keeffe GW, Dockery P, Sullivan AM. Effects of growth/differentiation factor 5 on the survival and morphology of embryonic rat midbrain dopaminergic neurones in vitro. J Neurocytol. 2004a;33:479–488. doi: 10.1007/s11068-004-0511-y. [DOI] [PubMed] [Google Scholar]
- O’Keeffe GW, Hanke M, Pohl J, Sullivan AM. Expression of growth differentiation factor-5 in the developing and adult rat brain. Brain Res Dev Brain Res. 2004b;151:199–202. doi: 10.1016/j.devbrainres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- O’sullivan DB, Harrison PT, Sullivan AM. Effects of GDF5 overexpression on embryonic rat dopaminergic neurones in vitro and in vivo. J Neural Transm. 2010;117:559–572. doi: 10.1007/s00702-010-0392-9. [DOI] [PubMed] [Google Scholar]
- Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg. 2005;102:216–222. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee SJ. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature. 1994;368:639–643. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- Sullivan AM, Pohl J, Blunt SB. Growth/differentiation factor 5 and glial cell line-derived neurotrophic factor enhance survival and function of dopaminergic grafts in a rat model of Parkinson's disease. Eur J Neurosci. 1998;10:3681–3688. doi: 10.1046/j.1460-9568.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- Sullivan AM, Opacka-Juffry J, Hotten G, Pohl J, Blunt SB. Growth/differentiation factor 5 protects nigrostriatal dopaminergic neurones in a rat model of Parkinson's disease. Neurosci Lett. 1997;233:73–76. doi: 10.1016/s0304-3940(97)00623-x. [DOI] [PubMed] [Google Scholar]
- Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- Toulouse A, Sullivan AM. Progress in Parkinson's disease-where do we stand? Prog Neurobiol. 2008;85:376–392. doi: 10.1016/j.pneurobio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Tseng JL, Bruhn SL, Zurn AD, Aebischer P. Neurturin protects dopaminergic neurons following medial forebrain bundle axotomy. Neuroreport. 1998;9:1817–1822. doi: 10.1097/00001756-199806010-00027. [DOI] [PubMed] [Google Scholar]


