Abstract
Alcohol use disorder (AUD), mild traumatic brain injury (mTBI), and posttraumatic stress disorder (PTSD) commonly co-occur (AUD + mTBI + PTSD). These conditions have overlapping symptoms which are, in part, reflective of overlapping neuropathology. These conditions become problematic because their co-occurrence can exacerbate symptoms. Therefore, treatments must be developed that are inclusive to all three conditions. Repetitive transcranial magnetic stimulation (rTMS) is non-invasive and may be an ideal treatment for co-occurring AUD + mTBI + PTSD. There is accumulating evidence on rTMS as a treatment for people with AUD, mTBI, and PTSD each alone. However, there are no published studies to date on rTMS as a treatment for co-occurring AUD + mTBI + PTSD. This review article advances the knowledge base for rTMS as a treatment for AUD + mTBI + PTSD. This review provides background information about these co-occurring conditions as well as rTMS. The existing literature on rTMS as a treatment for people with AUD, TBI, and PTSD each alone is reviewed. Finally, neurobiological findings in support of a theoretical model are discussed to inform TMS as a treatment for co-occurring AUD + mTBI + PTSD. The peer-reviewed literature was identified by targeted literature searches using PubMed and supplemented by cross-referencing the bibliographies of relevant review articles. The existing evidence on rTMS as a treatment for these conditions in isolation, coupled with the overlapping neuropathology and symptomology of these conditions, suggests that rTMS may be well suited for the treatment of these conditions together.
Keywords: transcranial magnetic stimulation, traumatic brain injury, posttraumatic stress disorder, treatment, neuroimaging, substance use disorders, addiction, co-morbidity, mental health disorders, behavioral health, neuroimaging, non-invasive brain stimulation
Introduction
Alcohol use disorder (AUD), mild traumatic brain injury (mTBI), and posttraumatic stress disorder (PTSD) share symptoms (Pape et al., 2013) and neuropathology. These conditions commonly co-occur, particularly in veteran and military populations. Unfortunately, there are few treatment options when these conditions co-occur. Treatment development, in fact, has involved the conduct of clinical trials with stringent eligibility criteria precluding the enrollment of people with co-occurring AUD, mTBI and PTSD (AUD + mTBI + PTSD). To address the need to begin developing treatments for AUD + mTBI + PTSD, this review paper elucidates the scientific evidence supporting use of rTMS. Repetitive TMS has potential as a treatment because there is evidence of beneficial effects for AUD (Mishra et al., 2010; De Ridder et al., 2011; Rapinesi et al., 2013; Mishra et al., 2014), TBI (Louise-Bender Pape et al., 2009; Cosentino et al., 2010; Bonni et al., 2013; Kreuzer et al., 2013; Koski et al., 2014), and PTSD (Grisaru et al., 1998; McCann et al., 1998; Rosenberg et al., 2002; Cohen et al., 2004; Osuch et al., 2009; Boggio et al., 2010; Watts et al., 2012; Isserles et al., 2013; Nakama et al., 2013) when occurring in isolation. To inform future development of rTMS as a treatment for AUD + mTBI + PTSD, we synthesize this evidence and develop a theory within the framework of a neurobiological model.
Each condition in isolation: definitions, prevalence and symptoms
Alcohol use disorder (AUD)
AUD is characterized by impaired control over drug seeking and use that often occurs in association with severe tolerance and episodes of withdrawal, social and occupational impairments, and irritability or intense cravings when alcohol is not available (American Psychiatric Association, 2004). Reports on the United States (US) population indicate that AUD rates reach as high as 9% (Falk et al., 2008) and that 17% of the US population engages in binge drinking (i.e., > four or five drinks in one sitting) (Kanny et al., 2013). Worldwide AUD prevalence rates range from 0% to 16% (World Health Organization, 2014). Hazardous drinking rates in US veterans returning from Iraq and Afghanistan are reported to be even higher, reaching 36% (Burnett-Zeigler et al., 2011). Recent studies indicate that AUD prevalence rates are also higher when mTBI (Carlson et al., 2010) co-occurs with PTSD (mTBI+PTSD) (Graham and Cardon, 2008; Polusny et al., 2011).
Alcohol craving, or the urge to drink, is a symptom of AUD and is important to assess because craving leads to relapse (Bottlender and Soyka, 2004; Chakravorty et al., 2010) and predicts drinking behavior (Flannery et al., 2003). Alcohol-related environmental cues can take on the rewarding properties of alcohol over time. In fact, repeated exposure to these cues in the presence of alcohol can induce craving in the absence of alcohol which can ultimately lead to excessive drinking and relapse (Lowman et al., 2000).
Traumatic brain injury (TBI)
TBI occurs after a blow to the head resulting in loss of or altered consciousness. Mild TBI is characterized by brief (≤ 30 minutes) loss of or alteration of (≤ 24 hours) consciousness due to a head injury resulting from external forces (ACRM, 1993; O’Neil et al., 2013). A brief period (≤ 24 hours) of posttraumatic amnesia can also occur (ACRM, 1993; O’Neil et al., 2013). The injury can be followed by cognitive impairments (e.g., difficulty with concentration and memory), physical symptoms (e.g., headache, nausea), or behavioral changes (e.g., irritability) which may or may not persist (ACRM, 1993). Mild TBI is also defined by normal brain morphology detected by a clinical computerized tomography (CT) or magnetic resonance imaging (MRI) scan (ACRM, 1993; O’Neil et al., 2013). Head injuries resulting in loss of consciousness for greater than 30 minutes, posttraumatic amnesia greater than 24 hours, and/or CT or MRI abnormalities are defined as moderate to severe TBI (O’Neil et al., 2013).
An estimated 1.2 million TBIs occur each year in the US with the majority being mild (National Center for Injury Prevention and Control, 2002). In the US population between 1998 and 2000, mTBI incidence rates were reported as 503 per 100,000 emergency room visits (Bazarian et al., 2005). The World Health Organization's mTBI Task Force also reports that the majority of head injuries are mild and further that the incidence rate of mTBI is estimated as 600 in 100,000 based on hospital and self-report survey data (Holm et al., 2005).
Because of increased exposure to explosions, US military veterans are even more vulnerable to mTBI. Nearly 20% of Iraq and Afghanistan conflict veterans experienced a mTBI (Hoge et al., 2008; Tanielian and Jaycox, 2008). Athletes engaged in sports are also at an increased risk for mTBI. Incidence rates of mTBI among athletes range from 0.1 to 21.5 in 1,000 worldwide (Clay et al., 2013).
Posttraumatic stress disorder (PTSD)
Mild TBI and mental health disorders, such as PTSD, have overlapping symptoms, making these commonly co-occurring conditions difficult to distinguish (Hoge et al., 2008; Elder and Cristian, 2009). Hallmark PTSD symptoms include intrusive thoughts, hyper-vigilance, avoidance, and response to trauma-related cues (American Psychiatric Association, 2004). These symptoms are caused by a traumatic event that resulted in extraordinary harm to themselves or others (American Psychiatric Association, 2013). Traumatic events, such as explosions, can also result in physical injury and mTBI. Therefore, it is not surprising, that PTSD rates among people with mTBI are relatively high, 17% and 26–44% in US civilian and veteran populations, respectively (Hoge et al., 2008; Brenner et al., 2010; Hoffman et al., 2012). Moreover, the co-occurrence of AUD, mTBI and PTSD exacerbates symptoms and prolongs recovery (Vanderploeg et al., 2009). Respondent-driven sampling estimates of co-occurring PTSD and TBI (severity not indicated) are 9%, co-occurring substance use disorder and mental health issue are 18% and co-occurring substance use disorders, PTSD, TBI and major depression disorder are 0.7% among US veterans.
Transcranial magnetic stimulation principles & therapeutic applications
Transcranial magnetic stimulation (TMS) principles
TMS is an intervention that is well suited for the treatment of co-occurring neurological and psychiatric conditions such as AUD + mTBI + PTSD, in part, because the TMS mechanism of action allows for non-invasive modulation of neural activity. The physical principles of TMS have been the subject of many previous publications (Ziad, 2002; Kobayashi and Pascual-Leone, 2003). In brief, TMS generates a magnetic field in a coil that is placed on the scalp (Ziad, 2002). The field from the coil induces an electrical current in the brain tissue beneath the coil, resulting in alterations of neural excitability (Ziad, 2002). Depending on stimulation parameters, rTMS is able to modulate cortical and subcortical function by increasing or decreasing cortical excitability (George, 2010).
Influence of TMS parameters on therapeutic effects
By adjusting TMS parameters, one can tailor the TMS intervention to be excitatory or inhibitory in accordance with the desired therapeutic effect. These parameters include, but are not limited to, intensity, frequency, number of stimuli, and number of sessions (Lisanby, 2008). Though not a parameter, coil shape also influences the neurophysiological properties and subsequent therapeutic effects of TMS.
Intensity is conventionally expressed as a function of the motor threshold (MT). The MT is determined by using single pulse TMS applied to the primary motor cortex and is defined as the minimum stimulation necessary to elicit a motor evoked potential (MEP) with a minimum amplitude in a specified muscle (e.g., abductor pollicis brevis muscle) in typically 5 of 10 stimulations (Rossini et al., 1994; Kobayashi and Pascual-Leone, 2003). TMS intensity is then set as a percentage of the MT. By using this convention, stimulation intensity is comparable between patients and between studies that use different coils or different generators. In general, increasing intensity above the MT or using a supra-threshold stimulation intensity will result in excitation of neural activity (Ziad, 2002). On the other hand, using a stimulation intensity below MT, or sub-threshold, will result in inhibition of neural activity (Ziad, 2002).
The frequency parameter is the number of pulses per second, or hertz (Hz), delivered over the same scalp site. The term repetitive TMS (rTMS) refers to TMS provided at a frequency greater than one pulse per second (1 Hz) (Kobayashi and Pascual-Leone, 2003). In general, low frequency (<1 Hz) stimulation will inhibit neuronal activity (Chen et al., 1997) and high frequency (> 1 Hz) stimulation will facilitate neuronal activity when provided at an intensity at or above the MT (Wassermann, 1998; Ziad, 2002; Kobayashi and Pascual-Leone, 2003).
In addition to excitatory and inhibitory effects, the effects of rTMS outlast the period of stimulation specifically in studies of the motor cortex (Chen et al., 1997; Esser et al., 2006; Hoogendam et al., 2010), prefrontal regions (Mottaghy et al., 2002) and parietal cortex (Hilgetag et al., 2001; Wang et al., 2014). The duration of induced excitability can range from seconds (Hoogendam et al., 2010) to 24 hours (Wang et al., 2014). Further evidence suggests that multiple rTMS sessions can have a cumulative effect. For example, Baumer et al. (2003) found that two rTMS sessions provided 24 hours apart prolonged the rTMS effects in motor cortex excitability (Baumer et al., 2003). A similar result was found by Maeda and colleagues, where two days of 1 Hz or 20 Hz rTMS protocols produced enhanced effects on motor cortex excitability compared to one day of stimulation (Maeda et al., 2000).
In addition to the above parameters, coil shape directly relates to the focality of TMS effects. Smaller coils will provide a more focal or targeted stimulation (Deng et al., 2014). Circular coils provide maximum current underneath the circumference of the circle and the weakest current within the coil center (Ziad, 2002) ultimately resulting in more diffuse stimulation. The figure-of-eight coil provides focal stimulation because the site of maximum stimulation is located at the intersection of the two circles or windings (Ziad, 2002). The double-cone coil and the H-coil are commonly used to stimulate deeper brain structures. The double-cone coil appears as a bent figure-of-eight coil. Neuroimaging evidence suggests that when applied to the medial prefrontal cortex (mPFC), the effects of the double-cone coil may reach the deeper anterior cingulate cortex (aCC) and affect function specific to the aCC (Hayward et al., 2007). Recent evidence suggests that using the double-cone coil at intensities necessary to penetrate deeper than 4cm may not be safe (Deng et al., 2014). The intensity of TMS stimulation is greatest directly underneath the coil and decays as a function of distance. The intensities required to reach more subcortical structures are very strong at the cortical surface, increasing the risk for seizure. Emerging evidence regarding the H-coil indicates that deeper brain structures are activated, compared to the figure-of-eight coil, at tolerable intensities (Zangen et al., 2005; Fadini et al., 2009). The Brainsway TMS device (Jerusalem, Israel), which uses the H-coil, recently received Food and Drug Administration (FDA) approval in the US for the treatment of major depressive disorder. It is important to consider that while the double-cone coil and H-coil may penetrate deeper brain structures, cortical surface structures will also be affected (Deng et al., 2014). Thus, the intensity of rTMS must be within safety parameters for surface cortical structures (Rossi et al., 2009).
Proximal and remote net neural effects of rTMS
Repetitive TMS-induced changes in excitatory and inhibitory neural processes occur at multiple levels, and techniques used to measure the overall net neural effects include electromyography (EMG), electroencephalography (EEG), Positron Emission Tomography (PET) and advanced Magnetic Resonance Imaging (MRI). Collectively, TMS studies using these multi-modal measures indicate that rTMS can induce change in neural activity in regions local to and remote from the stimulation site (Gersner et al., 2011) and within specific neural networks related to specific behaviors (Kozel et al., 2011; Allendorfer et al., 2012). The time course of a single pulse of TMS, characterized by an initial neuronal excitation followed by prolonged suppression, is thought to be the primary mechanism of how repeated TMS pulses result in long-lasting effects on neural activity (Ziad, 2002; Kobayashi and Pascual-Leone, 2003).
Evidence of changes local to or remote from the site of stimulation can be illustrated via studies of the primary motor cortex (M1), where both single pulse TMS and rTMS are used to elicit muscle contraction contralateral to the M1 area targeted (Pascual-Leone et al., 1994). Stimulation of M1 produces an immediate response in local neural activity as measured with motor evoked potentials. This activation spreads rapidly to both adjacent ipsilateral sites as well as homologous contralateral areas, revealing connectivity between the local and remote brain areas (Ilmoniemi et al., 1997; Casula et al., 2014).
Evidence derived from functional MRI (Pleger et al., 2006; Ruff et al., 2006; Sack et al., 2007; Blankenburg et al., 2008; Fox et al., 2012) and PET (Speer et al., 2003; Ferrarelli et al., 2004; Ohnishi et al., 2004) measures indicate that rTMS can be directed toward specific networks. The rTMS effect can be optimized by targeting a site that is a node of a single network or multiple networks. The capacity to target a network or networks makes TMS particularly relevant for AUD + mTBI + PTSD because the conditions share some common networks.
Methods: rTMS as a treatmentfor AUD, TBI, and PTSD
For the section ‘Review of existing literature for TMS as a treatment for each of the following conditions alone: AUD, mTBI, and PTSD’, targeted literature searches were conducted on each sub-section. Peer-reviewed articles written in English were identified using PubMed. Because of the novelty of this area and the relatively small number of peer-reviewed articles on this work, no date limits were set. Search terms were: “alcohol”, “non-invasive brain stimulation,” “posttraumatic stress disorder”, “PTSD”, “repetitive transcranial magnetic stimulation”, “rTMS”, “transcranial magnetic stimulation”, “TMS”, “traumatic brain injury”, and “TBI”. The literature search cross-referenced relevant review articles bibliographies. Only primary research articles where TMS was used as a treatment were included.
For all other sections, the most relevant and recent PubMed articles were used to provide supporting evidence.
Review of existing literature for rTMS as a treatment for each of the following conditions alone: AUD, TBI, and PTSD
rTMS as an AUD treatment
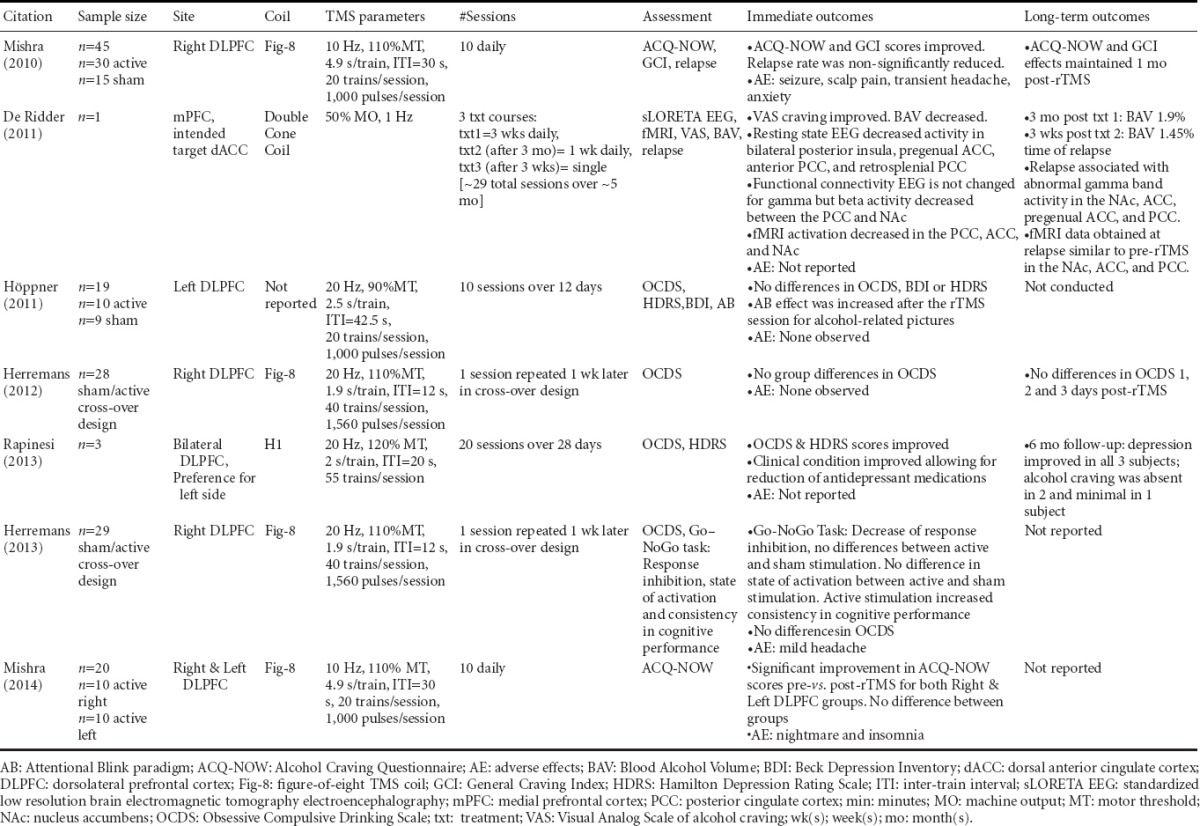
Repetitive TMS has been tested as a treatment for substance use disorders including alcohol use disorder (AUD). To date, there are seven published papers on the use of rTMS as an AUD treatment, five of which are randomized controlled trials (RCTs) (Mishra et al., 2010; Hoppner et al., 2011; Herremans et al., 2012; Herremans et al., 2013; Mishra et al., 2014), one case series (Rapinesi et al., 2013) and one case report (De Ridder et al., 2011). All seven studies assessed alcohol craving as an outcome but report inconsistent efficacy results. The findings of these studies are summarized in Table 1 according to site of stimulation, frequency, intensity, and number of stimulation sessions.
Table 1.
Summary of studies on repetitive transcranial magnetic stimulation (TMS) as a treatment among people with alcohol use disorder
Addiction treatment factors
When assessing treatment interventions for participants with AUD, factors that may affect alcohol craving outcomes such as alcohol use history, co-occurring conditions, and medication use should be considered. The alcohol use history of subjects who participated in the reviewed papers ranged from detoxified to actively drinking, which may have affected the influence of TMS treatment on observed outcomes. For example, the study by Höppner and the two studies by Herremans included participants who were within two to three weeks of detoxification (Hoppner et al., 2011; Herremans et al., 2012; 2013). Similarly, the case series by Rapinesi includes participants who had been abstinent for one month (Rapinesi et al., 2013). The case study by De Ridder included a participant who was actively drinking during the first rTMS course (De Ridder et al., 2011). Finally, while the 2010 study by Mishra contained information about the duration of alcohol use, the authors did not specify if participants were actively drinking before rTMS treatment or alcohol craving assessments (Mishra et al., 2010). There is evidence to suggest that alcohol craving levels decrease over time during withdrawal (Cordovil De Sousa Uva et al., 2010), therefore this factor should be controlled for or acknowledged if the goal of rTMS treatment is to decrease alcohol craving. The rate of relapse following rTMS treatment should be assessed as well.
People who have conditions that co-occur with AUD often take medications to treat one or more of their conditions, which is important to account for when providing treatments such as rTMS. The Rapinesi case series specifically included participants with AUD and dysthymia (Rapinesi et al., 2013), referred to in the Diagnostic and Statistical Manual of Mental Disorders V as persistent depression disorder (American Psychiatric Association, 2013). These participants were taking antidepressants and benzodiazepines. Benzodiazepines are commonly prescribed to treat alcohol withdrawal symptoms but have addictive liability (Williams and McBride, 1998). A single study found that benzodiazepine administration is associated with increases in alcohol craving levels among people with co-occurring AUD and bipolar disorder (Prisciandaro et al., 2011). In contrast, other evidence suggests that antidepressants such as mirtazapine reduce alcohol craving levels (Yoon et al., 2006); however, the antidepressant fluoxetine is without such effect (Janiri et al., 1996; Kabel and Petty, 1996). Following rTMS treatment, the participants in the Rapinesi study received lower doses of antidepressants due to rTMS-related improvements in their clinical condition, which remained improved six months after cessation of rTMS treatment (Rapinesi et al., 2013). Information on medication use, as well as medication interactions, is important to take into account when interpreting effects of rTMS on alcohol craving outcomes.
Summary and implications for future work
The studies conducted by Mishra are the only RCTs which proved efficacious for reducing alcohol craving (Mishra et al., 2010; Mishra et al., 2014). These studies involved provision of 10 daily sessions of high frequency (10 Hz) rTMS over the right DLPFC (Mishra et al., 2010; Mishra et al., 2014) and left DLPFC (Mishra et al., 2014) at an intensity of 110% MT. The most recent study conducted by Mishra provides evidence that provision of rTMS over the right or left DLPFC improves alcohol craving pre- compared to post-rTMS without a difference between groups (Mishra et al., 2010; Mishra et al., 2014). The high frequency supra-threshold stimulation most likely facilitated excitability in the DLPFC (Hoogendam et al., 2010). There is evidence that high frequency stimulation of the DLPFC can modulate dopamine release in deep-brain structures important for addiction and reward such as the nucleus accumbens (Erhardt et al., 2004), striatum (Strafella et al., 2001) and aCC (Cho and Strafella, 2009). Though there are some gaps as to the precise mechanism of these effects, the modulation of cortico-striatal dopamine release, presumably an effect of rTMS might explain the modulation in alcohol craving reported by Mishra. Some consensus can be reached by comparing stimulation intensities between studies that using a higher frequency of 20Hz which does not impact alcohol craving levels (Hoppner et al., 2011; Herremans et al., 2012; 2013; Rapinesi et al., 2013). More studies need to be conducted for a consensus on appropriate rTMS parameters prior to implementing rTMS as a treatment to reduce alcohol craving for AUD.
rTMS as a TBI treatment
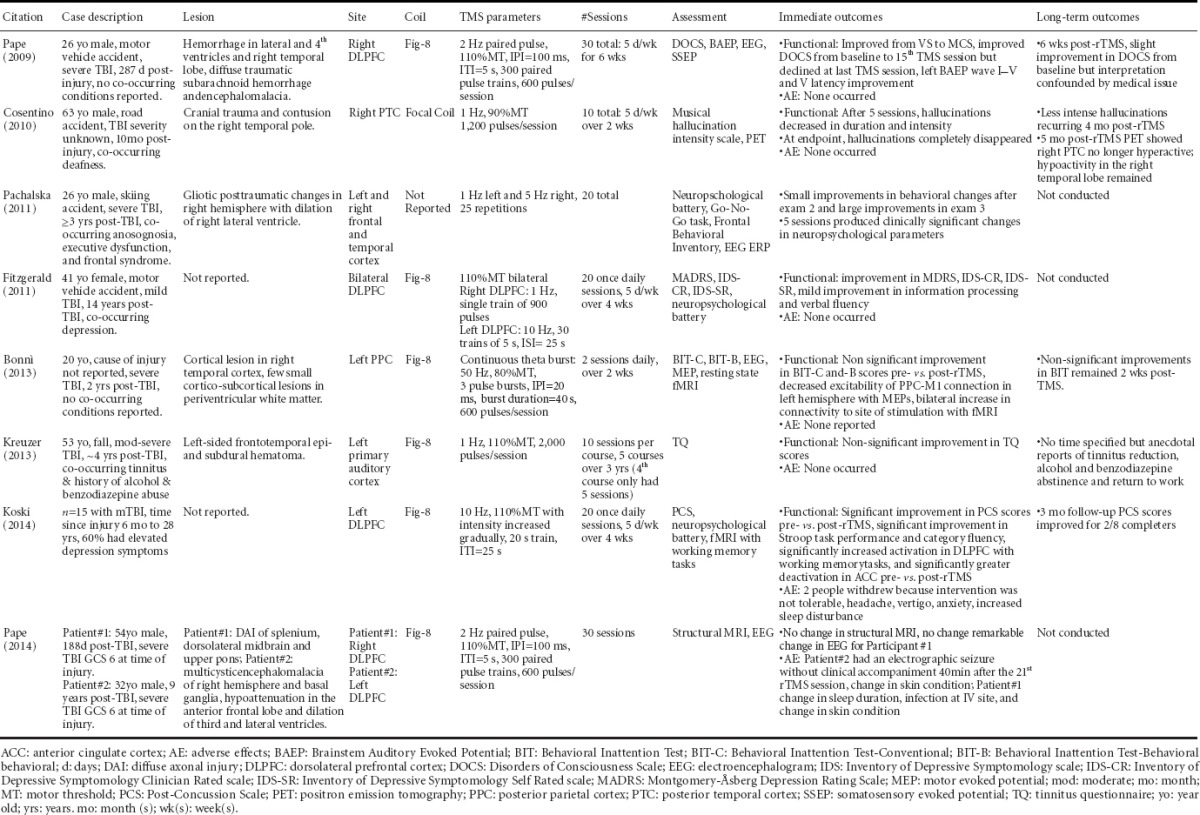
The existing literature regarding use of rTMS as a TBI treatment for the primary brain injury as well as neurological and psychiatric sequelae is in its infancy. To date, there are seven published case studies (Pape et al., 2009; Cosentino et al., 2010; Fitzgerald et al., 2011; Pachalska et al., 2011; Bonni et al., 2013; Kreuzer et al., 2013; Pape et al., 2014b) and one non-randomized pilot study on rTMS as a TBI treatment (Koski et al., 2014). The case studies are diverse in that rTMS was customized to treat a specified neurological sequelae ranging from overall neurobehavioral function (Pape et al., 2009), to a particular deficit (Bonni et al., 2013). The studies also range in TBI severity from severe to mild. Most recently, Koski et al. (2014) published a pilot study on a sample of 15 mTBI participants (Koski et al., 2014). This study did not include a sham control group, but it provides preliminary evidence that high frequency rTMS provided over the left DLPFC may improve symptoms associated with mTBI (Koski et al., 2014). Each study is summarized in Table 2 including collective contributions or impacts of research findings.
Table 2.
Summary of case reports on repetitive transcranial magnetic stimulation as a treatment among people with traumatic brain injury
Safety
One adverse event was reported in the eight TBI studies, but it should be noted that some of these studies did not explicitly address safety. For the TBI population, seizure is the greatest potential risk associated with rTMS. Pape et al. (2014b) reported the first rTMS related seizure for a patient who had remained in a state of disordered consciousness for nine years following a severe TBI. This patient was subsequently treated with 1,000 mg levetiracetam and it was reported that the patient safely tolerated a revised rTMS protocol while remaining on levetiracetam (750 mg BID). The study conducted by Kreuzer et al. (2013) included a subject with a history of a single seizure after injury, but no seizure activity was reported during the provision of rTMS. In this case, the patient was maintained on anti-seizure medications during the rTMS intervention, and no subsequent seizures occurred during the study (Kreuzer et al., 2013). In the pilot study conducted by Koski et al. (2014), two of 15 participants withdrew because they found the rTMS intervention intolerable. Other side effects are listed in Table 2 and, importantly, no seizures were reported in this study.
Co-occurring conditions
Primary injures due to TBI result in contusions, diffuse axonal injuries, hematomas and hemorrhages, but the location and extent of these lesions are heterogeneous (Pape, 2014a). Neurosensory and neurocognitive impairments can occur after the primary injury. For example, two studies reported using TMS to treat auditory impairments due to TBI. Kreuzer et al. (2013) reported use of rTMS as a treatment for tinnitus, and Cosentino et al. (2010) reported use of rTMS as a treatment for musical hallucinations. While aspects of the temporal cortex were sites of the primary lesions, Cosentino et al. (2010) stimulated the right posterior temporal cortex to reduce musical hallucinations and Kreuzer et al. (2013) stimulated the auditory cortex to reduce tinnitus symptoms.
Depression co-occurred for two of the TBI patients who received rTMS studied in this collective literature. The study reported by Kreuzer et al. (2013) describes a patient with a TBI and co-occurring tinnitus, depression, and a history of alcohol and benzodiazepine abuse. Fitzgerald et al. (2011) demonstrated that depression could be reduced in a TBI patient. These studies signify advancement in neuromodulation because the large-scale, double-blind randomized controlled trials on rTMS as a treatment for depression have routinely screened out patients who incurred TBI or have other co-occurring conditions such as substance use disorders. Most recently, George et al. (2014) assessed rTMS as a treatment among suicidal inpatients with PTSD, TBI or both. This randomized, sham-controlled pilot trial of high frequency (10 Hz) rTMS applied to the left prefrontal cortex demonstrated that both sham and active rTMS significantly improved suicidal ideation scores without a treatment effect (George et al., 2014). However, no PTSD or TBI-specific outcomes were reported.
Summary and implications for future work
The main limitation for this body of literature is the limited data. Seven TBI case studies and one pilot study in mTBI using rTMS as a treatment have been published to date. Each of these studies used different sites of stimulation and rTMS parameters. In addition, the rTMS paradigm for each of the TBI case studies was designed to address different sequelae. Therefore, there is insufficient evidence to determine the optimal stimulation site and TMS parameters for TBI patients. Larger-scale studies that rigorously test and optimize rTMS treatment parameters for the TBI population are needed. Given the heterogeneity of injuries within the TBI population, it may be beneficial to determine if rTMS can be optimized and tailored to each TBI patient or injury. Four reports reviewed above used neuroimaging-guided neuronavigation to identify the exact site of stimulation (Pape et al., 2009; Pachalska et al., 2011; Bonni et al., 2013; Pape et al., 2014b). Integration of advanced neuroimaging approaches with rTMS provision, to determine ideal site of stimulation and investigate neural mechanisms of recovery, will be vital to developing rTMS as a TBI treatment.
rTMS as a PTSD treatment
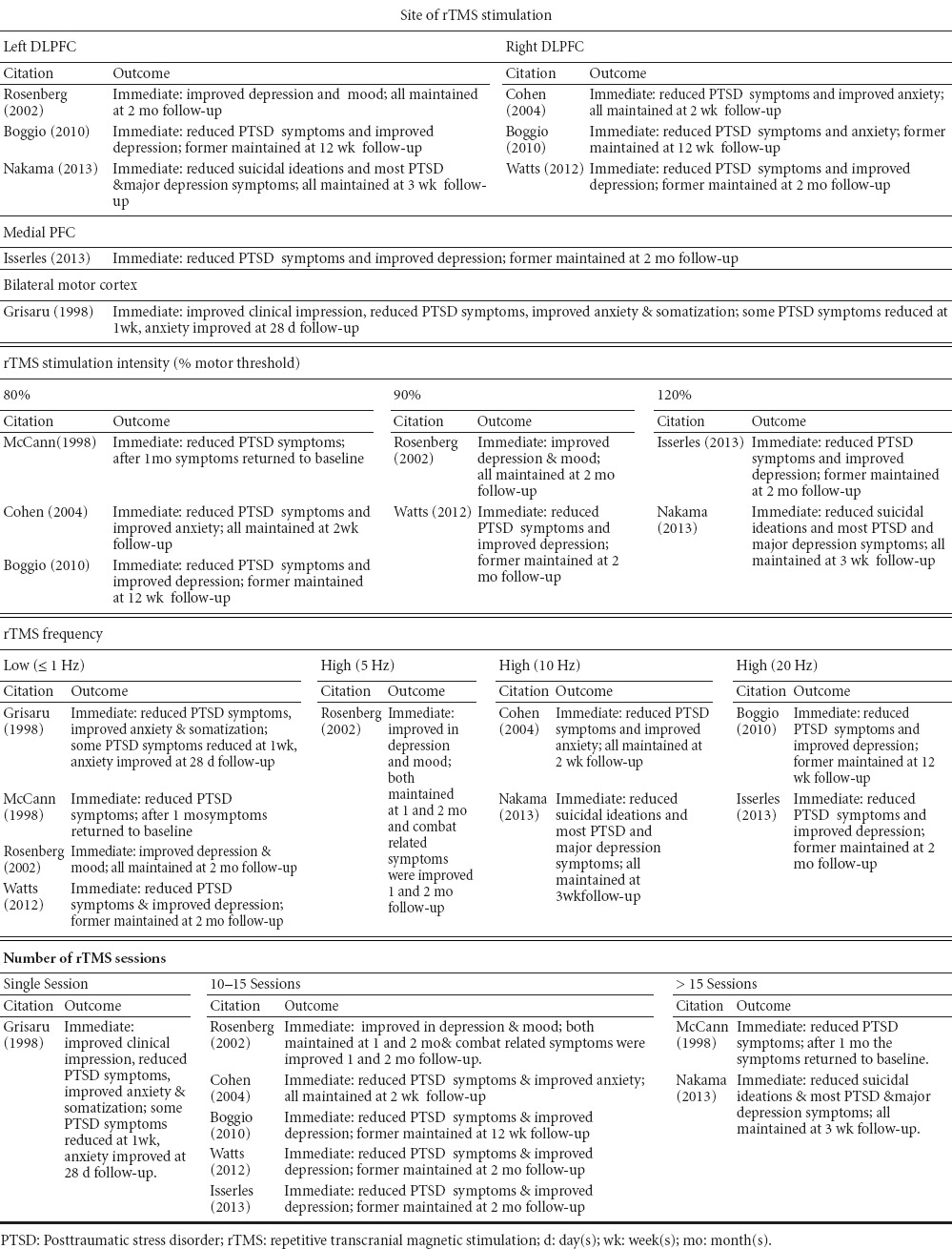
The potential of rTMS as a neuromodulatory treatment for PTSD has been investigated via a series of case studies, pilot studies and double-blind RCTs. Table 3 summarizes the efficacy studies according to rTMS parameters and Supplemental Table 1 summarizes all studies examining rTMS treatment for PTSD. Efficacy studies are those with statistically significant improvement in outcomes attributed to a rTMS protocol. Studies with questionable or unclear statistical measures were not included. A majority of these studies were also reviewed recently by Karsen et al. (2014) including a meta-analysis on three of these studies.
Table 3.
Summary of efficacy studies of repetitive transcranial magnetic stimulation as posttraumatic stress disorder treatment according to parameters
Supplemental Table 1.
Summary of studies on repetitive transcranial magnetic stimulation (TMS) as a treatment among people with posttraumatic stress disorder
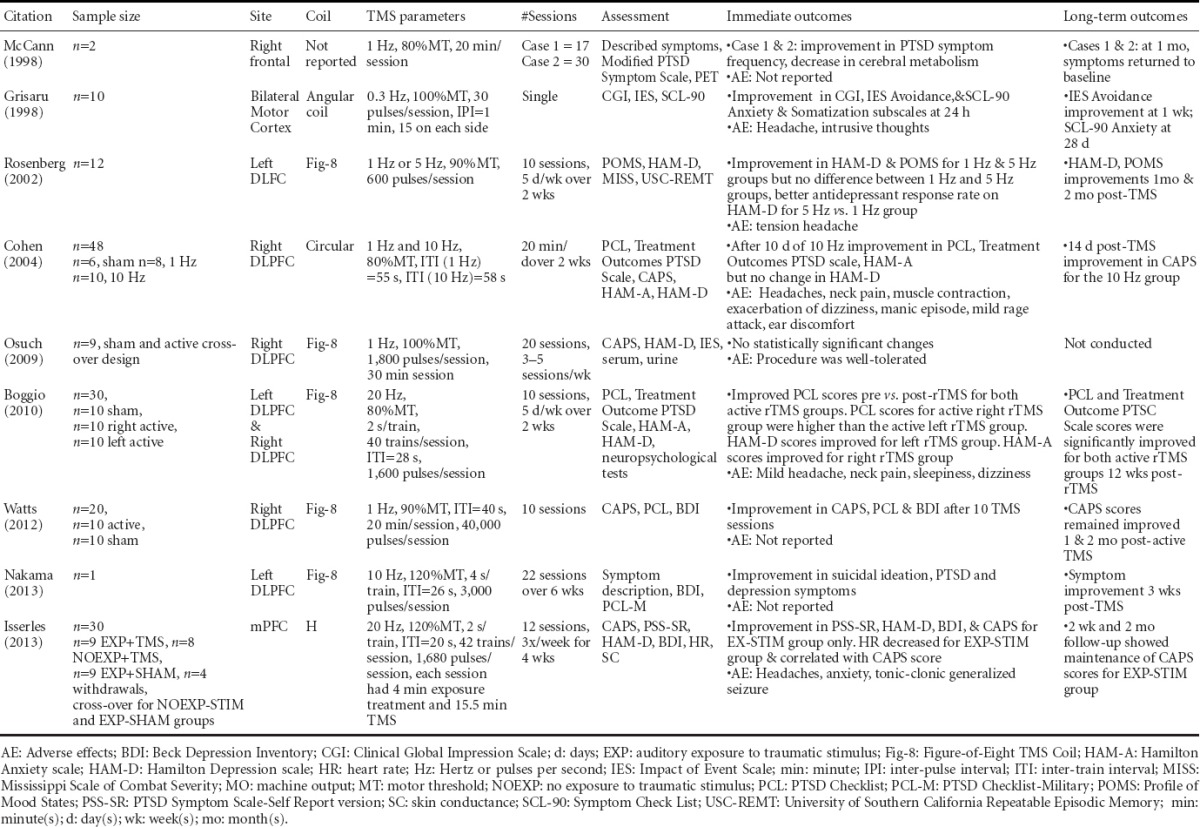
Co-occurring conditions
As PTSD and depression symptoms commonly co-occur, some of the PTSD studies summarized in Table 3 also included patients with co-occurring depression. Study findings reported by Boggio et al. (2010) indicate that depressive symptoms improved only for the active left DLPFC group, while anxiety symptoms improved only for the active right DLPFC group. The depression findings are consistent with the FDA-approved rTMS protocol for drug resistant depression (O’Reardon et al., 2007). A double-blind RCT conducted by Cohen et al. (2004) also found right DLPFC rTMS efficacious for PTSD and anxiety symptoms, but found no change in depressive symptoms. Furthermore, an open-label RCT conducted by Rosenberg et al. (2002) found that veterans with co-occurring PTSD and depression who received stimulation to the left DLPFC had significantly reduced depression (P < 0.001) and PTSD (P = 0.02) symptoms. Most recently, Isserles et al. (2013) demonstrated in a double-blind RCT that rTMS to the medial PFC using an H-coil significantly reduced PTSD (P < 0.001) and depression symptoms (P < 0.05). The evidence summarized suggests that the hemisphere of the DLPFC upon which rTMS is applied may, in part, determine the effects on PTSD and anxiety versus depression. All studies in which rTMS was administered over the left DLPFC found that depression and PTSD outcomes improved (Rosenberg et al., 2002; Boggio et al., 2010; Nakama et al., 2013). Additionally, Watts et al. (2012) found that 1Hz rTMS applied to the right DLPFC improved PTSD and depression symptoms. Furthermore, Isserles et al. (2013) found that 20 Hz rTMS applied to the mPFC using an H-coil improved both PTSD and depression symptoms. One neurophysiological explanation for this finding is that through the use of the H-coil, rTMS effects occurred at the site of stimulation, the mPFC, as well as the underlying deeper brain structure, the aCC (Hayward et al., 2007). This is important because the mPFC suppresses hypothalamic-pituitary-adrenal (HPA) axis (Diorio et al., 1993) and the amygdale (Lucassen et al., 2014) to regulate stress responses. Evidence from rodents indicates that chronic stress decreases dendritic arborization among mPFC and aCC pyramidal neurons (Radley et al., 2004). Therefore, excitation of glutamatergic mPFC pyramidal neurons using high frequency rTMS may ameliorate mPFC/aCC dysfunction and restore proper suppression of the HPA axis and amygdala.
In addition to the importance of site of stimulation, the meta-analysis conducted by Karsen et al. (2014) points out that there is a trend (P = 0.061) for a positive correlation between effect size and number of rTMS pulses. That is, as the number of rTMS pulses administered in three selected PTSD studies increases, effect size also increased. The study by Isserles et al. (2013) administered 20Hz rTMS with a total of approximately 20,000 pulses over the 12 treatment sessions, which is on the high end of rTMS studies for PTSD treatment.
Of particular relevance, the open-label trial conducted by Rosenberg et al. (2002) included 20 veterans with PTSD, depression and a history of alcohol use disorder. As per the exclusion criteria, all subjects had abstained from alcohol within three months of rTMS provision (Rosenberg et al., 2002). Although these investigators did not collect alcohol or addiction-related outcomes, the findings indicate that 10 sessions of rTMS provided over the left DLPFC at 1Hz and 5Hz improved PTSD and depression symptom (Rosenberg et al., 2002). The study reported no serious adverse events, which used rTMS among a population with three co-occurring conditions (i.e., PTSD, depression and AUD).
Summary and implications for future work
For the three conditions included in this review, the amount of evidence for rTMS as a treatment for people with PTSD is greater than for people with TBI or AUD. The reported evidence is derived not only from case reports, but also from rigorous double-blind RCTs. Collectively, the evidence suggests that high frequency, supra-threshold intensity rTMS applied to the DLPFC may hold promise for the treatment of PTSD. This evidence also illustrates that rTMS may be beneficial for the treatment of co-occurring conditions. The studies that examine the co-occurrence of PTSD with other mental health conditions measured a myriad of symptoms to understand the potential of rTMS to treat co-occurring conditions. These studies provide an excellent foundation towards developing rTMS as a treatment for co-occurring conditions.
Evidence to inform a neurobiological model
AUD, mTBI and PTSD each result in functional and structural changes to the brain. Commonly used diagnostic assessments involve neuropsychological testing, which include self-report questionnaires, interviews and standardized performance tests. While these diagnostic test results are informative, there is also a need for more direct neurophysiological evidence to provide information about how the neural mechanisms of repair differ for a single isolated condition versus co-occurring conditions. An advanced neuroimaging technique, functional magnetic resonance imaging (fMRI), can be used to investigate mechanism of repair because it acquires information regarding neurophysiological changes within the whole brain that can be further refined by regions and neural networks of interest. This evidence can provide information about how activation within brain regions and neural networks change in response to cues or tasks as well as treatments.
To examine the neural circuitry of AUD alone, fMRI protocols can be used (Schacht et al., 2013) to examine neural activation in response to visual alcohol cues. This is important clinically because contextual cues within common environmental settings are related to alcohol-induced craving (Schacht et al., 2013), which is associated with relapse (Bottlender and Soyka, 2004; Chakravorty et al., 2010). Understanding changes in neural activation in response to cue-elicited craving within established AUD-related regions provides the information necessary to develop craving and relapse prevention treatments for people with AUD + mTBI + PTSD.
In addition to task-based fMRI protocols, measurement of brain activation at rest through resting state functional connectivity (rsFC) also allows for further characterization of the underlying neural circuitry (Fox and Greicius, 2010).
There are many advantages to using rsFC to inform treatment development for the AUD + mTBI + PTSD population. First, rsFC is well suited for examining how brain networks function together and is ideal for examining a co-morbid brain state that involves both unique and overlapping networks. Second, rsFC is free from task-related confounds such as differing levels of attention and cognitive abilities (Fox and Greicius, 2010). Finally, clinical treatment often occurs in an outpatient setting devoid of alcohol-related contextual cues making it important to determine how the AUD + mTBI + PTSD brain functions at rest.
Diffusion tensor imaging (DTI), another advanced imaging technique, is an indirect assessment of the structural integrity of white matter fiber tract (Arfanakis et al., 2002). Data regarding the structural connectivity of the brain can inform the interpretation of rsFC findings because DTI provides insights into the structural alterations that occur in brain microstructure, which are not detectable with conventional imaging.
Neurobiological findings supportive of a theoretical model of the AUD + mTBI + PTSD brain state
The underlying circuitry implicated in AUD has been well characterized. What is unknown is how co-occurring mTBI and PTSD affects the AUD brain state. While some brain regions and networks implicated in AUD alone overlap with that of mTBI alone and PTSD alone, some regions and networks are unique to each disorder. Characterization of the AUD + mTBI + PTSD brain state, relative to AUD alone, will inform the neural circuitry specific to these disorders when they co-occur. An understanding of the neural circuitry will help advance development of targeted neurotherapeutic treatments to reduce craving and prevent relapse. In this section, we discuss relevant neuroimaging findings and integrate these findings with pre-clinical work to inform a theoretical model of the AUD + mTBI + PTSD brain state.
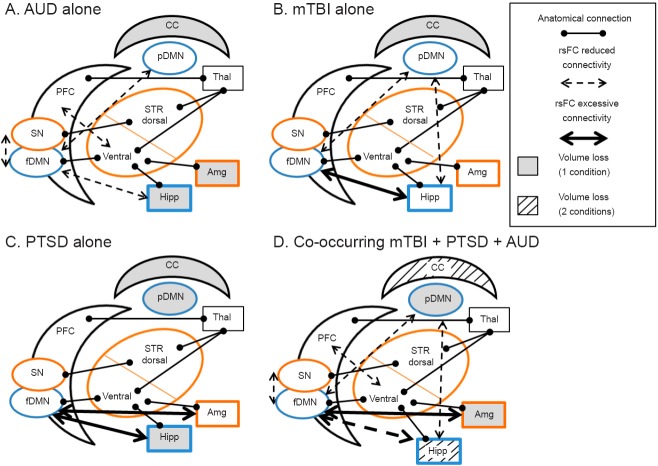
Figure 1 illustrates the neurobiological model for each condition alone and for co-occurring conditions. The brain regions are color coded according to their involvement in the Default Mode Network (DMN) and Salience Network (SN) because of well-established evidence that these two networks are impaired with AUD, mTBI and PTSD (Park et al., 2010; Schmaal et al., 2013). The blue regions indicate the DMN and the orange regions indicate the SN. Gray matter regions known to be important for each condition alone are outlined in black font.
Figure 1.
Neural regions and networks implicated in (A) alcohol use disorder (AUD), (B) mild traumatic brain injury (mTBI), (C) posttraumatic stress disorder (PTSD), and (D) their co-occurrence.
PFC: Prefrontal cortex; fDMN: frontal default mode network; pDMN: posterior default mode network; STR: striatum; Hipp: hippocampus; Amg: amygdala; Thal: thalamus; CC: corpus callosum. Blue outlines indicate Default Mode Network (DMN); orange outlines indicate salience network (SN).
Alcohol use disorder (AUD) pathways
AUD is driven by circuitry involving the cortico-striatal system [prefrontal cortex (PFC), dorsal and ventral striatum (STR)] classically involved in addiction (Kelley, 2004; Chambers et al., 2007a; Haber and Knutson, 2010; Schacht et al., 2013) and co-occurring substance use and mental health disorders (Chambers et al., 2001; Chambers and Self, 2002; Chambers et al., 2010). These structures are anatomically connected, and addiction behaviors are modified by additional structural and functional connections with limbic brain regions such as the hippocampus (Hipp) and amygdala (Amg) as well as the thalamus (Thal) (Chambers et al., 2007b; Haber and Knutson, 2010).
As illustrated in Figure 1A the corpus callosum (CC) is degenerated for AUD alone. Similarly, it has also been shown that the hippocampus and amygdala have reduced volume. Figure 1A illustrates the corpus callosum (CC) that is degenerated with AUD and the gray matter regions activated in response to alcohol cues (Pfefferbaum et al., 1996; Pfefferbaum et al., 2000).
Addiction circuitry has been characterized for people with AUD alone according to changes in neural activation as measured with fMRI. Alcohol cue-elicited activation increases in regions comprising the cortical-striatal system and those that modify it. This increased activation in specified regions is also significantly correlated with increased alcohol craving and AUD severity (George et al., 2001; Grusser et al., 2004; Myrick et al., 2004; Hermann et al., 2006; Park et al., 2007; Wrase et al., 2007; Filbey et al., 2008; Vollstadt-Klein et al., 2010; Claus et al., 2011; Schacht et al., 2013). Collectively, evidence indicates that excessive activity in the cortico-striatal system (PFC and STR) and modifiers of this system (Hipp, Amg, Thal) occur in response to alcohol cues in AUD alone.
Although there are few studies examining rsFC among people with AUD alone, we do know that impaired brain networks include the salience network (SN, Figure 1, Orange outlined regions). Salience, an important feature of reward that drives craving, includes sub-regions of the PFC, STR and Amg (Seeley et al., 2007). Specifically, impaired connectivity between the PFC and the ventral STR is associated with alcohol craving among people with AUD alone (Park et al., 2010). Most recently, Seo demonstrated that the PFC among people with AUD alone is excessively active at rest, and that this excessive activation was positively associated with increased craving and relapse risk (Seo et al., 2013).
Another key and impaired network with AUD alone is the default mode network (DMN, Figure 1, Blue outlined regions). The DMN, involving sub-regions of the cortex and the Hipp (Raichle et al., 2001; Buckner et al., 2008), is active at rest but is suppressed during task performance (e.g., memory retrieval, planning) (Buckner et al., 2008; Fox and Greicius, 2010). The DMN hinges on cortical regions overlapping with the SN. In AUD alone, evidence suggests that connectivity between the DMN and SN is impaired and that connectivity, along with indices of cognitive task performance, can be improved with pharmacotherapy (Schmaal et al., 2013). This illustrates how a better understanding of neural network connectivity and between network interactions can be used to develop treatments for AUD alone.
AUD alone has been linked with structural changes in the brain involving addiction circuitry. Examination of post-mortem brain tissue reveals that as lifetime alcohol consumption increases, overall white matter volume decreases (Kril et al., 1997). MRI findings of brain morphology demonstrate corpus callosum volume reduction (Pfefferbaum et al., 1996). Reduced tissue volume of limbic brain regions (i.e., Hipp, Amg) is associated with significantly higher levels of alcohol craving (Wrase et al., 2008). This finding is recapitulated by rodent hippocampal lesion models, which are characterized by enhanced sensitivity to the effects of alcohol (Conroy et al., 2007; Berg et al., 2011; Jeanblanc et al., 2014). DTI studies confirm these early reports and demonstrate that the corpus callosum and many other white matter tracts are affected by AUD alone (Pfefferbaum et al., 2000; Arnone et al., 2006; Monnig et al., 2013).
In summary, neuroimaging studies collectively show impaired neural activity and network connectivity of addiction circuitry with AUD alone both in response to alcohol cues and at rest. The evidence suggests that the PFC, in particular, is excessively active in response to cues (Grusser et al., 2004) and during rest (Seo et al., 2013). DTI studies implicate a wide-spread loss of white matter fiber tract integrity, and that the corpus callosum in particular could be vulnerable (Schacht et al., 2013).
Mild traumatic brain injury (mTBI) pathways
While Figure 1B illustrates only evidence derived from mTBI, there is also growing evidence among the moderate and severe TBI population. Therefore, we discuss recent neuroimaging findings about mild, moderate and severe TBI in this section.
Similar to AUD alone, evidence suggests functional and structural connectivity of the DMN (Figure 1B, Blue outlines) is altered with mTBI alone. Two studies examining rsFC after mTBI indicate increased frontal DMN and reduced posterior DMN (posterior cingulate cortex) rsFC (Johnson et al., 2012; Zhou et al., 2012a), which is consistent with known vulnerability of the frontal cortex with mTBI (McAllister, 2008). Decreased cognitive task performance is also associated with reduced rsFC in the posterior DMN after mTBI (Zhou et al., 2012a). With a greater number of mTBI events experienced, evidence also indicates weaker connections between frontal cortical sub-regions of the DMN (Johnson et al., 2012), suggesting that multiple mTBI events may result in cumulative alterations in neural networks, thus compounding deficits.
While conventional neuroimaging techniques reveal no structural changes after mTBI, DTI studies indicate that white matter integrity is decreased in tracts including the corpus callosum, internal capsule, corona radiata, and thalamic radiation (Arfanakis et al., 2002; Lipton et al., 2012) and within the PFC (Lipton et al., 2009). Matthews found as many as 14 specific regions (e.g., corpus callosum) with diminished white matter integrity for Iraq and Afghanistan veterans experiencing loss of consciousness vs. veterans experiencing only altered consciousness (Matthews et al., 2012). Notably, damage to the white matter in the corpus callosum is negatively correlated with executive function among Iraq and Afghanistan veterans (Jorge et al., 2012). DTI is ideal for elucidating mTBI unique microstructural changes that correlate with clinical outcomes.
Compared to mTBI, moderate and severe TBI result in morphological changes to the brain that can be detected using conventional, structural neuroimaging methods. Each moderate and severe TBI is different and results in heterogeneous lesions (Maas et al, 2007; Pape, 2014a). There is a burgeoning body of evidence utilizing rsFC and DTI techniques which shed light on neural network connectivity for moderate and severe TBI. The rsFC approach is particularly well suited for the moderate to severe TBI population because no task is required during the acquisition of fMRI data which removes the confound of varying degrees of cognitive impairment. A number of recent studies have found abnormalities in rsFC among participants with moderate to severe TBI (Hillary et al., 2011; Sharp et al., 2011; Maki-Marttunen et al., 2013; Pandit et al., 2013; Ham et al., 2014). Reduced connectivity within the fronto-parietal control network and regions overlapping with the SN (Ham et al., 2014) are reported after moderate and severe TBI relative to healthy control participants. Also, these rsFC alterations are associated with impairments in attention and self-awareness (Ham et al., 2014). However, connectivity within the DMN was found to be increased six months after moderate to severe TBI (Hillary et al., 2011; Sharp et al., 2011).
Structural connectivity is also altered following moderate to severe TBI with DTI findings suggest wide-spread white matter loss (Sharp et al., 2011; Pandit et al., 2013). Some studies have directly compared rsFC and DTI findings among participants with moderate to severe TBI (Sharp et al., 2011; Pandit et al., 2013). Within the corpus callosum, lower DMN connectivity was associated with lower indices of white matter integrity (Sharp et al., 2011).
Posttraumatic stress disorder (PTSD)
PTSD is characterized by functional abnormalities in the Amg and Hipp, which are two limbic brain regions shown to modify addiction circuitry for AUD alone (Kelley, 2004; Haber and Knutson, 2010). More specifically, rsFC evidence among Iraq and Afghanistan veterans with PTSD alone (Rabinak et al., 2011) demonstrates altered functional connectivity between the Amg and cortical structures. Specifically, there is increased connectivity between the Amg and anterior insula which is part of the SN (Rabinak et al., 2011) and the fDMN (Brown et al., 2014). However, there are inconsistencies in the literature regarding the connectivity between the Amg and aCC, a hub of the SN: one study reports no change (Rabinak et al., 2011), another an increase (Brown et al., 2014) in connectivity, and yet another reports a decrease in anti-correlated connectivity among people with PTSD relative to control participants (Sripada et al., 2012). This is important clinically because the Amg is involved with fear-conditioned responses relevant to PTSD symptoms (Mahan and Ressler, 2012). Increased or excessive connectivity between the fDMN and the Amg may be related to increased self-referential thoughts related to trauma (Brown et al., 2014). Though results were inconsistent regarding connectivity between the aCC and Amg, the study conducted by Sripada and colleagues showed a decrease in anti-correlated connectivity suggesting a dysfunction in top-down regulation of the Amg by prefrontal structures. Resting-state functional connectivity data also reveal that connections between the Amg and the posterior portion of the DMN are negatively correlated with anxiety symptoms (Zhou et al., 2012b).
Regarding structural abnormalities for people with PTSD alone, conventional MRI studies show decreases in Hipp volume and other cortical regions (e.g., posterior cingulate cortex of the posterior DMN) (Shin and Liberzon, 2010). DTI studies indicate more focal decreases in white matter connectivity. DTI findings in persons with PTSD elucidate abnormalities in PFC regions implicated in the SN and DMN as well as AUD addiction circuitry (Kim et al., 2005; Kim et al., 2006; Schuff et al., 2011; Zhang et al., 2011; Fani et al., 2012). Furthermore, integrity of white matter tracts that innervate sub-cortical regions of AUD addiction circuitry are decreased with PTSD (Kim et al., 2005; Schuff et al., 2011).
Co-occurring AUD + mTBI + PTSD
Well-established cognitive neuroscience evidence suggests that PFC modulation of executive function, cognitive control and information processing is compromised in mTBI alone, PTSD alone and AUD alone (Bechara et al., 2001; Dolan et al., 2012). The corpus callosum is also affected by both mTBI and AUD, and reduced integrity in this region is correlated with further impaired cognitive task performance. Mild TBI and PTSD compound the decreases in structural white matter integrity found in AUD alone. It is therefore plausible that the inability to effectively inhibit craving is compounded with AUD + mTBI + PTSD.
While key connections that enable inhibition have compound damage, the PFC in AUD alone is excessively active in response to alcohol cues and at rest. This excessive activation is associated with increased craving (Grusser et al., 2004) and relapse (Seo et al., 2013). Thus, people with AUD + mTBI + PTSD have reduced ability to inhibit craving and have a disorder where craving levels are increased. Since the STR is involved with habit and expression of motivated behaviors, the disconnection at rest between the PFC and the STR means that the loss of inhibitory control from the PFC may leave the brain vulnerable to relapse.
The DMN is thought to be critical for information processing and attention and should be more engaged during rest and less engaged during task performance (Buckner et al., 2008), but with mTBI inferential evidence suggests over-engagement of the DMN during rest and task performance (Zhou et al., 2012a). Therefore, because the DMN overlaps with addiction circuitry and the SN, fatigue within this system could also leave the AUD + mTBI + PTSD brain even more vulnerable to relapse. Vulnerability to relapse is plausible because damage to the Amg and Hipp occur. Rodent models involving Hipp lesions provide further evidence for alcohol addiction vulnerability (Conroy et al., 2007; Berg et al., 2011; Jeanblanc et al., 2014). The Amg regulates stress- and fear-conditioned responses like those symptomatic of PTSD (Mahan and Ressler, 2012). The Hipp is important for episodic and contextual memory (Mesulam, 2000). In PTSD, it is thought that there is a deficit in the top-down regulation from the PFC to the Amg and Hipp, which may account for hyperarousal and the inability to extinguish traumatic memories. This means that PTSD and AUD related decreases in Hipp volume might lead to different responses to salient contextual cues. For people with AUD + mTBI + PTSD, exacerbated impairment in the PFC by mTBI and PTSD may lead to compounded diminished ability to control craving responses elicited by alcohol cues where responses to salient cues are different, all of which contribute to relapse vulnerability.
Optimal rTMS parameters and stimulation sites for AUD + mTBI + PTSD
The evidence reviewed earlier indicates that a high frequency, supra-threshold intensity stimulation to the right DLPFC was the most efficacious for reducing alcohol craving. The most efficacious studies of rTMS to reduce alcohol craving, conducted by Mishra and colleagues, applied high frequency, supra-threshold intensity stimulation to the right DLPFC (Mishra et al., 2010; Mishra et al., 2014). Furthermore, the rTMS case study conducted by De Ridder et al. (2011) provided evidence that normalization of brain activity in the PFC coincided with reductions in alcohol craving and use. This evidence, combined with the neuroimaging evidence, suggests that the PFC may be the optimal stimulation site for reducing alcohol craving. However, white matter loss among people with AUD + mTBI + PTSD could affect the transmission of the rTMS signal. The intensity and possibly the frequency of rTMS may need modifications to suit the AUD population with co-occurring mTBI and PTSD.
There exist substantial gaps in our understanding of the precise neurophysiological mechanisms of rTMS on behavior. However, in order to further theorize about the neurophysiological response to rTMS applied to the DLPFC, we must synthesize information about the effects of rTMS on transmitter release, excitability and the neural environment with an expansion of Figure 1D.
There is evidence that high frequency rTMS alters gamma oscillations in the DLPFC which are mediated through GABA (Barr et al., 2013). rTMS applied to the DLPFC also increases levels of dopamine and glutamate in the ventral STR, an area critical for addiction (Zangen and Hyodo, 2002). Furthermore, rTMS alters brain-derived neurotrophic factor (BDNF) (Yukimasa et al., 2006; Feng et al., 2012) which plays a role in plasticity and may promote a more healthy neural environment. This information does not provide the empirical evidence necessary to fully understand the neurophysiological mechanism of rTMS. However, this as well as the neurobiological findings detailed in this section provides us enough evidence to postulate that high frequency (above 5 Hz), supra-threshold (above 100%MT) stimulation applied to the right DLPFC holds promise. An excitatory rTMS protocol could modulate dopamine and glutamate altered in addiction (Koob and Volkow, 2010), excite projections to the amygdala that could reduce the stress response (Etkin et al., 2011) and promote a healthy neural environment that could improve recovery following brain injury.
Discussion
Current AUD treatment consists of pharmacotherapy and/or cognitive behavioral therapy. Preliminary evidence indicate that these interventions are effective for AUD and co-occurring mTBI + PTSD, but the effects are modest reductions in alcohol craving and relapse (Maisel et al., 2013). RCTs on AUD routinely exclude people with mTBI and PTSD. Innovative studies designed to examine alternative and complementary treatments to pharmacology, as well as modulate neural activity are needed for the AUD + mTBI + PTSD population.
rTMS technology is versatile and can be used to target specific brain regions to induce or inhibit local and remote neural activity as well as activity within distributed neural networks (Ziad, 2002). Well-established evidence of neuronal networks involved with neurological and psychiatric disorders led to the development of rTMS as a neurotherapy for mental health and addiction disorders. The neuropathology of AUD + mTBI + PTSD affects multiple overlapping neural networks. rTMS has long-term remote and proximal effects on neurophysiology and therefore has the potential to modulate and ameliorate neural maladaptation that has occurred as a result of co-occurring AUD + mTBI + PTSD.
rTMS may be targeted to a specific region such as the DLPFC, an area known to be affected in all three of these conditions. By targeting a region common to all conditions, rTMS effects can be distributed throughout the multiple networks involved when these conditions co-occur. Current treatments often target one symptom or one network, but based on the evidence there are multiple symptoms and multiple networks impaired. Since rTMS has the potential to affect multiple networks, it may ameliorate multiple symptoms. Moreover, the symptoms of co-occurring AUD + mTBI + PTSD overlap and can exacerbate one another and if a single symptom is improved, others may improve.
The formative rTMS studies in AUD, PTSD and TBI populations reviewed above open avenues for research to develop neurotherapeutics complementing behavioral therapy and pharmacotherapy. Furthermore, these studies demonstrate the potential of rTMS to normalize activation in the regions within AUD circuitry. Empirical evidence from neuroimaging techniques will provide a solid foundation that will inform the theoretical model (Figure 1). This information will inform decisions regarding the site of stimulation and other rTMS parameters including frequency, intensity, inter-pulse interval, number of trains, inter-train interval and treatment duration. If the site of stimulation is identified as the DLPFC, which is consistent with an earlier rTMS study conducted in AUD alone (Mishra et al., 2010; Mishra et al., 2014), then rTMS intensity may need to be the parameter that is adjusted. Examining neural activation both to alcohol cues and at rest will determine whether the magnitude of brain impairment with mTBI + PTSD + AUD is different from that of AUD alone, which would provide information about how to alter the rTMS parameters from that of the published literature.
Information gained from preliminary neuroimaging and rTMS studies among the AUD + mTBI + PTSD population could inform key experiments further elucidating the underlying neurophysiological mechanisms responsible for the effects of rTMS on behaviors such as alcohol craving. Experiments might include rodent models for alcohol addiction, blast-induced TBI and chronic stress each in isolation and together. The use of microdialysis, fast-scan cyclic voltammetry or magnetic resonance spectroscopy before and after rTMS treatment would allow for the detection of how rTMS may alter neurotransmitters in specific brain regions and how these alterations correlate with behavioral readouts relevant to AUD + mTBI + PTSD. Furthermore, electrophysiological recordings on rodents in these behavioral models both at the site of stimulation and remote areas relevant to addiction such as the vSTR, Amg or Hipp both before and after rTMS would also inform the neurophysiological effects of rTMS.
Conclusion
In summary, the co-occurrence of AUD + mTBI + PTSD is prevalent, exacerbates symptoms of the three conditions alone and current treatment options for these co-occurring conditions are limited. The use of a non-invasive neuromodulatory treatment such as rTMS is well suited for the treatment of these co-occurring conditions. Advanced neuroimaging techniques will inform the theoretical model of a unique brain state, thereby informing development of targeted treatments such as rTMS. Comparing human neuroimaging data with rodent neuroimaging, neurochemical, and neurophysiological data will further inform our understanding of neural mechanisms and aid in rTMS treatment optimization.
Footnotes
Funding: This material is the result of work supported with resources by Department of Veterans Affairs (VA), Health Services Research and Development Service and the Office of Academic Affiliations (TPP 42-013) at Edward Hines VA Hospital. This manuscript was supported by the following: VA OAA Polytrauma Fellowship to AAH, NIDRR Merit Switzer Research Fellowship Award H133F-130011to AAH and the VA RR & D CDA-II RX000949-01A2 to AAH. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the VA or the United States government.
Conflicts of interest: None declared.
References
- Allendorfer JB, Storrs JM, Szaflarski JP. Changes in white matter integrity follow excitatory rTMS treatment of post-stroke aphasia. Restor Neurol Neurosci. 2012;30:103–113. doi: 10.3233/RNN-2011-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Congress of Rehabilitation Medicines (1993) Definition of mild traumatic brain injury. J Head Trauma Rehabil. 8:86–87. [Google Scholar]
- American Psychiatric Association. 4th Edition. Washington, D.C: American Psychiatric Association; 2004. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. Text Revision. [Google Scholar]
- American Psychiatric Association. 5th Edition. Washington, D.C: American Psychiatric Association; 2013. Diagnostic and Statistical Manual of Mental Disorders DSM-V. [Google Scholar]
- Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- Arnone D, Abou-Saleh MT, Barrick TR. Diffusion tensor imaging of the corpus callosum in addiction. Neuropsychobiology. 2006;54:107–113. doi: 10.1159/000096992. [DOI] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Davis KD, Fitzgerald PB, Daskalakis ZJ. Measuring GABAergic inhibitory activity with TMS-EEG and its potential clinical application for chronic pain. J Neuroimmune Pharmacol. 2013;8:535–546. doi: 10.1007/s11481-012-9383-y. [DOI] [PubMed] [Google Scholar]
- Baumer T, Lange R, Liepert J, Weiller C, Siebner HR, Rothwell JC, Munchau A. Repeated premotor rTMS leads to cumulative plastic changes of motor cortex excitability in humans. NeuroImage. 2003;20:550–560. doi: 10.1016/s1053-8119(03)00310-0. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, McClung J, Shah MN, Cheng YT, Flesher W, Kraus J. Mild traumatic brain injury in the United States, 1998--2000. Brain Inj. 2005;19:85–91. doi: 10.1080/02699050410001720158. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Berg SA, Czachowski CL, Chambers RA. Alcohol seeking and consumption in the NVHL neurodevelopmental rat model of schizophrenia. Behav Brain Res. 2011;218:346–349. doi: 10.1016/j.bbr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenburg F, Ruff CC, Bestmann S, Bjoertomt O, Eshel N, Josephs O, Weiskopf N, Driver J. Interhemispheric effect of parietal TMS on somatosensory response confirmed directly with concurrent TMS-fMRI. J Neurosci. 2008;28:13202–13208. doi: 10.1523/JNEUROSCI.3043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Rocha M, Oliveira MO, Fecteau S, Cohen RB, Campanha C, Ferreira-Santos E, Meleiro A, Corchs F, Zaghi S, Pascual-Leone A, Fregni F. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. J Clin Psychiatry. 2010;71:992–999. doi: 10.4088/JCP.08m04638blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnì S, Mastropasqua C, Bozzali M, Caltagirone C, Koch G. Theta burst stimulation improves visuo-spatial attention in a patient with traumatic brain injury. Neurol Sci. 2013;34:2053–2056. doi: 10.1007/s10072-013-1412-y. [DOI] [PubMed] [Google Scholar]
- Bottlender M, Soyka M. Impact of craving on alcohol relapse during, and 12 months following, outpatient treatment. Alcohol Alcohol. 2004;39:357–361. doi: 10.1093/alcalc/agh073. [DOI] [PubMed] [Google Scholar]
- Brenner LA, Ivins BJ, Schwab K, Warden D, Nelson LA, Jaffee M, Terrio H. Traumatic brain injury, posttraumatic stress disorder, and postconcussive symptom reporting among troops returning from iraq. J Head Trauma Rehabil. 2010;25:307–312. doi: 10.1097/HTR.0b013e3181cada03. [DOI] [PubMed] [Google Scholar]
- Brown VM, LaBar KS, Haswell CC, Gold AL, McCarthy G, Morey RA. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology. 2014;39:351–359. doi: 10.1038/npp.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burnett-Zeigler I, Ilgen M, Valenstein M, Zivin K, Gorman L, Blow A, Duffy S, Chermack S. Prevalence and correlates of alcohol misuse among returning Afghanistan and Iraq veterans. Addict Behav. 2011;36:801–806. doi: 10.1016/j.addbeh.2010.12.032. [DOI] [PubMed] [Google Scholar]
- Carlson KF, Nelson D, Orazem RJ, Nugent S, Cifu DX, Sayer NA. Psychiatric diagnoses among Iraq and Afghanistan war veterans screened for deployment-related traumatic brain injury. J Trauma Stress. 2010;23:17–24. doi: 10.1002/jts.20483. [DOI] [PubMed] [Google Scholar]
- Casula EP, Tarantino V, Basso D, Arcara G, Marino G, Toffolo GM, Rothwell JC, Bisiacchi PS. Low-frequency rTMS inhibitory effects in the primary motor cortex: Insights from TMS-evoked potentials. NeuroImage. 2014;98:225–232. doi: 10.1016/j.neuroimage.2014.04.065. [DOI] [PubMed] [Google Scholar]
- Chakravorty S, Kuna ST, Zaharakis N, O’Brien CP, Kampman KM, Oslin D. Covariates of craving in actively drinking alcoholics. Am J Addict. 2010;19:450–457. doi: 10.1111/j.1521-0391.2010.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Self DW. Motivational responses to natural and drug rewards in rats with neonatal ventral hippocampal lesions: an animal model of dual diagnosis schizophrenia. Neuropsychopharmacology. 2002;27:889–905. doi: 10.1016/S0893-133X(02)00365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biol Psychiatry. 2001;50:71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Bickel WK, Potenza MN. A scale-free systems theory of motivation and addiction. Neurosci Biobehav Rev. 2007a;31:1017–1045. doi: 10.1016/j.neubiorev.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Sajdyk TJ, Conroy SK, Lafuze JE, Fitz SD, Shekhar A. Neonatal amygdala lesions: co-occurring impact on social/fear-related behavior and cocaine sensitization in adult rats. Behav Neurosci. 2007b;121:1316–1327. doi: 10.1037/0735-7044.121.6.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Sentir AM, Conroy SK, Truitt WA, Shekhar A. Cortical-striatal integration of cocaine history and prefrontal dysfunction in animal modeling of dual diagnosis. Biol Psychiatry. 2010;67:788–792. doi: 10.1016/j.biopsych.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PloS One. 2009;4:e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36:2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay MB, Glover KL, Lowe DT. Epidemiology of concussion in sport: a literature review. J Chiropr Med. 2013;12:230–251. doi: 10.1016/j.jcm.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Kaplan Z, Kotler M, Kouperman I, Moisa R, Grisaru N. Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2004;161:515–524. doi: 10.1176/appi.ajp.161.3.515. [DOI] [PubMed] [Google Scholar]
- Conroy SK, Rodd Z, Chambers RA. Ethanol sensitization in a neurodevelopmental lesion model of schizophrenia in rats. Pharmacol Biochem Behav. 2007;86:386–394. doi: 10.1016/j.pbb.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordovil De Sousa Uva M, Luminet O, Cortesi M, Constant E, Derely M, De Timary P. Distinct effects of protracted withdrawal on affect, craving, selective attention and executive functions among alcohol-dependent patients. Alcohol Alcohol. 2010;45:241–246. doi: 10.1093/alcalc/agq012. [DOI] [PubMed] [Google Scholar]
- Cosentino G, Giglia G, Palermo A, Panetta ML, Lo Baido R, Brighina F, Fierro B. A case of post-traumatic complex auditory hallucinosis treated with rTMS. Neurocase. 2010;16:267–272. doi: 10.1080/13554790903456191. [DOI] [PubMed] [Google Scholar]
- De Ridder D, Vanneste S, Kovacs S, Sunaert S, Dom G. Transient alcohol craving suppression by rTMS of dorsal anterior cingulate: an fMRI and LORETA EEG study. Neurosci Lett. 2011;496:5–10. doi: 10.1016/j.neulet.2011.03.074. [DOI] [PubMed] [Google Scholar]
- Deng ZD, Lisanby SH, Peterchev AV. Coil design considerations for deep transcranial magnetic stimulation. Clin Neurophysiol. 2014;125:1202–1212. doi: 10.1016/j.clinph.2013.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan S, Martindale S, Robinson J, Kimbrel NA, Meyer EC, Kruse MI, Morissette SB, Young KA, Gulliver SB. Neuropsychological sequelae of PTSD and TBI following war deployment among OEF/OIF veterans. Neuropsychol Rev. 2012;22:21–34. doi: 10.1007/s11065-012-9190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GA, Cristian A. Blast-related mild traumatic brain injury: mechanisms of injury and impact on clinical care. Mt Sinai J Med. 2009;76:111–118. doi: 10.1002/msj.20098. [DOI] [PubMed] [Google Scholar]
- Erhardt A, Sillaber I, Welt T, Muller MB, Singewald N, Keck ME. Repetitive transcranial magnetic stimulation increases the release of dopamine in the nucleus accumbens shell of morphine-sensitized rats during abstinence. Neuropsychopharmacology. 2004;29:2074–2080. doi: 10.1038/sj.npp.1300493. [DOI] [PubMed] [Google Scholar]
- Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G. A direct demonstration of cortical LTP in humans: a combined TMS/EEG study. Brain Res Bull. 2006;69:86–94. doi: 10.1016/j.brainresbull.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini T, Matthaus L, Rothkegel H, Sommer M, Tergau F, Schweikard A, Paulus W, Nitsche MA. H-coil: Induced electric field properties and input/output curves on healthy volunteers, comparison with a standard figure-of-eight coil. Clin Neurophysiol. 2009;120:1174–1182. doi: 10.1016/j.clinph.2009.02.176. [DOI] [PubMed] [Google Scholar]
- Falk D, Yi H, Hiller-Sturmhofel S. The epidemiological analysis of co-ocurring alcohol and other drug use and disorders. Alcohol Res Health. 2008;31:155. [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Jovanovic T, Glover EM, Bradley B, Choi K, Ely T, Gutman DA, Ressler KJ. White matter integrity in highly traumatized adults with and without post-traumatic stress disorder. Neuropsychopharmacology. 2012;37:2740–2746. doi: 10.1038/npp.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng SF, Shi TY, Fan Y, Wang WN, Chen YC, Tan QR. Long-lasting effects of chronic rTMS to treat chronic rodent model of depression. Behav Brain Res. 2012;232:245–251. doi: 10.1016/j.bbr.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Haraldsson HM, Barnhart TE, Roberts AD, Oakes TR, Massimini M, Stone CK, Kalin NH, Tononi G. A [17F]-fluoromethane PET/TMS study of effective connectivity. Brain Res Bull. 2004;64:103–113. doi: 10.1016/j.brainresbull.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33:1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Hoy KE, Maller JJ, Herring S, Segrave R, McQueen S, Peachey A, Hollander Y, Anderson JF, Daskalakis ZJ. Transcranial magnetic stimulation for depression after a traumatic brain injury: a case study. J ECT. 2011;27:38–40. doi: 10.1097/YCT.0b013e3181eb30c6. [DOI] [PubMed] [Google Scholar]
- Flannery BA, Poole SA, Gallop RJ, Volpicelli JR. Alcohol craving predicts drinking during treatment: an analysis of three assessment instruments. J Stud Alcohol. 2003;64:120–126. doi: 10.15288/jsa.2003.64.120. [DOI] [PubMed] [Google Scholar]
- Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. NeuroImage. 2012;66c:151–160. doi: 10.1016/j.neuroimage.2012.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS. Transcranial magnetic stimulation for the treatment of depression. Expert Rev Neurother. 2010;10:1761–1772. doi: 10.1586/ern.10.95. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry. 2001;58:345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- George MS, Raman R, Benedek DM, Pelic CG, Grammer GG, Stokes KT, Schmidt M, Spiegel C, Dealmeida N, Beaver KL, Borckardt JJ, Sun X, Jain S, Stein MB. A two-site pilot randomized 3 day trial of high dose left prefrontal repetitive transcranial magnetic stimulation (rTMS) for suicidal inpatients. Brain Stimul. 2014;7:421–431. doi: 10.1016/j.brs.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Gersner R, Kravetz E, Feil J, Pell G, Zangen A. Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: differential outcomes in anesthetized and awake animals. J Neurosci. 2011;31:7521–7526. doi: 10.1523/JNEUROSCI.6751-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DP, Cardon AL. An update on substance use and treatment following traumatic brain injury. Ann N Y Acad Sci. 2008;1141:148–162. doi: 10.1196/annals.1441.029. [DOI] [PubMed] [Google Scholar]
- Grisaru N, Amir M, Cohen H, Kaplan Z. Effect of transcranial magnetic stimulation in posttraumatic stress disorder: a preliminary study. Biol Psychiatry. 1998;44:52–55. doi: 10.1016/s0006-3223(98)00016-x. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham TE, Bonnelle V, Hellyer P, Jilka S, Robertson IH, Leech R, Sharp DJ. The neural basis of impaired self-awareness after traumatic brain injury. Brain. 2014;137:586–597. doi: 10.1093/brain/awt350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G, Mehta MA, Harmer C, Spinks TJ, Grasby PM, Goodwin GM. Exploring the physiological effects of double-cone coil TMS over the medial frontal cortex on the anterior cingulate cortex: an H2(15)O PET study. Eur J Neurosci. 2007;25:2224–2233. doi: 10.1111/j.1460-9568.2007.05430.x. [DOI] [PubMed] [Google Scholar]
- Hermann D, Smolka MN, Wrase J, Klein S, Nikitopoulos J, Georgi A, Braus DF, Flor H, Mann K, Heinz A. Blockade of cue-induced brain activation of abstinent alcoholics by a single administration of amisulpride as measured with fMRI. Alcohol Clin Exp Res. 2006;30:1349–1354. doi: 10.1111/j.1530-0277.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- Herremans SC, Baeken C, Vanderbruggen N, Vanderhasselt MA, Zeeuws D, Santermans L, De Raedt R. No influence of one right-sided prefrontal HF-rTMS session on alcohol craving in recently detoxified alcohol-dependent patients: results of a naturalistic study. Drug Alcohol Depend. 2012;120:209–213. doi: 10.1016/j.drugalcdep.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Herremans SC, Vanderhasselt MA, De Raedt R, Baeken C. Reduced intra-individual reaction time variability during a Go-NoGo task in detoxified alcohol-dependent patients after one right-sided dorsolateral prefrontal HF-rTMS session. Alcohol Alcohol. 2013;48:552–557. doi: 10.1093/alcalc/agt054. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Theoret H, Pascual-Leone A. Enhanced visual spatial attention ipsilateral to rTMS-induced ‘virtual lesions’ of human parietal cortex. Nat Neurosci. 2001;4:953–957. doi: 10.1038/nn0901-953. [DOI] [PubMed] [Google Scholar]
- Hillary FG, Slocomb J, Hills EC, Fitzpatrick NM, Medaglia JD, Wang J, Good DC, Wylie GR. Changes in resting connectivity during recovery from severe traumatic brain injury. Int J Psychophysiol. 2011;82:115–123. doi: 10.1016/j.ijpsycho.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Hoffman JM, Dikmen S, Temkin N, Bell KR. Development of posttraumatic stress disorder after mild traumatic brain injury. Arch Phys Med Rehabil. 2012;93:287–292. doi: 10.1016/j.apmr.2011.08.041. [DOI] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Holm L, Cassidy JD, Carroll LJ, Borg J. Summary of the WHO collaborating centre for neurotrauma task force on mild traumatic brain injury. J Rehabil Med. 2005;37:137–141. doi: 10.1080/16501970510027321. [DOI] [PubMed] [Google Scholar]
- Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3:95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Höppner J, Broese T, Wendler L, Berger C, Thome J. Repetitive transcranial magnetic stimulation (rTMS) for treatment of alcohol dependence. World J Biol Psychiatry 12 Suppl. 2011;1:57–62. doi: 10.3109/15622975.2011.598383. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Naatanen R, Katila T. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8:3537–3540. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- Isserles M, Shalev AY, Roth Y, Peri T, Kutz I, Zlotnick E, Zangen A. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder--a pilot study. Brain Stimul. 2013;6:377–383. doi: 10.1016/j.brs.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Janiri L, Gobbi G, Mannelli P, Pozzi G, Serretti A, Tempesta E. Effects of fluoxetine at antidepressant doses on short-term outcome of detoxified alcoholics. Int Clin Psychopharmacol. 1996;11:109–117. [PubMed] [Google Scholar]
- Jeanblanc J, Balguerie K, Coune F, Legastelois R, Jeanblanc V, Naassila M. Light alcohol intake during adolescence induces alcohol addiction in a neurodevelopmental model of schizophrenia. Addict Biol. 2014 doi: 10.1111/adb.12146. doi: 10.1111/adb.12146. [DOI] [PubMed] [Google Scholar]
- Johnson B, Zhang K, Gay M, Horovitz S, Hallett M, Sebastianelli W, Slobounov S. Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. NeuroImage. 2012;59:511–518. doi: 10.1016/j.neuroimage.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge RE, Acion L, White T, Tordesillas-Gutierrez D, Pierson R, Crespo-Facorro B, Magnotta VA. White matter abnormalities in veterans with mild traumatic brain injury. Am J Psychiatry. 2012;169:1284–1291. doi: 10.1176/appi.ajp.2012.12050600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabel DI, Petty F. A placebo-controlled, double-blind study of fluoxetine in severe alcohol dependence: adjunctive pharmacotherapy during and after inpatient treatment. Alcohol Clin Exp Res. 1996;20:780–784. doi: 10.1111/j.1530-0277.1996.tb01686.x. [DOI] [PubMed] [Google Scholar]
- Kanny D, Liu Y, Brewer RD, Garvin WS, Balluz L. Vital Signs: Binge Drinking Prevalence, Frequency, and Intensity Among Adults - United States, 2010. MMWR Morb Mortal Wkly Rep. 2013;62:14–19. [PubMed] [Google Scholar]
- Karsen EF, Watts BV, Holtzheimer PE. Review of the effectiveness of transcranial magnetic stimulation for post-traumatic stress disorder. Brain Stimul. 2014;7:151–157. doi: 10.1016/j.brs.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Lyoo IK, Kim SJ, Sim M, Kim N, Choi N, Jeong DU, Covell J, Renshaw PF. Disrupted white matter tract integrity of anterior cingulate in trauma survivors. Neuroreport. 2005;16:1049–1053. doi: 10.1097/00001756-200507130-00004. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Jeong DU, Sim ME, Bae SC, Chung A, Kim MJ, Chang KH, Ryu J, Renshaw PF, Lyoo IK. Asymmetrically altered integrity of cingulum bundle in posttraumatic stress disorder. Neuropsychobiology. 2006;54:120–125. doi: 10.1159/000098262. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski L, Kolivakis T, Yu C, Chen JK, Delaney S, Ptito A. Noninvasive brain stimulation for persistent postconcussion symptoms in mild traumatic brain injury. J Neurotrauma In press. 2014 doi: 10.1089/neu.2014.3449. [DOI] [PubMed] [Google Scholar]
- Kozel FA, Johnson KA, Nahas Z, Nakonezny PA, Morgan PS, Anderson BS, Kose S, Li X, Lim KO, Trivedi MH, George MS. Fractional anisotropy changes after several weeks of daily left high-frequency repetitive transcranial magnetic stimulation of the prefrontal cortex to treat major depression. J ECT. 2011;27:5–10. doi: 10.1097/YCT.0b013e3181e6317d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer PM, Landgrebe M, Frank E, Langguth B. Repetitive transcranial magnetic stimulation for the treatment of chronic tinnitus after traumatic brain injury: a case study. J Head Trauma Rehabil. 2013;28:386–389. doi: 10.1097/HTR.0b013e318254736e. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Lipton ML, Gulko E, Zimmerman ME, Friedman BW, Kim M, Gellella E, Gold T, Shifteh K, Ardekani BA, Branch CA. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology. 2009;252:816–824. doi: 10.1148/radiol.2523081584. [DOI] [PubMed] [Google Scholar]
- Lipton ML, Kim N, Park YK, Hulkower MB, Gardin TM, Shifteh K, Kim M, Zimmerman ME, Lipton RB, Branch CA. Robust detection of traumatic axonal injury in individual mild traumatic brain injury patients: intersubject variation, change over time and bidirectional changes in anisotropy. Brain Imaging Behav. 2012;6:329–342. doi: 10.1007/s11682-012-9175-2. [DOI] [PubMed] [Google Scholar]
- Lisanby SH. New York: Oxford University Press; 2008. The Oxford Handbook of Transcranial Stimulation. [Google Scholar]
- Lowman C, Hunt WA, Litten RZ, Drummond DC. Research perspectives on alcohol craving: an overview. Addiction. 2000;95(Suppl 2):S45–54. doi: 10.1080/09652140050111636. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Pruessner J, Sousa N, Almeida OF, Van Dam AM, Rajkowska G, Swaab DF, Czeh B. Neuropathology of stress. Acta Neuropathol. 2014;127:109–135. doi: 10.1007/s00401-013-1223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas AI, Marmarou A, Murray GD, Teasdale SG, Steyerberg EW. Prognosis and clinical trial design in traumatic brain injury: the IMPACT study. J Neurotrauma. 2007;24:232–238. doi: 10.1089/neu.2006.0024. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275–293. doi: 10.1111/j.1360-0443.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki-Marttunen V, Diez I, Cortes JM, Chialvo DR, Villarreal M. Disruption of transfer entropy and inter-hemispheric brain functional connectivity in patients with disorder of consciousness. Front Neuroinform. 2013;7:24. doi: 10.3389/fninf.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SC, Spadoni AD, Lohr JB, Strigo IA, Simmons AN. Diffusion tensor imaging evidence of white matter disruption associated with loss versus alteration of consciousness in warfighters exposed to combat in Operations Enduring and Iraqi Freedom. Psychiatry Res. 2012;204:149–154. doi: 10.1016/j.pscychresns.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW. Neurobehavioral sequelae of traumatic brain injury: evaluation and management. World Psychiatry. 2008;7:3–10. doi: 10.1002/j.2051-5545.2008.tb00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Kimbrell TA, Morgan CM, Anderson T, Geraci M, Benson BE, Wassermann EM, Willis MW, Post RM. Repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Arch Gen Psychiatry. 1998;55:276–279. doi: 10.1001/archpsyc.55.3.276. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. ed Second. New York: Oxford University Press, Inc; 2000. Principles of Behavioral and Cognitive Neurology. [Google Scholar]
- Mishra BR, Nizamie SH, Das B, Praharaj SK. Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction. 2010;105:49–55. doi: 10.1111/j.1360-0443.2009.02777.x. [DOI] [PubMed] [Google Scholar]
- Mishra BR, Praharaj SK, Katshu MZ, Sarkar S, Nizamie SH. Comparison of Anticraving Efficacy of Right and Left Repetitive Transcranial Magnetic Stimulation in Alcohol Dependence: A Randomized Double-Blind Study. J Neuropsychiatry Clin Neurosci. 2014 doi: 10.1176/appi.neuropsych.13010013. doi: 10.1176/appi.neuropsych.13010013. [DOI] [PubMed] [Google Scholar]
- Monnig MA, Caprihan A, Yeo RA, Gasparovic C, Ruhl DA, Lysne P, Bogenschutz MP, Hutchison KE, Thoma RJ. Diffusion tensor imaging of white matter networks in individuals with current and remitted alcohol use disorders and comorbid conditions. Psychol Addict Behav. 2013;27:455–465. doi: 10.1037/a0027168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottaghy FM, Gangitano M, Sparing R, Krause BJ, Pascual-Leone A. Segregation of areas related to visual working memory in the prefrontal cortex revealed by rTMS. Cereb Cortex. 2002;12:369–375. doi: 10.1093/cercor/12.4.369. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- Nakama H, Garcia A, O’Brien K, Ellis N. Case report of a 24-year-old man with resolution of treatment-resistant major depressive disorder and comorbid PTSD using rTMS. J ECT. 2014;30:e9–10. doi: 10.1097/YCT.0b013e182a2705d. [DOI] [PubMed] [Google Scholar]
- National Center for Injury Prevention and Control. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem; Atlanta, GA, Centers for Disease Control and Prevention. 2002 [Google Scholar]
- O’Neil ME, Carlson K, Storzbach D, Brenner L, Freeman M, Quinones A, Motu’apuaka M, Ensley M, Kansagara D. Complications of Mild Traumatic Brain Injury in Veterans and Military Personnel: A Systematic Review. Washington (DC): Department of Veterans Affairs (US); VA Evidence-based Synthesis Program Reports. [PubMed] [Google Scholar]
- O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, Demitrack MA, George MS, Sackeim HA. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Matsuda H, Imabayashi E, Okabe S, Takano H, Arai N, Ugawa Y. rCBF changes elicited by rTMS over DLPFC in humans. Suppl Clin Neurophysiol. 2004;57:715–720. doi: 10.1016/s1567-424x(09)70412-x. [DOI] [PubMed] [Google Scholar]
- Osuch EA, Benson BE, Luckenbaugh DA, Geraci M, Post RM, McCann U. Repetitive TMS combined with exposure therapy for PTSD: a preliminary study. J Anxiety Disord. 2009;23:54–59. doi: 10.1016/j.janxdis.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachalska M, Lukowicz M, Kropotov JD, Herman-Sucharska I, Talar J. Evaluation of differentiated neurotherapy programs for a patient after severe TBI and long term coma using event-related potentials. Med Sci Monit. 2011;17:Cs120–128. doi: 10.12659/MSM.881970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit AS, Expert P, Lambiotte R, Bonnelle V, Leech R, Turkheimer FE, Sharp DJ. Traumatic brain injury impairs small-world topology. Neurology. 2013;80:1826–1833. doi: 10.1212/WNL.0b013e3182929f38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape T. Acute neurorehabilitation for disorders of consciousness. In: Selzer ME, Clarke S, Cohen LG, Kwakkel G, Miller RH, editors. The Textbook of Neural Repair and Rehabilitation. Cambridge, UK: Cambridge University Press; 2014a. pp. 385–404. [Google Scholar]
- Pape TL, High WM, Jr, St Andre J, Evans C, Smith B, Shandera-Ochsner AL, Wingo J, Moallem I, Baldassarre M, Babcock-Parziale J. Diagnostic accuracy studies in mild traumatic brain injury: a systematic review and descriptive analysis of published evidence. PM R. 2013;5:856–881. doi: 10.1016/j.pmrj.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Pape T, Rosenow J, Lewis G, Ahmed G, Walker M, Guernon A, Roth H, Patil V. Repetitive transcranial magnetic stimulation-associated neurobehavioral gains during coma recovery. Brain Stimul. 2009;2:22–35. doi: 10.1016/j.brs.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Pape TL, Rosenow JM, Patil V, Steiner M, Harton B, Guernon A, Herrold A, Pacheco M, Crisan E, Ashley WW, Jr, Odle C, Park Y, Chawla J, Sarkar K. RTMS safety for two subjects with disordered consciousness after traumatic brain injury. Brain Stimul. 2014b;7:620–622. doi: 10.1016/j.brs.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Park MS, Sohn JH, Suk JA, Kim SH, Sohn S, Sparacio R. Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol Alcohol. 2007;42:417–422. doi: 10.1093/alcalc/agl117. [DOI] [PubMed] [Google Scholar]
- Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J, Heinz A. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosci. 2010;30:7749–7753. doi: 10.1523/JNEUROSCI.5587-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond JE, Sullivan EV. Thinning of the corpus callosum in older alcoholic men: a magnetic resonance imaging study. Alcohol Clin Exp Res. 1996;20:752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin Exp Res. 2000;24:1214–1221. [PubMed] [Google Scholar]
- Pleger B, Blankenburg F, Bestmann S, Ruff CC, Wiech K, Stephan KE, Friston KJ, Dolan RJ. Repetitive transcranial magnetic stimulation-induced changes in sensorimotor coupling parallel improvements of somatosensation in humans. J Neurosci. 2006;26:1945–1952. doi: 10.1523/JNEUROSCI.4097-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polusny MA, Kehle SM, Nelson NW, Erbes CR, Arbisi PA, Thuras P. Longitudinal effects of mild traumatic brain injury and posttraumatic stress disorder comorbidity on postdeployment outcomes in national guard soldiers deployed to Iraq. Arch Gen Psychiatry. 2011;68:79–89. doi: 10.1001/archgenpsychiatry.2010.172. [DOI] [PubMed] [Google Scholar]
- Prisciandaro JJ, Brown DG, Brady KT, Tolliver BK. Comorbid anxiety disorders and baseline medication regimens predict clinical outcomes in individuals with co-occurring bipolar disorder and alcohol dependence: Results of a randomized controlled trial. Psychiatry Res. 2011;188:361–365. doi: 10.1016/j.psychres.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B, Phan KL. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry. 2011;2:62. doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapinesi C, Kotzalidis GD, Serata D, Del Casale A, Bersani FS, Solfanelli A, Scatena P, Raccah RN, Brugnoli R, Digiacomantonio V, Carbonetti P, Fensore C, Tatarelli R, Angeletti G, Ferracuti S, Girardi P. Efficacy of add-on deep transcranial magnetic stimulation in comorbid alcohol dependence and dysthymic disorder: three case reports. Prim Care Companion CNS Disord. 2013 doi: 10.4088/PCC.12m01438. 15pii: PCC.12m01438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg PB, Mehndiratta RB, Mehndiratta YP, Wamer A, Rosse RB, Balish M. Repetitive transcranial magnetic stimulation treatment of comorbid posttraumatic stress disorder and major depression. J Neuropsychiatry Clin Neurosci. 2002;14:270–276. doi: 10.1176/jnp.14.3.270. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, Rees G, Josephs O, Deichmann R, Driver J. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Sack AT, Kohler A, Bestmann S, Linden DE, Dechent P, Goebel R, Baudewig J. Imaging the brain activity changes underlying impaired visuospatial judgments: simultaneous FMRI, TMS, and behavioral studies. Cereb Cortex. 2007;17:2841–2852. doi: 10.1093/cercor/bhm013. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addiction Biol. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Goudriaan AE, Joos L, Kruse AM, Dom G, van den Brink W, Veltman DJ. Modafinil modulates resting-state functional network connectivity and cognitive control in alcohol-dependent patients. Biol Psychiatry. 2013;73:789–795. doi: 10.1016/j.biopsych.2012.12.025. [DOI] [PubMed] [Google Scholar]
- Schuff N, Zhang Y, Zhan W, Lenoci M, Ching C, Boreta L, Mueller SG, Wang Z, Marmar CR, Weiner MW, Neylan TC. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: an MRI study. NeuroImage. 2011;54(Suppl 1):S62–68. doi: 10.1016/j.neuroimage.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA psychiatry. 2013;70:727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Beckmann CF, Greenwood R, Kinnunen KM, Bonnelle V, De Boissezon X, Powell JH, Counsell SJ, Patel MC, Leech R. Default mode network functional and structural connectivity after traumatic brain injury. Brain. 2011;134:2233–2247. doi: 10.1093/brain/awr175. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer AM, Willis MW, Herscovitch P, Daube-Witherspoon M, Shelton JR, Benson BE, Post RM, Wassermann EM. Intensity-dependent regional cerebral blood flow during 1-Hz repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers studied with H215O positron emission tomography: II. Effects of prefrontal cortex rTMS. Biol Psychiatry. 2003;54:826–832. doi: 10.1016/s0006-3223(03)00324-x. [DOI] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012;37:241–249. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21:Rc157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanielian T, Jaycox LH. RAND Corporation; 2008. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. [Google Scholar]
- Vanderploeg RD, Belanger HG, Curtiss G. Mild traumatic brain injury and posttraumatic stress disorder and their associations with health symptoms. Arch Phys Med Rehabil. 2009;90:1084–1093. doi: 10.1016/j.apmr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Wichert S, Rabinstein J, Buhler M, Klein O, Ende G, Hermann D, Mann K. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010;105:1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Wang JX, Rogers LM, Gross EZ, Ryals AJ, Dokucu ME, Brandstatt KL, Hermiller MS, Voss JL. Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science. 2014;345:1054–1057. doi: 10.1126/science.1252900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5-7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Watts BV, Landon B, Groft A, Young-Xu Y. A sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Brain Stimul. 2012;5:38–43. doi: 10.1016/j.brs.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Williams D, McBride AJ. The drug treatment of alcohol withdrawal symptoms: a systematic review. Alcohol Alcohol. 1998;33:103–115. doi: 10.1093/oxfordjournals.alcalc.a008365. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global Information System on Alcohol and Health (GISAH) 2014 [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. NeuroImage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, Caviness VS, Hodge SM, Tang L, Albaugh M, Ziegler DA, Davis OC, Kissling C, Schumann G, Breiter HC, Heinz A. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Yoon SJ, Pae CU, Kim DJ, Namkoong K, Lee E, Oh DY, Lee YS, Shin DH, Jeong YC, Kim JH, Choi SB, Hwang IB, Shin YC, Cho SN, Lee HK, Lee CT. Mirtazapine for patients with alcohol dependence and comorbid depressive disorders: a multicentre, open label study. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1196–1201. doi: 10.1016/j.pnpbp.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Yukimasa T, Yoshimura R, Tamagawa A, Uozumi T, Shinkai K, Ueda N, Tsuji S, Nakamura J. High-frequency repetitive transcranial magnetic stimulation improves refractory depression by influencing catecholamine and brain-derived neurotrophic factors. Pharmacopsychiatry. 2006;39:52–59. doi: 10.1055/s-2006-931542. [DOI] [PubMed] [Google Scholar]
- Zangen A, Hyodo K. Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in the nucleus accumbens. Neuroreport. 2002;13:2401–2405. doi: 10.1097/00001756-200212200-00005. [DOI] [PubMed] [Google Scholar]
- Zangen A, Roth Y, Voller B, Hallett M. Transcranial magnetic stimulation of deep brain regions: evidence for efficacy of the H-coil. Clin Neurophysiol. 2005;116:775–779. doi: 10.1016/j.clinph.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang Y, Li L, Li Z, Li W, Ma N, Hou C, Zhang Z, Zhang Z, Wang L, Duan L, Lu G. Different white matter abnormalities between the first-episode, treatment-naive patients with posttraumatic stress disorder and generalized anxiety disorder without comorbid conditions. J Affect Disord. 2011;133:294–299. doi: 10.1016/j.jad.2011.03.040. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Milham MP, Lui YW, Miles L, Reaume J, Sodickson DK, Grossman RI, Ge Y. Default-mode network disruption in mild traumatic brain injury. Radiology. 2012a;265:882–892. doi: 10.1148/radiol.12120748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Z, Qin LD, Wan JQ, Sun YW, Su SS, Ding WN, Xu JR. Early altered resting-state functional connectivity predicts the severity of post-traumatic stress disorder symptoms in acutely traumatized subjects. PLoS One. 2012b;7:e46833. doi: 10.1371/journal.pone.0046833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziad N. New York, NY: Oxford University Press Inc; 2002. Handbook of Transcranial Magnetic Stimulation. [Google Scholar]