ABSTRACT
BACKGROUND
Novel nicotine delivery products, such as electronic cigarettes (e-cigarettes), have dramatically grown in popularity despite limited data on safety and benefit. In contrast, the similar U.S. Food and Drug Administration (FDA)-approved nicotine inhaler is rarely utilized by smokers. Understanding this paradox could be helpful to determine the potential for e-cigarettes as an alternative to tobacco smoking.
OBJECTIVE
To compare the e-cigarette with the nicotine inhaler in terms of perceived benefits, harms, appeal, and role in assisting with smoking cessation.
DESIGN
A cross-over trial was conducted from 2012 to 2013
PARTICIPANTS/INTERVENTIONS
Forty-one current smokers age 18 and older used the e-cigarette and nicotine inhaler each for 3 days, in random order, with a washout period in between. Thirty-eight participants provided data on product use, perceptions, and experiences.
MAIN MEASURES
The Modified Cigarette Evaluation Questionnaire (mCEQ) measured satisfaction, reward, and aversion. Subjects were also asked about each product’s helpfulness, similarity to cigarettes, acceptability, image, and effectiveness in quitting smoking. Cigarette use was also recorded during the product-use periods.
KEY RESULTS
The e-cigarette had a higher total satisfaction score (13.9 vs. 6.8 [p < 0.001]; range for responses 3–21) and higher reward score (15.8 vs. 8.7 [p < 0.001]; range for responses 5–35) than the inhaler. The e-cigarette received higher ratings for helpfulness, acceptability, and “coolness.” More subjects would use the e-cigarette to make a quit attempt (76 %) than the inhaler (24 %) (p < 0.001). Eighteen percent (7/38) of subjects abstained from smoking during the 3-day periods using the e-cigarette vs. 10 % (4/38) using the inhaler (p = 0.18).
CONCLUSION
The e-cigarette was more acceptable, provided more satisfaction, and had higher perceived benefit than the inhaler during this trial. E-cigarettes have the potential to be important nicotine delivery products owing to their high acceptance and perceived benefit, but more data are needed to evaluate their actual efficacy and safety. Providers should be aware of these issues, as patients will increasingly inquire about them.
KEY WORDS: e-cigarette, nicotine, tobacco, smoking, cessation
INTRODUCTION
Tobacco use remains the leading cause of preventable death in the U.S. Nicotine replacement therapies (NRTs) have well-demonstrated safety and efficacy, and are the most commonly used tobacco dependence treatments. One of these, the nicotine inhaler is an evidence-based proven treatment for tobacco dependence, approved by the U.S. Food and Drug Administration (FDA) in 1997, that delivers nicotine vapor absorbed through the oral mucosa while substituting the hand-mouth behavior of cigarette smoking. Despite their effectiveness, these medications have not been highly utilized in the general population,1–3 possibly due to their limited marketing or relatively low nicotine delivery compared with cigarettes.
New nicotine delivery products have recently entered the market, the majority of which are not approved NRTs. Some, including the electronic cigarette or e-cigarette, have gained popularity among the general public. The e-cigarette delivers vaporized nicotine through the heating of a nicotine-containing solution, producing a visible “vapor” that can be inhaled and exhaled. Awareness and use of this product is growing dramatically,4 with 60 % of adults reporting familiarity.5 There are many types of e-cigarettes with varying characteristics and nicotine delivery.6 As the FDA has not finalized its regulatory process for the e-cigarette, at this time it is readily available in convenience stores and is used by hundreds of thousands of consumers. Not surprisingly, as one of the most trusted sources of health information,7 physicians are increasingly being asked by their patients about these products.8
Evaluation of these new products is critical in terms of their perceived benefit and risk, appeal, and context of use. Moreover, contrasting the e-cigarette with an approved NRT product, such as the similarly designed nicotine inhaler, could suggest why the e-cigarette is highly popular despite no regulatory evaluation, while the nicotine oral inhaler is not. Observational studies suggest that smokers use e-cigarettes to help them stop using tobacco. At this early stage, most studies are surveys (self-reported or convenience samples) of current e-cigarette users.9–11 Recent cohort studies suggest possible smoking cessation and reduction benefit with e-cigarette use,12–14 and a single randomized-controlled-trial has been published suggesting the beneficial effects of e-cigarettes on smoking cessation;15 however, the low rates of cessation in the NRT control group has raised questions about this study’s generalizability. Further studies are needed to more clearly determine perceptions and use practices of e-cigarettes. This study attempted to evaluate how the e-cigarette compared to the nicotine inhaler, chosen as the NRT of interest due to its design similarity to the e-cigarette, in terms of perceived benefits, harms, appeal, ease of use, enjoyment, and perceived role in assisting with smoking cessation.
METHODS
This study was a trial of current smokers asked to use the e-cigarette and the nicotine oral inhaler each for 3 days and to provide data regarding their perceptions and experiences. The study was approved by the Rutgers University institutional review board (IRB) and all subjects provided informed consent.
Setting/Participants
Subjects were 41 daily cigarette smokers aged 18 years or older, who had never used either the e-cigarette or the nicotine inhaler, recruited from central New Jersey communities via flyers, email, and word-of-mouth. Eligibility was assessed via telephone screening. Intention to quit was not an eligibility requirement but was assessed. Exclusion criteria included recent (within 2 weeks) myocardial infarction or angina, poorly controlled asthma/chronic obstructive pulmonary disease (COPD), active substance abuse, pregnancy, or current use of any other cessation medications.
Study Products
The “blu” brand of disposable e-cigarette16 was purchased September 2012 through March, 2013. This brand is one of the most popular and represents a starter product among the various brands available.17 These are cigarette-shaped devices that include a battery, electronic circuit, vaporizer, cartridge, and a mouthpiece. According to the manufacturer, the cartridge contains distilled water, nicotine (approximately 20–24 mg per disposable e-cigarette), vegetable glycerin, natural flavors, artificial flavors, and citric acid.16 Participants were given three disposable, regular-flavor blu e-cigarettes, instructed to use a new e-cigarette each day and to puff the device as they would their usual cigarettes, as recommended by the “blu” instruction manual.16
Nicotine oral inhalers (Pfizer) are plastic, pen-shaped containers with cartridges inserted. The cartridge houses a nicotine-containing porous plug (contains 10 mg per cartridge; delivers up to 2 mg per cartridge).18 With puffing, the plug produces an invisible nicotine vapor absorbed through the oral mucosa. These products were provided in packs of six inhaler cartridges, with instructions to use freely up to a maximum of sixteen cartridges per day as recommended by the manufacturer. Participants were instructed to inhale deeply into back of throat or puff in short breaths, trying to use 80 inhalations over 20 minutes, as described in the package insert.18
Procedures
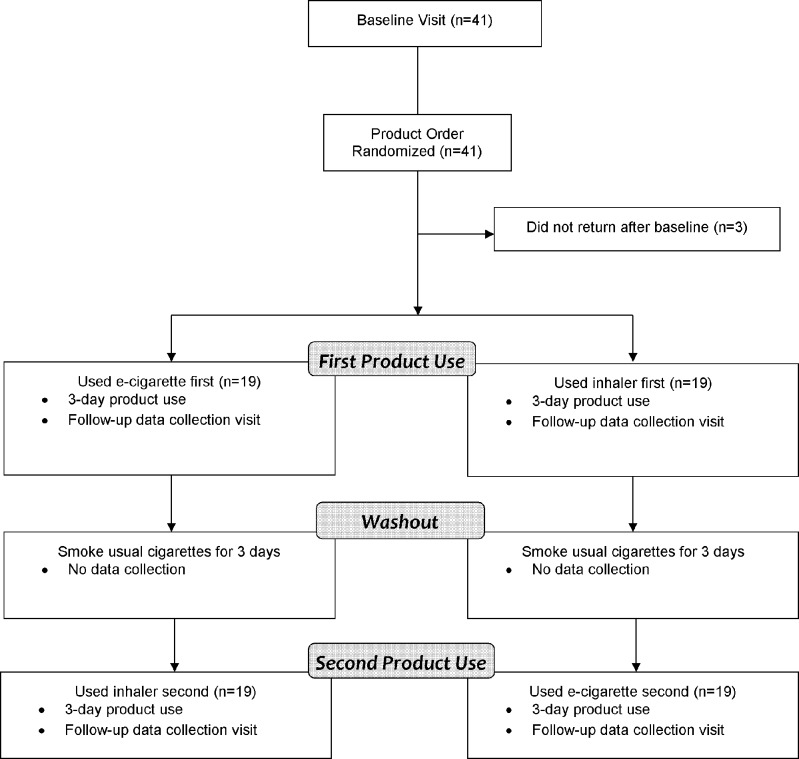
Participant procedures are illustrated in Figure 1. At baseline, subjects were assigned via predetermined, computer-generated, randomized sequence to the order in which they used each of the nicotine delivery products. The randomization order was not blinded. Analyses confirmed that the order of use had no significant effect on the outcomes. Subjects were provided the e-cigarette and the nicotine inhaler to use as they wished for a 3-day period, providing adequate time for subjects to learn the optimal ways to use the devices. After the first post-product-use visit, subjects were instructed to smoke their usual cigarettes as they wished for 3 days prior to using the next product (washout period). During the product-use periods, subjects were encouraged not to smoke their own cigarettes to gain insight into craving and satisfaction. Specifically, they were instructed to use the products as cigarette substitutes, but were told that cigarette smoking was permissible if absolutely necessary.
Figure 1.
Study protocol flow diagram.
Data Collection
Data were collected at three time points: baseline, post-e-cigarette use (3-day period) and post-inhaler use (3-day period). Subjects received a $25 gift card for attending each data collection session.
Baseline Data Collection/Outcomes
Subjects completed a baseline interview that gathered demographic information, tobacco use and cessation history, and if they had previously heard of each nicotine delivery device. They were then asked what they had heard about each product (open-ended). Subjects completed the validated Modified Cigarette Evaluation Questionnaire (mCEQ)19 to measure domains of reinforcement (smoking satisfaction, psychological rewards, and aversion). As an example, subjects were asked “Was the product satisfying?” on a 7-point scale (e.g., 1 [not at all], 4 [moderately], 7 [extremely]). The mCEQ instrument was the study’s primary outcome. Exhaled carbon-monoxide was not collected, as subjects were not able to be seen at standardized times of day for the baseline and follow-up sessions.
Following the baseline interview, subjects were given the first product (e-cigarette or inhaler first, randomly-assigned) and instructed on its use by the research assistant (see Study Products, above). Subjects began use of the product the morning following the baseline data collection visit.
First Post-Product Use Data Collection
After using the first product for 3 days, subjects returned for a data-collection visit. Subjects completed the mCEQ as well as questions about perceptions of product use on a scale of 1 (lowest) to 10 (highest), including: how helpful was the product at keeping you from smoking, how similar was it to cigarettes, how acceptable would the product be to smokers, how favorable or cool is the image of the product, how effective would the product be at helping people quit smoking. Subjects were also asked if they would use the product to make a quit attempt.
Washout Period
On the washout days, participants smoked their usual cigarettes and did not take part in any study procedures.
Second Post-product Use Data Collection
Following the 3-day washout period, subjects used the other nicotine delivery product that they had not yet used (either e-cigarette or inhaler) for a 3-day period. After that use-period, subjects returned for their second post-product data-collection visit. Those procedures were identical to the first post-product data collection visit (above).
Statistical Analysis
This pilot was modeled after the Bullen study,20 which was powered to detect an approximate one-point difference on an 11-point Likert-type scale, assuming a within-participant SD of the response variable of 1.5 points and statistical power of 90 % at a two-sided significance level of 5 % with a sample size of 40.
Data were analyzed using SPSS Software (Version 20.0). Frequencies of demographic variables are reported. Student t-tests were used to determine differences between the inhaler and the e-cigarette for number of cigarettes smoked per day, and product perception measures: helpfulness, similarity to cigarettes, acceptability, image, and usefulness in quitting, as well as “would use product to quit smoking.” Repeated measures ANOVA with Bonferroni corrections were used to compare the mCEQ (Satisfaction Scores, Psychological Reward Scores, and Aversion Scores) for cigarettes, e-cigarettes and the inhaler. Significance was defined as p value < 0.05.
RESULTS
Subject Characteristics
A total of 41 subjects were enrolled and completed the baseline visit (Table 1). The majority of subjects were female (27/41; 66 %) with a mean age of 47 (range 20–70). Most were White (25/41; 61 %), but many were African-American (10/41; 24 %) or Latino (4/41; 10 %). Subjects smoked a mean of 15 cigarettes per day (CPD) (SD 6.9), and over 80 % (33/41) smoked within 30 minutes of waking. Just over half (21/41; 51 %) had ever used NRTs in the past, and there was no significant relationship between prior NRT use and outcomes.
Table 1.
Characteristics of Participants (N = 41)
| n (%) or Mean | |
|---|---|
| Gender | |
| Male | 14 (34.1 %) |
| Female | 27 (65.9 %) |
| Age | Mean 47 (range 20–70; SD 12.6) |
| Race | |
| White | 25 (61.0 %) |
| African American | 10 (24.4 %) |
| Latino | 4 (9.8 %) |
| Other | 2 (4.8 %) |
| Education | |
| No HS Degree | 3 (7.3 %) |
| High School Graduate/GED | 14 (34.1 %) |
| Some College | 17 (41.5 %) |
| Bachelor’s Degree or higher | 7 (17.1 %) |
| Cigarettes per day (CPD) | Mean 15 (range 3–30; SD 6.9) |
| CPD Category | |
| 10 or fewer | 12 (29.3 %) |
| 11–20 | 23 (56.1 %) |
| 21–30 | 6 (14.6 %) |
| Time to first cigarette (TTFC) in minutes | Mean 24 (range 0–120; SD 27.9) |
| TTFC Category | |
| Within 5 minutes | 14 (34.1 %) |
| 5–30 minutes | 19 (46.3 %) |
| 31 or more minutes | 8 (19.5 %) |
| Heaviness of Smoking Index (HSI) (0 lowest – 6 highest) | |
| Light Addiction (0–2) | 9 (22.0 %) |
| Moderate Addiction (3, 4) | 31 (75.6 %) |
| Heavy Addiction (5, 6) | 1 (2.4 %) |
| Lifetime Quit Attempts | Mean 5 (range 0–20; SD 4.5) |
| Ever Use of Cessation Medications | |
| Patch | 19 (46.3 %) |
| Gum | 12 (29.3 %) |
| Varenicline (Chantix) | 12 (29.3 %) |
| Bupropion (Zyban) | 7 (17.1 %) |
| Lozenge | 3 (7.3 %) |
| Ever Use of Other Treatment | |
| Cold Turkey | 32 (78.0 %) |
| Hypnosis | 2 (4.9 %) |
| Acupuncture | 1 (2.4 %) |
| Individual Counseling | 1 (2.4 %) |
| Group Counseling | 1 (2.4 %) |
| Brochure | 1 (2.4 %) |
| Stage of Change | |
| Precontemplation (not interested in quitting) | 2 (4.9 %) |
| Contemplation (considering quitting but not within 30 days) | 14 (34.1 %) |
| Preparation (considering quitting within 30 days) | 13 (31.7 %) |
| Want to reduce but not quit | 10 (24.4 %) |
| Declined to answer | 2 (4.9 %) |
| Importance of quitting (1 lowest – 10 highest) | Mean 8.1 (SD 2.0) |
| Confidence to quit | Mean 5.2 (SD 2.7) |
| Readiness to quit | Mean 6.6 (SD 3.0) |
Baseline Product Knowledge and Beliefs
A majority of subjects (38/41; 93 %) reported having heard about the e-cigarette at baseline, but only 12/41 (29 %) reported previously hearing about the nicotine inhaler. In response to “what have you heard about these products?” (open-ended), 15/41 (37 %) of respondents noted that using an e-cigarette reduces the harm of smoking and 14/41 (34 %) that it helps smokers to quit, while only 2/41 (5 %) of participants noted that the inhaler reduces harm and 0/41 (0 %) reported that it helped people quit smoking.
Follow-up Data Collection—Loss to Follow-up
Of the 41 baseline subjects, three subjects did not return to complete the product-use sessions (two from the e-cigarette-first group and one from the inhaler-first group) (Fig. 1). Thus, 38 subjects remained during follow-up (19 from each sequence of use).
Follow-up Product Experiences—Modified Cigarette Evaluation Questionnaire
Smokers completed the mCEQ at baseline and after each use period (Table 2). The Satisfaction scores were similar between tobacco cigarettes and e-cigarettes, both of which were higher than the inhaler. The total Psychological Rewards scores were higher for the tobacco cigarette and e-cigarette compared to the inhaler. For total Aversion scores, the e-cigarette scored significantly lower than the tobacco cigarette . Compared to the inhaler, the e-cigarette scored significantly higher in each of the individual measures that make up the Satisfaction and Psychological Rewards Scores.
Table 2.
Mean scores (SD) from the Modified Cigarette Evaluation Questionnaire * (n = 38)
| Variable | Product Type | Within subject effects | |||
|---|---|---|---|---|---|
| Tobacco Cigarette | E-cigarette | Inhaler | F ratio | p value | |
| Satisfying †, ‡ | 4.7 (1.4) | 5.0 (1.4) | 2.6 (1.6) | 26.4 | < 0.001 |
| Tastes good†, ‡ | 3.8 (1.9) | 3.8 (1.9) | 2.0 (1.6) | 11.5 | < 0.001 |
| Enjoy †, ‡ | 2.8 (1.9) | 4.0 (2.0) | 1.6 (1.4) | 16.1 | < 0.001 |
| Total Satisfaction Score †, ‡ | 13.3 (4.2) | 13.9 (4.8) | 6.8 (4.5) | 25.5 | < 0.001 |
| Calms you down†, ‡ | 4.6 (1.9) | 4.2 (1.8) | 2.2 (1.4) | 30.6 | < 0.001 |
| Makes more awake†, ‡ | 2.9 (2.0) | 2.6 (1.9) | 1.4 (1.0) | 12.7 | < 0.001 |
| Makes less | 4.2 (2.1) | 3.3 (2.0) | 2.2 (1.5) | 14.7 | < 0.001 |
| irritable†, ‡,§ | |||||
| Helps concentrate†, ‡ | 2.7 (1.9) | 2.7 (1.8) | 1.4 (0.7) | 10.4 | < 0.001 |
| Reduces hunger†, ‡,§ | 3.8 (2.2) | 2.3 (1.6) | 1.5 (1.2) | 23.3 | < 0.001 |
| Total Psychological Rewards Score†, ‡ | 18.3 (7.5) | 15.8 (7.8) | 8.7 (4.2) | 35.7 | < 0.001 |
| Makes dizzy§ | 1.8 (1.5) | 1.2 (0.7) | 1.5 (1.1) | 3.6 | 0.032 |
| Makes nauseated | 1.2 (0.8) | 1.2 (0.8) | 1.8 (1.8) | 4.1 | 0.021 |
| Total Aversion Score § | 3.0 (1.7) | 2.4 (1.1) | 3.3 (2.5) | 3.2 | 0.047 |
* Rating scale = 1 (not at all)–7 (extremely) for individual scores
† Inhaler and tobacco cigarette significantly differed (p < 0.05)
‡ Inhaler and e-cigarette significantly differed (p < .05)
§ Tobacco cigarette and e-cigarette significantly differed (p < 0.05)
Note: Bonferroni adjustments for multiple comparisons
Follow-up Product Experiences—Perceptions
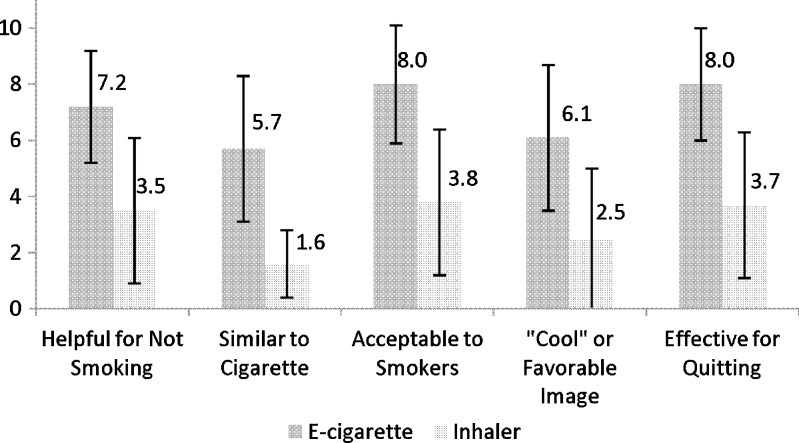
After using both products, subjects were asked about the helpfulness, similarity to cigarettes, acceptability, image, and usefulness in quitting on a scale of 1 (lowest) to 10 (highest). The e-cigarette scored higher on all measures compared to the inhaler (Fig. 2).
Figure 2.
Mean (SD) product ratings on scale of 1–10 (lowest–highest) (n = 38; p < 0.001 for all comparisons).
Follow-up Product Experiences—Intended Use
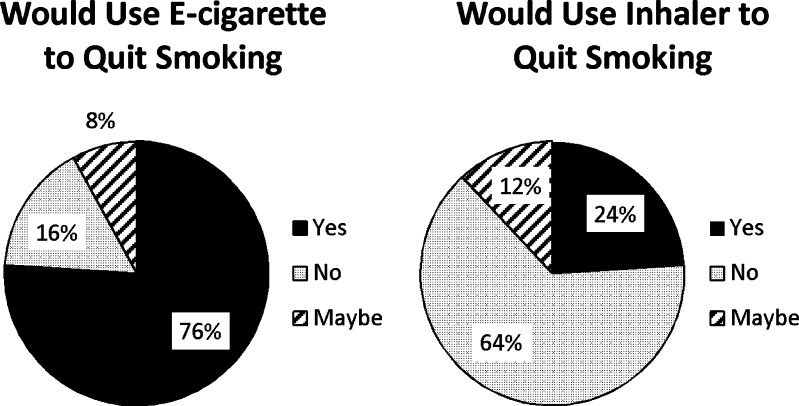
Subjects were asked if they would use these products to make a quit attempt (Fig. 3), with a large majority (29/38; 76 %) answering affirmative for the e-cigarette compared to a minority (9/38; 24 %) for the inhaler (t = 5.1; p < 0.001).
Figure 3.
Product use preferences for quit attempt (n = 38).
Follow-up Product and Cigarette Use
Subjects used each of the nicotine delivery products a mean of eight times per day. Although this was not a study of tobacco cessation, changes in number of cigarettes smoked were recorded. During the 3-day product use periods, 7/38 (18 %) reported abstinence from tobacco cigarettes for the entire 3-day e-cigarette period versus 4/38 (10 %) during the 3-day inhaler period (t = 1.4; p = 0.18). The mean cigarettes per day (CPD) smoked while using each of the two study products was 2.3 CPD (SD 2.1) during the e-cigarette-use period and 2.8 CPD (SD 2.3) during the inhaler-use period (t = 1.3; p = 0.22).
DISCUSSION
This study compared experiences and perceptions of the e-cigarette to those of the nicotine inhaler. At baseline, a higher proportion of subjects were aware of and had more positive preconceptions of the e-cigarette compared to the inhaler, possibly reflecting better marketing and social media exposure. The nicotine inhaler, available since 1997, is a relatively minor pharmaceutical product by market share, and has not recently received a great deal of advertising exposure. On the contrary, e-cigarettes have been some of the most highly publicized products released in the past few years. Television and internet advertisements, Facebook, and Twitter are examples of how these new products have been presented to the public in dramatic fashion. Unfortunately, this type of rapid exposure is not always factually-based and could perpetuate unsubstantiated beliefs.9,21,22 Hopefully balancing these beliefs are physicians, who remain one of the most trusted sources of health information and thus, have the potential to be an important source for answers about e-cigarettes that may influence the public’s perceptions and use of these products.
After the product-use period, the e-cigarette was perceived as superior to the inhaler in many respects. It was felt to be more helpful for people trying to quit, more acceptable and “cool”, and believed to be more effective. Many more participants would use the e-cigarette to quit smoking than the inhaler. This compares similarly to data from New Zealand, where more than half of e-cigarette users considered it an acceptable cessation aid.23 The e-cigarette had superior satisfaction scores compared to the inhaler, even on par with the participants’ own tobacco cigarette, and was superior to the inhaler in terms of psychological reward. These favorable perceptions are consistent with a brief trial of another brand of e-cigarette (NJOY).24 From a product dissemination standpoint, our results suggest promise for the e-cigarette as an acceptable and well-perceived nicotine delivery product with the potential for rapid adoption.
Although not a trial designed to evaluate changes in smoking behavior, use of both products resulted in a similar proportion of subjects (18 % vs. 10 %) remaining abstinent from their tobacco cigarettes during their use. Novel tobacco dependence treatment interventions are needed to improve abstinence rates and reduce the burden of tobacco use in our society. Varying levels of nicotine delivery may have impacted perceived effectiveness of these products in the current study. The nicotine inhaler and most of the disposable e-cigarettes typically do not deliver high levels of nicotine. Therefore, the comparison of inhaler to disposable e-cigarette was appropriate in this design. Data indicate that some brands of e-cigarette, often the rechargeable brands, can deliver reliable blood nicotine levels.6,25,26 Gradually, cohort studies12–14 and RCTs15 suggesting benefit of e-cigarettes for cessation are emerging. However, determinations of efficacy will require larger, randomized clinical trials and will need to address any concerns regarding safety.
The study has some limitations. First, as a pilot, the sample size was relatively small. In addition, there were only 38 subjects as opposed to the 40 subjects for which the study was originally powered. Second, this was not a cessation trial, although it is conceivable that the conditions experienced by subjects might reflect those of cigarette smokers experimenting with such products on their own. Lastly, the withdrawal measures could be impacted by concurrent use of tobacco cigarettes. Data collection sessions were scheduled at participants’ convenience, not at standardized times, and a notable proportion did smoke cigarettes during each products’ 3-day period. For these reasons, CO measures were not collected, which did not allow for biochemical validation. The study’s strengths included its cross-over design, which allowed subjects to compare each of the two products directly and the randomization of product sequence to reduce order bias.
In conclusion, during this brief trial, the e-cigarette was found to be more acceptable, provided more satisfaction and rewards, and had higher perceived benefit than the nicotine inhaler. These findings may explain why the e-cigarette has become popular among smokers while the inhaler has not achieved the same favorability. Based on this difference, e-cigarettes could have the potential to become “tobacco cigarette substitutes,” owing to their high acceptance and perceived effectiveness. While toxicants have been identified in e-cigarettes, they are present at orders of magnitude lower than tobacco cigarettes. As such, e-cigarettes may hold value as a harm reduction strategy among those unwilling or unable to quit. However, given the large variation in the market with respect to brands, more data are needed to demonstrate their efficacy and safety, and to allow physicians to more appropriately inform their patients about these products.
Acknowledgements
This study was funded through a pilot grant from the Rutgers–Cancer Institute of New Jersey (P30CA072720).
Conflict of Interest
Michael B. Steinberg – none; Mia Hanos Zimmermann – none; M. Jane Lewis – none; Cristine D. Delnevo – none; Parth Shukla – none; Elliot Coups – none; Jonathan Foulds – primarily funded by National Institutes of Health grants: P50-DA-036107-01 & P50-DA-036105-01 and by Penn State Cancer Institute and Social Science Research Institute. He has done paid consulting for pharmaceutical companies involved in producing smoking cessation medications, including GSK, Pfizer, Novartis, J&J, and Cypress Bioscience.
REFERENCES
- 1.Steinberg MB, Akincigil A, Delnevo CD, Crystal S, Carson JL. Gender and age disparities for tobacco dependence treatment: Results of the 2001–2002 National Ambulatory Medical Care Survey. Am J Prev Med. 2006;30:405–412. doi: 10.1016/j.amepre.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville: U.S. Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- 3.Steinberg MB, Evans RM, Hughes JR, Leone FT, Lipsky M. Treatment of Tobacco Dependence; AMA Therapeutic Insights; 2011. Available at http://www.ama-assn.org/ama/pub/education-careers/continuing-medical-education/cme-credit-offerings/therapeutic-insights/treatment-tobacco-dependence.page (accessed 25 April 2014).
- 4.Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the 'e-cigarette' in the USA. Tob Control. 2013;22(1):19–23. doi: 10.1136/tobaccocontrol-2011-050044. [DOI] [PubMed] [Google Scholar]
- 5.King BA, Alam S, Promoff G, Arrazola R, Dube SR. Awareness and ever-use of electronic cigarettes among U.S. Adults, 2010–2011. Nicotine Tob Res. 2013;15:1623–1627. doi: 10.1093/ntr/ntt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2013; [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 7.Smith D. Health care consumers use and trust of health information sources. J Commun Healthc. 2011;4(3):200–210. doi: 10.1179/1753807611Y.0000000010. [DOI] [Google Scholar]
- 8.Pepper JK, McRee AL, Gilkey MB. Healthcare providers’ beliefs and attitudes about electronic cigarettes and preventative counseling for adolescent patients. J Adolesc Health. 2013; [Epub ahead of print], doi: 10.1016/j.jadohealth.2013.10.001. [DOI] [PMC free article] [PubMed]
- 9.Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction. 2011;106:2017–2028. doi: 10.1111/j.1360-0443.2011.03505.x. [DOI] [PubMed] [Google Scholar]
- 10.Siegel MB, Tanwar KL, Wood KS. Electronic cigarettes as a smoking-cessation: tool results from an online survey. Am J Prev Med. 2011;40:472–475. doi: 10.1016/j.amepre.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Foulds J, Veldheer S, Berg A. Electronic cigarettes (e-cigs): views of aficionados and clinical/public health perspectives. Int J Clin Pract. 2011;65:1037–1042. doi: 10.1111/j.1742-1241.2011.02751.x. [DOI] [PubMed] [Google Scholar]
- 12.Polosa R, Morjaria JB, Caponnetto P, et al. Effectiveness and tolerability of electronic cigarette in real-life: a 24-month prospective observational study. Int Emerg Med. 2013; [Epub ahead of print]. [DOI] [PubMed]
- 13.Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, Polosa R. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One. 2013;8(6):e66317. doi: 10.1371/journal.pone.0066317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav. 2014;39(2):491–494. doi: 10.1016/j.addbeh.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomized controlled trial. Lancet. 2013;382(9905):1629–1637. doi: 10.1016/S0140-6736(13)61842-5. [DOI] [PubMed] [Google Scholar]
- 16.Blu Cigs website, 2013. Disposable eCig Features, http://www.blucigs.com/disposables (accessed 25 April 2014).
- 17.Wells Fargo Securities, 2013. Tobacco—Nielsen C-Store Data Including E-Cigs. Equity Research.
- 18.Nicotrol Inhaler Website; 2014; https://www1.pfizerpro.com/hcp/nicotrol/nicotrol-Inhaler (accessed 25 April 2014).
- 19.Cappelleri JC, Bushmakin AG, Baker CL, Merikle E, Olufade AO, Gilbert DG. Confirmatory factor analyses and reliability of the modified cigarette evaluation questionnaire. Addict Behav. 2007;32:912–923. doi: 10.1016/j.addbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 20.Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tob Control. 2010;2:98–103. doi: 10.1136/tc.2009.031567. [DOI] [PubMed] [Google Scholar]
- 21.Choi K, Forster J. Characteristics associated with awareness, perceptions, and use of electronic nicotine delivery systems among young US Midwestern adults. Am J Public Health. 2013;103:556–561. doi: 10.2105/AJPH.2012.300947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cobb NK, Brookover J, Cobb CO. Forensic analysis of online marketing for electronic nicotine delivery systems. Tob Control 2013; [Epub ahead of print]. [DOI] [PubMed]
- 23.Li J, Bullen C, Newcombe R, Walker N, Walton D. The use and acceptability of electronic cigarettes among New Zealand smokers. N Z Med J. 2013;126:48–57. [PubMed] [Google Scholar]
- 24.Nides MA, Leischow SJ, Bhatter M, Simmons M. Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. Am J Health Behav. 2014;38:265–274. doi: 10.5993/AJHB.38.2.12. [DOI] [PubMed] [Google Scholar]
- 25.Polosa R, Caponnetto P, Morjaria JB, Papale G, Campagna D, Russo C. Effect of an electronic nicotine delivery device (e-Cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. B.M.C. Public Health. 2011;11:786. doi: 10.1186/1471-2458-11-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology. 2014;231(2):401–407. doi: 10.1007/s00213-013-3249-8. [DOI] [PubMed] [Google Scholar]