Abstract
Neural stem cells are characterized by the ability to differentiate and stably express exogenous ge-nes. Vascular endothelial growth factor plays a role in protecting local blood vessels and neurons of newborn rats with hypoxic-ischemic encephalopathy. Transplantation of vascular endothelial growth factor-transfected neural stem cells may be neuroprotective in rats with cerebral palsy. In this study, 7-day-old Sprague-Dawley rats were divided into five groups: (1) sham operation (control), (2) cerebral palsy model alone or with (3) phosphate-buffered saline, (4) vascular endothelial growth factor 165 + neural stem cells, or (5) neural stem cells alone. The cerebral palsy model was established by ligating the left common carotid artery followed by exposure to hypoxia. Phosphate-buffered saline, vascular endothelial growth factor + neural stem cells, and neural stem cells alone were administered into the sensorimotor cortex using the stereotaxic instrument and microsyringe. After transplantation, the radial-arm water maze test and holding test were performed. Immunohistochemistry for vascular endothelial growth factor and histology using hematoxylin-eosin were performed on cerebral cortex. Results revealed that the number of vascular endothelial growth factor-positive cells in cerebral palsy rats transplanted with vascular endothelial growth factor-transfected neural stem cells was increased, the time for finding water and the finding repetitions were reduced, the holding time was prolonged, and the degree of cell degeneration or necrosis was reduced. These findings indicate that the transplantation of vascular endothelial growth factor-transfected neural stem cells alleviates brain damage and cognitive deficits, and is neuroprotective in neonatal rats with hypoxia ischemic-mediated cerebral palsy.
Keywords: nerve regeneration, vascular endothelial growth factor, neural stem cells, cerebral palsy, animal model, transplantation, neuroprotection, NSFC grant, neural regeneration
Introduction
Children with cerebral palsy exhibit numerous dysfunctions, including mental retardation, epilepsy, behavioral disorders, disturbances in sensation and perception, and language deficits–all of which represent a serious threat to the child's health and development (Liu et al., 2012; Wang et al., 2012). Great progress has recently been made for the treatment and management of cerebral palsy. However, this treatment only provides symptomatic relief. Therefore, cerebral palsy still remains a very important topic in pediatric neurodevelopmental research (Novak et al., 2012).
Neural stem cells (NSCs) isolated from embryonic rodents, adult rodents, and human embryonic brain tissue have a strong ability for self-renewal and proliferation and the potential to differentiate into various cell types. NSCs in brain tissue contribute to the repair of neuronal function (Okano et al., 2002; Kim et al., 2007). However, because they are low in number and have a limited distribution range in the brain, developing NSCs exhibit a limited capacity for repairing neural damage (Li et al., 2010). NSCs are considered to be a delivery vehicle for gene therapy for diseases of the central nervous system (Liang et al., 2012; Zhang et al., 2012). Using transgenic technology, exogenous therapeutic genes are introduced into NSCs (Park et al., 2002), which are transplanted into the damaged region of the brain to replace apoptotic and necrotic neuronal cells (Zhang et al., 2009). These cells then express the exogenous therapeutic target gene for a prolonged period, combine the benefits of stem cell transplantation with gene therapy, and thereby have a broad application potential (Liu et al., 2010).
Vascular endothelial growth factor (VEGF)-mediated effects on vascular endothelial cells include accelerated proliferation and migration of these cells, the formation of new blood vessels, and an increase in vascular permeability (Zhang et al., 2000). Preliminary experiments of our group have confirmed that adenoviral vector-mediated transduction of VEGF165 improves neural functional recovery after hypoxic-ischemic brain damage in neonatal rats (Zheng et al., 2010a, b). However, other studies have demonstrated that transplantation of VEGF-transfected NSCs can be used to treat cerebral ischemia in adult rats. NSCs have been found to migrate to the host brain tissue and express VEGF gene products. This transplantation therapy has a protective effect on local blood vessels and neurons (Wang et al., 2007; Pimentel-Coelho et al., 2010). Our group has previously constructed a recombinant lentivirus vector containing the VEGF165 gene, pGC-FU-VEGF165, which transfects cultured NSCs and can be transplanted into the brain (Zhang et al., 2011). In the present study, we examined the neuroprotective effect of the transplantation of VEGF-transfected NSCs in neonatal rats with cerebral palsy to enable the development of a new treatment strategy for cerebral palsy.
Materials and Methods
Rat embryonic NSC culture and identification
Pregnant (gestation days 14–16) Sprague-Dawley rats (weighing 256 g) were provided by the Department of Animal Experiments in Xiangya Hospital of Central South University, China (license No. SCXK (Xiang) 2010-0002). The embryos were removed under sterile conditions and cells were dissociated with trypsin (0.25% trypsin and 0.02% ethylene diamine tetraacetic acid) for 10 minutes. Samples were then filtered and centrifuged at 1,000 r/min for 5 minutes to collect cells. The supernatant was removed and cells were then incubated in NSC-conditioned medium (DMEM/F12, basic fibroblast growth factor 20 ng/mL, epidermal cell growth factor 20 ng/mL, B27 1:50, insulin 100 ng/mL, heparin 5 μg/mL, glutamine 2.5 mmol/L), at 37°C in an atmosphere of 5% CO2. NSCs were harvested after primary culture and examined for the NSC marker, nestin, using immunofluorescence. As previously shown (Liu et al., 2009), these cells and the clones derived from them both expressed nestin, thus confirming these cells as NSCs (data not shown).
Construction of pGC-FU-VEGF165 and transfection into NSCs
The recombinant lentiviral vector, pGC-FU-VEGF165, containing VEGF165 (Wuhan Boster Biological Technology Co., Ltd., Wuhan, Hubei Province, China), was constructed in our laboratory and used to transfect NSCs. Briefly, primers were designed using the human VEGF165 gene sequence (AF486837) from GenBank (http://www.ncbi.nlm.nih.gov/genbank). The target gene was amplified using polymerase chain reaction (PCR) and cloned into the lentiviral vector, pGC-FU, to produce pGC-FU-VEGF carrying VEGF165 with green fluorescent protein as a marker. NSCs were transfected with pGC-FU-VEGF165, and green fluorescence was observed under a fluorescence microscope (Olympus, Tokyo, Japan). Reverse transcription PCR and sequencing confirmed that the target gene was correctly cloned into the lentiviral vector. NSCs transfected with recombinant lentivirus exhibited green fluorescence.
Animals and treatments
Male and female Sprague-Dawley rats (n = 150; 7 days old; 12–15 g) were randomly divided into five groups (n = 30/group) as follows: (1) sham operation (control group), (2) cerebral palsy model with or without (3) phosphate-buffered saline (PBS) transplantation, (4) VEGF-NSCs transplantation (VEGF + NSCs), and (5) NSCs transplantation alone (NSCs). Sham-operated rats only underwent a left common carotid artery isolation without ligation or hypoxia. All transplantations were performed 3 days after the cerebral palsy model was established.
Cerebral palsy model establishment
A unilateral occlusion of carotid artery, followed by exposure to hypoxia, is an accepted cerebral palsy animal model, named the Rice-way model (Walter et al., 2000). Establishment of this model is performed on 7-day-old rats because at this age, the surge in brain growth in the rat is comparable to that occurring in newborn humans (Walter et al., 2000; Kim et al., 2007). To eliminate pain and suffering, the 7-day-old rats were exposed to ether inhalation-mediated anesthesia, then placed in a supine position. A median neck incision was made and the left common carotid artery was ligated. The skin incision was then sutured. Rats were then returned to the cages containing their mothers for 2 hours and then placed in a 1 L plexiglass hypoxia chamber at 37°C. A nitrogen-oxygen mixture of 8.0 ± 0.1% oxygen was injected into the chamber at 1.0–2.0 L/min for 2 hours. The rats were then returned to their cages and fed by their mothers. Rats used for behavioral tests were weaned at 21 days and fed in separate cages according to their gender.
Intracerebral transplantation
Rats were fixed onto a stereotaxic apparatus. The transplantation site was located on the left sensorimotor cortex (coordinates AP: −0.3 mm, ML: −2 mm, DV: −1.5 mm [corresponding to 0.3 mm posterior to the bregma, 2 mm lateral to the bregma, and at a depth of 1.5 mm, respectively]) (Zheng et al., 2010a, b). Aliquots (2 μL) were injected using a perpendicular microsyringe (Bi-sen Electrical and Mechanical Equipment Engineering, Shanghai, China).
PBS, VEGF + NSCs and NSCs groups received a stereotaxic injection of 2 μL PBS buffer, NSCs transfected with recombinant lentivirus vector containing VEGF165 (lentivirus MOI = 50 TU/cell, 5 × 104 NSCs/μL), and normal NSCs (5 × 104 cells/μL) (Zhang et al., 2011, 2012), respectively, into the left sensorimotor cortex 3 days after the cerebral palsy model was established.
The injection process took more than 5 minutes, and the needle was kept for 5 minutes after the injection, then gradually pulled out over 5 minutes. The scalp was sutured and rats were returned to their cages after warming and awakening. Neurobehavioral tests were performed in a double blind manner after treatment.
Immunohistochemistry for VEGF using diaminobenzidine
Immunohistochemical detection was performed 7 days after transplantation. Rats were anesthetized with chloral hydrate, perfused with 4% paraformaldehyde and fixed. After decapitation, the left brain tissue was paraffin embedded and cut (coronal serial sections of 3 μm) from the bregma to 3 mm posterior to the bregma. Immunostaining was performed according to the VEGF immunohistochemistry kit instructions (Beijing Zhongshan Golden Bridge Biotechnology, Beijing, China). Slices were incubated with rabbit anti-mouse VEGF polyclonal antibody (1:100) or rabbit anti-mouse CD34 polyclonal antibody (1:50) at 4°C overnight. Biotin-labeled goat anti-rabbit antibody was then added and sections were incubated at 37°C for 15 minutes, followed by horseradish peroxidase-labeled streptavidin at 37°C for 15 minutes. Slices were rinsed three times with 0.01 mol/L PBS for 5 minutes. Immunoreactivity was detected with diaminobenzidine (Wuhan Boster Biological), then sections were counter-stained with hematoxylin-eosin and mounted with gum. Negative controls were incubated with 0.01 mol/L PBS, instead of primary antibody. VEGF-positive cells were counted from five fields of each slice (n = 2) under a fluorescence microscope (Leica, Solms, Germany). Overall, the mean number of positive cells for each group was obtained from a total of ten fields per rat from each group.
Radial arm water maze test
This test was performed on 30-day-old rats to assess their ability to observe, study, and memorize the environment. The eight-arm radial maze consisted of a central platform (30 cm in diameter) from which eight arms extended symmetrically (50 cm long and 12 cm wide).
A well was present at the outer end of each arm. Animals were deprived of water for 48 hours before testing. At the end of each daily session, the animals were allowed to drink for 30 minutes, and were then put back into their home cages. Before spatial discrimination testing, animals were allowed to freely explore the maze with all arms containing water-filled wells (baited) (50 μL per well) on 2 consecutive days before test days. For spatial discrimination testing, wells in only three arms were baited, and the sequence of angles between them was 135°, 90° and 135°.
Rats were tested for acquisition over three daily sessions consisting of five trials separated by 1-minute intervals. Each trial began with the placement of the animal on the central platform, facing arm 3, and ended when the rat had visited the three baited arms. The following data were recorded: (1) the time taken to visit the three baited arms, (2) the number of working memory errors (the re-entries into previously visited baited arms), and (3) the number of reference memory errors (the each entry into a non-baited arm).
Holding test
The holding test was used to evaluate rat sensorimotor function. The rat's forepaws grasped a hollow plastic tube (0.6 cm in diameter) placed horizontally and hung 45 cm above a desk. The time spent suspended (maximum 60 seconds) was recorded. A time greater than 60 seconds was recorded as 60 seconds.
Hematoxylin-eosin staining
Hematoxylin-eosin staining was performed on 35-day-old rats after the completion of the behavioral tests. Rats were deeply anesthetized with 10% chloral hydrate (600 mg/kg), the heart was exposed after thoracotomy, and a needle attached to a 20 mL syringe was inserted into the left ventricle. The right atrium was cut and sterile saline was injected until the perfusate cleared. The animal was then perfused with 50 mL of 4% paraformaldehyde in 0.1 mol/L PBS and the brain removed and immediately stored in the same solution. After 72 hours of fixation, the tissue was dehydrated, cleared, paraffin embedded, and cut into continuous coronal slices (at a thickness of 5 μm), 3 mm posterior to the bregma. The sections were transferred onto pre-treated glass slides, dewaxed, and morphological changes in the brain were examined with hematoxylin-eosin staining.
Statistical analysis
All data are expressed as the mean ± SD and were analyzed by one-way analysis of variance followed by the Student-Newman-Keuls post hoc test. Significance was reached at values of P < 0.05. Statistical analysis was performed using SPSS 17.0 software (SPSS, Chicago, IL, USA).
Results
Effect of VEGF165-transfected NSC transplantation on VEGF labeling and number of VEGF-positive cells in cerebral cortex of cerebral palsy rats
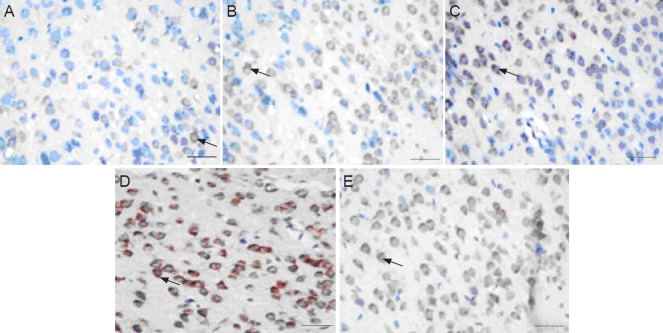
Immunoreactivity for VEGF in the cerebral cortex was very low in the control group but notably higher in the cerebral palsy group (Table 1, Figure 1), suggesting that hypoxia and ischemia increased VEGF expression. Quantification analysis of the number of VEGF-positive cells in the cerebral cortex revealed that the number of VEGF-positive cells did not differ between both cerebral palsy and PBS groups but significantly (P < 0.05) increased in both VEGF + NSCs and NSCs groups compared with the cerebral palsy or PBS groups. This finding indicates that transplantation of either NSCs alone or VEGF-transfected NSCs increases the amount of VEGF in the cortex. Furthermore, the number of VEGF-positive cells in the VEGF + NSCs group was significantly (P < 0.05) higher com-pared with the NSCs alone group, suggesting that in neonatal rats with cerebral palsy, VEGF levels are enhanced when these rats are transplanted with VEGF-transfected NSCs compared with the transplantation of NSCs alone.
Table 1.
Effect of VEGF165-transfected NSC transplantation on the number of VEGF-positive cells (n/400-fold field of view) in 7-day-old cerebral palsy rats
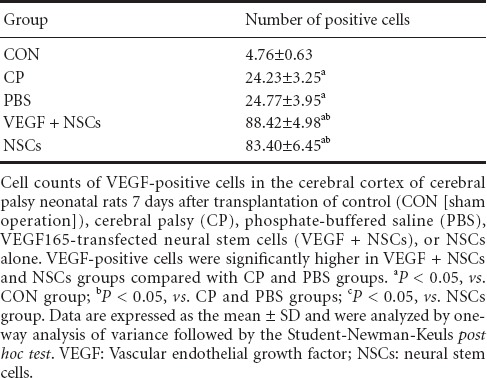
Figure 1.
Photomicrographs showing increased VEGF immunohistochemical staining in the VEGF + NSCs group.
Immunohistochemistry for VEGF using diaminobenzidine in cerebral cortex neurons of cerebral palsy neonatal rats 7 days after transplantation of control (A, sham operation), cerebral palsy (B), phosphate-buffered saline (C), VEGF165-transfected neural stem cells (D), or NSCs alone (E). VEGF-positive cells are shown with arrows. Scale bars: 100 μm. VEGF: Vascular endothelial growth factor; NSCs: neural stem cells.
Effect of VEGF165-transfected NSC transplantation on learning and memory in cerebral palsy rats
In the radial arm test (Table 2), significant (P < 0.05) differences were found between the control group and the remaining four groups.
Table 2.
Effect of VEGF165-transfected NSC transplantation on learning and memory in 30-day-old cerebral palsy rats
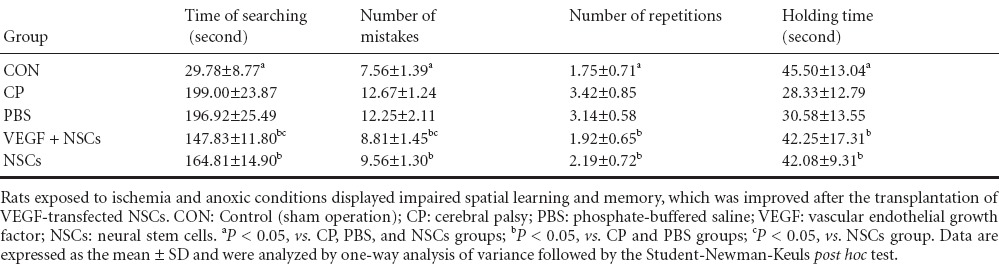
The cerebral palsy group and PBS group had similar times for searching in the water. The searching times in both VEGF + NSCs and NSCs groups were significantly (P < 0.05) shortened compared with both the cerebral palsy and PBS groups. However, animals in the NSCs group had markedly (P < 0.05) longer searching times in the water compared with the VEGF + NSCs group.
The average number of mistakes (Table 2) was similar between the cerebral palsy and PBS groups. The average number of mistakes were found to be significantly (P < 0.05) different between the remaining groups with mistakes found in the following groups (from fewer to greater): VEGF + NSCs group < NSCs group < PBS group. The average number of repetitions (Table 2) in the VEGF + NSCs group was not significantly different from that of the control group.
However, significant (P < 0.05) differences were found between the control group and the other four groups. No significant difference was detected between the cerebral palsy group and PBS group. The average number of repetitions was markedly (P < 0.05) lower in both VEGF + NSCs and NSCs groups compared with both cerebral palsy and PBS groups.
No significant difference was found between the VEGF + NSCs group and control group. The average number of repetitions in both cerebral palsy and PBS groups was significantly (P < 0.05) higher compared with both VEGF+ NSCs and control groups.
The holding times (Table 2) were significantly (P < 0.05) different between the control group and the other groups, except for the NSCs group. The holding times in both VEGF + NSCs and NSCs groups were significantly (P < 0.05) longer compared with both cerebral palsy and PBS groups. No significant difference was detected between the cerebral palsy group and PBS group, and between the VEGF + NSCs group and control group.
Effect of VEGF165-transfected NSC transplantation on cortical cells in cerebral palsy rats
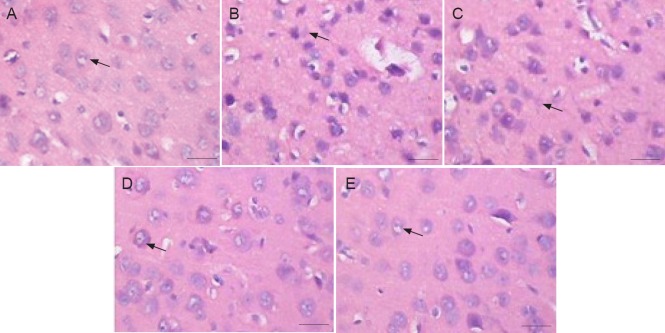
Cortical cells in the control group were neatly arranged and at a high density (Figure 2). Their intense staining revealed a normal and clear morphology and structure. In contrast, cells of both cerebral palsy and PBS groups were arranged in a disorderly manner and were less prominent. Furthermore, the cells showed signs of degeneration, necrosis, and local cystic degeneration. We also observed karyopyknosis, structural areas of cell bodies that were unclear or not visible, and cells with a gap-like cavity. This damage was typical of animal models of ischemia-hypoxia-mediated cerebral palsy. In both VEGF + NSCs and NSCs groups, degenerated or necrotic cells were not as abundant, and most cells exhibited a relatively normal cell structure with a clear morphology. Furthermore, cells were more prominent and karyopyknosis was not predominantly visible in these groups compared with both cerebral palsy and PBS groups.
Figure 2.
Effect of VEGF165-transfected NSC transplantation on cortical cell morphology in 35-day-old cerebral palsy rats (hematoxylin-eosin staining).
In the CON group (A), cells were neatly arranged. In both CP and PBS groups (B, C), cells were less prominent. In both VEGF + NSCs and NSCs groups (D, E), degenerated or necrotic cells were less prominent. Cells in both groups (D, E) mainly exhibited a clear morphology and were more prominent compared with both the CP and PBS groups. Arrows represent NSCs. Scale bars: 25 μm. CON: Control (sham operation); CP: cerebral palsy; PBS: phosphate-buffered saline; VEGF: vascular endothelial growth factor; NSCs: neural stem cells.
Discussion
After transplantation, NSCs proliferate, migrate, and undergo self-renewal. Moreover, they differentiate into various mature neuronal cells to establish correct synaptic connections with the host neuronal cells, form ion channels, replace both injured and necrotic neuronal cells, and reconstruct neural circuits (Huang et al., 2004; Schänzer et al., 2004). Therefore, NSCs contribute to brain damage repair. For acute and subacute stages of neonatal brain injury, treatments aim to provide neuroprotection, and for the chronic stage, neurorestoration has been applied (Mibel and Cesar, 2013). Transplanted NSCs secrete essential factors that promote myelin formation and anti-inflammatory cytokines that inhibit autoimmune reactions. These secreted factors help damaged neurons to restore normal morphology, promote formation of the extracellular matrix, inhibit neuronal apoptosis, and promote the functional restoration of the nervous system (Park et al., 2006). Genetically-modified NSCs carrying exogenous genes which are transplanted into the brain can effectively express the target gene, thus combining the benefits of stem cell transplantation and gene therapy (Hwang et al., 2009).
VEGF is a cytokine that induces angiogenesis, promotes the proliferation of endothelial cells, and also acts directly on neuronal cells as a neurotrophic factor promoting regeneration (Wang et al., 2007; Lee et al., 2010). Application of exogenous VEGF to the brain surface of rats with transient ischemia reduced infarct volume, and alleviated brain edema and neuronal injury within 24 hours (Hayashi et al., 1998). Endothelial cells have been shown to generally proliferate a few days after ischemia, with neovascularization occurring a few months later (Jin et al., 2002; Ma et al., 2011).
Early in ischemia-reperfusion, VEGF is more conducive to neurogenesis than angiogenesis (Zhang et al., 2011). Furthermore, VEGF plays a neuroprotective role in the acute phase of ischemia (Zachary et al., 2005; Jin et al., 2006). Prior to the formation of new blood vessels, VEGF directly affects nerve tissue, which helps to prolong cell survival until new blood vessels form. Moreover, it reduces brain edema and neuronal damage after cerebral ischemia-reperfusion.
In the acute phase of cerebral ischemia-reperfusion, rapid expression of VEGF may not primarily serve to promote angiogenesis but may be neuroprotective, thereby shielding the cells from ischemic damage (Matsuzaki et al., 2001; Zachary et al., 2005). Findings from some studies suggest that VEGF has a direct neuroprotective effect on neurons, which is independent of angiogenesis (Jin et al., 2002; Ma et al., 2011).
The newborn brain is still at the developmental stage and its NSCs have a powerful capacity for proliferation and differentiation. Therefore, the transplantation of NSCs carrying the VEGF gene has a broad therapeutic potential in newborn rats with cerebral palsy (Park et al., 2006). These authors evaluated the therapeutic efficacy of VEGF-transfected NSCs for the treatment of cerebral palsy in newborn rats.
Our results revealed that the expression of VEGF was higher in the cerebral palsy group compared with control, suggesting that brain hypoxia and ischemia increases the level of VEGF. The number of VEGF-positive cells was significantly higher in both VEGF + NSCs and NSCs groups compared with both the cerebral palsy and PBS groups, indicating that transplantation of NSCs alone or VEGF-transfected NSCs can enhance the level of VEGF in the cerebral cortex. VEGF-positive cells were higher in the VEGF + NSCs group compared with the NSCs group, suggesting that VEGF-transfected NSCs further elevated VEGF. Taken together, our findings demonstrate that VEGF-transfected NSCs effectively increase VEGF protein levels.
In the radial arm water maze task, rats of both the cerebral palsy and PBS groups spent a prolonged period of time searching, had higher error rates, and a higher number of repetitions. These results indicate that hypoxia-ischemia impairs spatial learning and memory. Compared with both the cerebral palsy and PBS groups, rats of both the VEGF + NSCs and NSCs groups reached the target faster in the radial maze, with a reduced number of errors and repetitions. This finding suggests that transplantation of transfected NSCs or NSCs alone significantly improves spatial learning and memory in the cerebral palsy model. A more significant improvement occurred in the VEGF + NSCs group compared with the NSCs group.
The holding test showed that the sensory motor functions of rats in both VEGF + NSCs and NSCs groups were significantly improved compared with both PBS and cerebral palsy groups. No significant difference was found between the VEGF + NSCs group and control group. These results indicate that sensorimotor function in cerebral palsy rats can return to a normal level after transplantation with VEGF-transfected NSCs.
Hematoxylin-eosin staining in the ischemic hemisphere of rats in both cerebral palsy and PBS groups revealed that cortical cells were arranged in a disordered manner. Cell stomata appeared shrunken, cellular structure was affected, and nuclear pyknosis was present.
Necrosis and degeneration were alleviated in both NSCs and VEGF + NSCs groups with more prominent presence of neuronal cells compared with both cerebral palsy and PBS groups. These results demonstrate that the transplantation of NSCs or VEGF-transfected NSCs improve neuronal loss and are neuroprotective.
This study shows that NSC transplantation combined with VEGF gene therapy increases VEGF protein levels, and is neuroprotective in rat brain tissue. This strategy effectively improved oxygen deprivation-mediated brain damage and long-term learning and memory function.
Footnotes
Funding: This work was supported by grants from the National Natural Science Foundation of China, No. 81070523, 81270728.
Conflicts of interest: None declared.
Copyedited by Mark F, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
References
- Hayashi T, Abe K, Itoyama Y. Reduction of ischemic damage by application of vascular endothelial growth factor in rat brain after transient ischemia. J Cereb Blood Flow Metab. 1998;18:887–895. doi: 10.1097/00004647-199808000-00009. [DOI] [PubMed] [Google Scholar]
- Huang YF, Zhuang SQ, Chen DP, Liang YJ, Li XY. Angiogenesis and its regulatory factors in brain tissue of neonatal rat hypoxic-ischemic encephalopathy. Zhonghua Er Ke Za Zhi. 2004;42:210–214. [PubMed] [Google Scholar]
- Hwang DH, Kim BG, Kim EJ, Lee SI, Joo IS, Suh-Kim H. Transplantation of human neural stem cells transduced with Olig2 transcription factor improves locomotor recovery and enhances myelination in the white matter of rat spinal cord following contusive injury. BMC Neurosci. 2009;10:117. doi: 10.1186/1471-2202-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci U S A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol. 2006;66:236–242. doi: 10.1002/neu.20215. [DOI] [PubMed] [Google Scholar]
- Kim SU. Genetically engineered human neural stem cells for brain repair in neurological diseases. Brain Dev. 2007;29:193–201. doi: 10.1016/j.braindev.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Lee JM, Bae JS, Jin HK. Intracerebellar transplantation of neural stem cells into mice with neurodegeneration improves neuronal networks with functional synaptic transmission. J Vet Med Sci. 2010;72:999–1009. doi: 10.1292/jvms.09-0514. [DOI] [PubMed] [Google Scholar]
- Li B, Piao CS, Liu XY, Guo WP, Xue YQ, Duan WM, Gonzalez-Toledo ME, Zhao LR. Brain self-protection: the role of endogenous neural progenitor cells in adult brain after cerebral cortical ischemia. Brain Res. 2010;1327:91–102. doi: 10.1016/j.brainres.2010.02.030. [DOI] [PubMed] [Google Scholar]
- Liang W, Xu ZE, Ke JL, Chen JJ, Li H. Differentiation of bone marrow-derived neural stem cells transfected by nuclear receptor-related factor 1 gene into dopaminergic neurons in vitro. Zhongguo Zuzhi Gongcheng Yanjiu. 2012;16:4247–4252. [Google Scholar]
- Liu CJ, Yin F, Zheng XR, Zhang SS, Tan JL. The isolating culture of neural stem cells from rat's fetus brain and transfection by electroporation. Zhonghua Yi Xue Za Zhi. 2009;89:3007–3009. [PubMed] [Google Scholar]
- Liu ML, Oh JS, An SS, Pennant WA, Kim HJ, Gwak SJ, Yoon DH, Kim KN, Lee M, Ha Y. Controlled nonviral gene delivery and expression using stable neural stem cell line transfected with a hypoxia-inducible gene expression system. J Gene Med. 2010;12:990–1001. doi: 10.1002/jgm.1527. [DOI] [PubMed] [Google Scholar]
- Liu YS, Wang XD, Yang J, Hua RR, Cheng HB, Liu YF, An YH. Effect evaluation of autologous bone marrow mesenchymal stem cell transplantation on motor function in children with cerebral palsy. Zhongguo Zuzhi Gongcheng Yanjiu. 2012;16:4290–4295. [Google Scholar]
- Ma Y, Qu Y, Fei Z. Vascular endothelial growth factor in cerebral ischemia. J Neurosci Res. 2011;89:969–978. doi: 10.1002/jnr.22628. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Tamatani M, Yamaguchi A, Namikawa K, Kiyama H, Vitek MP, Mitsuda N, Tohyama M. Vascular endothelial growth factor rescues hippocampal neurons from glutamate-induced toxicity: signal transduction cascades. FASEB J. 2001;15:1218–1220. [PubMed] [Google Scholar]
- Mibel MP, Cesar VB. Advances in the cell-based treatment of neonatalhypoxic-ischemic brain injury. Future Neurol. 2013;8:193–203. doi: 10.2217/fnl.12.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak I, Hines M, Goldsmith S, Barclay R. Clinical prognostic messages from a systematic review on cerebral palsy. Pediatrics. 2012;130:e1285–1312. doi: 10.1542/peds.2012-0924. [DOI] [PubMed] [Google Scholar]
- Okano H. Neural stem cells: their identification, isolation and potential therapeutic application. Seikagaku. 2002;74:17–26. [PubMed] [Google Scholar]
- Park KI, Ourednik J, Ourednik V, Taylor RM, Aboody KS, Auguste KI, Lachyankar MB, Redmond DE, Snyder EY. Global gene and replacement strategies via stem cells. Gene Ther. 2002;9:813–824. doi: 10.1038/sj.gt.3301721. [DOI] [PubMed] [Google Scholar]
- Park KI, Himes BT, Stieg PE, Tessler A, Fischer I, Snyder EY. Neural stem cells may be uniquely suited for combined gene therapy and cell replacement: evidence from engraftment of neurotrophin-3-expressing stem cells in hypoxic-ischemic brain injury. Exp Neurol. 2006;199:179–190. doi: 10.1016/j.expneurol.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Pimentel-Coelho PM, Mendez-Otero R. Cell therapy for neonatal hypoxic-ischemic encephalopathy. Stem Cells Dev. 2010;19:299–310. doi: 10.1089/scd.2009.0403. [DOI] [PubMed] [Google Scholar]
- Schänzer A1, Wachs FP, Wilhelm D, Acker T, Cooper-Kuhn C, Beck H, Winkler J, Aigner L, Plate KH, Kuhn HG. Direct stimulation of adult neural stem cells in vitro and neurogenesis in vivo by vascular endothelial growth factor. Brain Pathol. 2004;14:237–248. doi: 10.1111/j.1750-3639.2004.tb00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter B, Valerio DA, Erika M, Mauro C. Long-lasting behavioral alterations following a hypoxicrischemic brain injury in neonatal rats. Brain Res. 2000;859:318–325. doi: 10.1016/s0006-8993(00)01997-1. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jin K, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA. VEGF-overexpressing transgenic mice show enhanced post-ischemic neurogenesis and neuromigration. J Neurosci Res. 2007;85:740–747. doi: 10.1002/jnr.21169. [DOI] [PubMed] [Google Scholar]
- Wang YQ, Sun FY. Neuroprotective mechanisms of vascular endothelial growth factor. Sheng Li Ke Xue Jin Zhan. 2007;38:202–207. [PubMed] [Google Scholar]
- Wang YX, Yu T, Chu Q, Wang Y, Yu LY, Zhang JH, Zhao P. Three-dimensional gait analysis of temporal and spatial parameters and the action of the pelvic during gait in children with spastic diplegic cerebral palsy. Zhongguo Zuzhi Gongcheng Yanjiu. 2012;16:4039–4043. [Google Scholar]
- Zachary I. Neuroprotective role of vascular endothelial growth factor: signalling mechanisms, biological function, and therapeutic potential. Neurosignals. 2005;14:207–221. doi: 10.1159/000088637. [DOI] [PubMed] [Google Scholar]
- Zhang L, Yang Y, Wang Y, Gao X. Astragalus membranaceus extract promotes neovascularisation by VEGF pathway in rat model of ischemic injury. Pharmazie. 2011;66:144–150. [PubMed] [Google Scholar]
- Zhang P, Li J, Liu Y, Chen X, Kang Q, Zhao J, Li W. Human neural stem cell transplantation attenuates apoptosis and improves neurological functions after cerebral ischemia in rats. Acta Anaesthesiol Scand. 2009;53:1184–1191. doi: 10.1111/j.1399-6576.2009.02024.x. [DOI] [PubMed] [Google Scholar]
- Zhang SS, Zheng XR, Yin F, Tan JL, Yang YJ. Lentiviral-mediated vascular endothelial growth factor 165 gene transfer into neural stem cells promotes proliferation. Neural Regen Res. 2011;6:1457–1461. [Google Scholar]
- Zhang YH, Lu L, Dong J, Song L, He CK, Zhang Y. Effects of X binding protein-1 gene-modified neural stem cells transplantation on expression of related neurotransmitters and nucleoprotein in the substantia nigra of rat Parkinson's disease model. Zhongguo Zuzhi Gongcheng Yanjiu. 2012;16:8510–8513. [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen NV, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XR, Zhang SS, Yang YJ, Tan JL. Construction of vector specifically expressed vascular endothelial growth factor gene in endothelial cells and research of its expressive character. Zhongguo Xiandai Yixue Zazhi. 2010a;20:3205–3209, 3213. [Google Scholar]
- Zheng XR, Zhang SS, Yang YJ, Yin F, Wang X, Zhong L, Yu XH. Adenoviral vector-mediated transduction of VEGF improves neural functional recovery after hypoxia-ischemic brain damage in neonatal rats. Brain Res Bull. 2010b;81:372–377. doi: 10.1016/j.brainresbull.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Zheng XR, Zhang SS, Yin F, Tang JL, Yang YJ, Wang X, Zhong L. Neuroprotection of VEGF-expression neural stem cells in neonatal cerebral palsy rats. Behav Brain Res. 2012;230:108–115. doi: 10.1016/j.bbr.2012.01.026. [DOI] [PubMed] [Google Scholar]