ABSTRACT
Background
Hypertension is a major risk factor for peripheral artery disease (PAD). Little is known about relative efficacy of antihypertensive treatments for preventing PAD.
Objectives
To compare, by randomized treatment groups, hospitalized or revascularized PAD rates and subsequent morbidity and mortality among participants in the Antihypertensive and Lipid-Lower Treatment to Prevent Heart Attack Trial (ALLHAT).
Design
Randomized, double-blind, active-control trial in high-risk hypertensive participants.
Participants
Eight hundred thirty participants with specified secondary outcome of lower extremity PAD events during the randomized phase of ALLHAT.
Interventions/events
In-trial PAD events were reported during ALLHAT (1994–2002). Post-trial mortality data through 2006 were obtained from administrative databases. Mean follow-up was 8.8 years.
Main Measures
Baseline characteristics and intermediate outcomes in three treatment groups, using the Kaplan-Meier method to calculate cumulative event rates and post-PAD mortality rates, Cox proportional hazards regression model for hazard ratios and 95 % confidence intervals, and multivariate Cox regression models to examine risk differences among treatment groups.
Key Results
Following adjustment for baseline characteristics, neither participants assigned to the calcium-channel antagonist amlodipine nor to the ACE-inhibitor lisinopril showed a difference in risk of clinically advanced PAD compared with those in the chlorthalidone arm (HR, 0.86; 95 % CI, 0.72–1.03 and HR, 0.98; 95 % CI, 0.83–1.17, respectively). Of the 830 participants with in-trial PAD events, 63 % died compared to 34 % of those without PAD; there were no significant treatment group differences for subsequent nonfatal myocardial infarction, coronary revascularizations, strokes, heart failure, or mortality.
Conclusions
Neither amlodipine nor lisinopril showed superiority over chlorthalidone in reducing clinically advanced PAD risk. These findings reinforce the compelling need for comparative outcome trials examining treatment of PAD in high-risk hypertensive patients. Once PAD develops, cardiovascular event and mortality risk is high, regardless of type of antihypertensive treatment.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-014-2947-1) contains supplementary material, which is available to authorized users.
KEY WORDS: cardiovascular disease, hypertension, clinical trials
Peripheral arterial disease (PAD) is an increasingly prevalent and costly disease affecting 8–12 million Americans. It is a slowly progressive manifestation of atherosclerotic disease and is particularly prevalent among those 65 years and older (12–15 %; estimated to be 20 % in those over 70), Black Americans (8 %, compared to 4 % in White Americans), and diabetics (approximately 20 % of symptomatic PAD patients).1–5 Patients with lower extremity PAD are three to six times more likely to die of cardiovascular disease than those without PAD, and they carry a risk of cardiovascular events as high as that of patients with coronary heart disease, including myocardial infarction.6–8
Hypertension is among the strongest and most modifiable risk factors for PAD, yet little is known about the relative efficacy of antihypertensive treatments for lowering PAD risk.9–11 While major studies with potential to impact clinical practice have compared the efficacy of antihypertensive regimens in reducing risk of such major cardiovascular disease events as stroke, myocardial infarction, and heart failure,12–14 few active-comparator trials have compared the efficacy of specific antihypertensive drugs in reducing risk of PAD or risk of cardiovascular disease in people with PAD.15 The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), a randomized, double-blind, active-controlled trial of 42,418 high-risk hypertensive patients, provides an opportunity to analyze the relative efficacy of antihypertensive drugs in preventing lower extremity PAD hospitalization and outpatient surgical treatment events. ALLHAT also affords the opportunity to evaluate long-term clinical outcomes after PAD hospitalization or revascularization. We previously reported a trend toward reduced risk of PAD events (treated as out-patient or hospitalized; with or without vascularization procedures) in the calcium-channel antagonist amlodipine group (RR, 0.87; 95 % CI, 0.75–1.01; P = 0.06), but no difference in the ACE-inhibitor lisinopril group (RR, 1.04; 95 % CI, 0.90–1.19; P = 0.63) compared with the diuretic chlorthalidone.16 Here we use stricter criteria to analyze first in-trial PAD: we look at events that were centrally verified through review of hospital or operative reports and, in addition, look at morbidity and mortality following those events. Aims of this study are to: (1) describe characteristics of patients who developed clinically advanced PAD, i.e., requiring hospitalization or revascularization, by treatment group; (2) compare first occurrence of clinically advanced PAD during ALLHAT by treatment group; (3) compare in-trial morbidity following PAD events by treatment group; (4) compare mortality (cardiovascular, non-cardiovascular, and all-cause) in patients with PAD by treatment group.17
METHODS
Design and In-Trial Follow-Up
ALLHAT was designed to determine whether occurrence of fatal coronary heart disease or non-fatal myocardial infarction (primary outcome) is lower for hypertensive patients (BP of 140–180/90–110 for untreated or ≤160/100 for treated patients), age ≥55, assigned to an angiotensin-converting enzyme inhibitor (ACEI; lisinopril), a calcium channel-blocker (CCB; amlodipine), or an alpha-receptor-blocker (doxazosin) compared with assignment to treatment with a thiazide-type diuretic (chlorthalidone), who had at least one additional cardiovascular disease risk factor, only one of which was required to be entered on the screening form (even if more were present). Risk factors included old myocardial infarction or stroke, history of coronary revascularization procedure, other documented atherosclerotic cardiovascular disease (category contains PAD, including history of intermittent claudication, peripheral artery revascularization, or peripheral artery angioplasty/stent), type 2 diabetes, current cigarette smoking, HDL < 0.90 mmol/l, left ventricular hypertrophy, major ST depression, or T-wave inversion. History of PAD was not otherwise collected at baseline. Randomized treatment assignments were allocated in a 1.7:1:1:1 ratio to chlorthalidone, amlodipine, lisinopril, and doxazosin, respectively. Participants (n = 42,418) were recruited between February 1994 and January 1998. All participants gave written informed consent, and all centers obtained institutional review board approval. The JNC-5 provided blood pressure (BP) guidelines for ALLHAT.18 Each of the masked, randomly assigned (step 1) study drugs had three dosage levels. If participants failed to meet the BP goal at the maximum tolerated dose, second- (step 2) and third-line (step 3) drugs were available to be added to the blinded study drug at the site investigator’s discretion: step 2, atenolol, 25–100 mg/day; reserpine, 0.05–0.2 mg/day; and clonidine 0.1–0.3 mg/day, titrated in three doses, and step 3, hydralazine, 25–100 mg twice/day. Low doses of open-label step 1 classes were permitted if clear medical indications developed. Site coordinators were trained and regularly tested in BP measurement; participants’ BPs were measured at 3–4-month follow-up visits using standard mercury sphygmomanometers.19 The doxazosin arm was stopped early because of futility and increased cardiovascular disease, especially heart failure, in the doxazosin arm compared with the chlorthalidone arm.20 Results of the other two treatment group comparisons were published in 2002.16 Mean in-trial follow-up for amlodipine and lisinopril versus chlorthalidone comparisons was 4.9 years (3.2 years in the doxazosin comparison with chlorthalidone). Due to differential follow-up times of participants assigned to the alpha-1 antagonist doxazosin treatment group, the doxazosin-chlorthalidone comparison was not included in current analyses, and the remainder of this report reflects experiences for the 33,357 participants in the chlorthalidone, amlodipine, and lisinopril arms.
PAD, a component of a major secondary outcome, is defined in the ALLHAT protocol as: (1) hospitalized, with or without a revascularization procedure (lower extremity angioplasty, stent, or bypass surgery documented by hospital discharge summary, procedure sheet, or face sheet); or (2) outpatient revascularization procedure (documented by procedure sheet); or, (3) treated medically, as per the attending physician’s usual care regimen, as an outpatient (documented by check box on endpoint questionnaire).21 Hospitalizations and revascularizations were reported by clinical site investigators to the Coordinating Center, where a physician-led endpoints group centrally reviewed all reports and corresponding documentation, including discharge summaries for hospitalizations and operative reports for non-hospitalized revascularization procedures. Pre-specified subgroups included: (1) men vs. women, (2) participants <65 vs. ≥65 years, (3) Black vs. non-Black participants, and (4) diabetic vs. non-diabetic participants. Post-hoc subgroups included presence or absence of coronary heart disease at baseline. In the main results publication, PAD was reported as per the protocol-specified definition.16 For PAD events reported in this article, we restricted analyses to verifiable lower extremity arterial disease events, i.e., documented hospitalizations and revascularization procedures that occurred during the randomized phase of ALLHAT. We further limited review to participants for whom post-trial mortality data were available.17 Hereafter, PAD events will refer to the first post-randomization clinically advanced PAD event, i.e., hospitalization or revascularization procedure.
Post-Trial Follow-Up
Detailed descriptions of post-trial follow-up aims and procedures as well as main results of the extended follow-up through 2006 for the amlodipine and lisinopril comparisons with chlorthalidone have been published.17 Briefly, following the in-trial phase, and per prior Institutional Review Board permission, we conducted an extended follow-up of ALLHAT participants through national administrative databases for an average of 4 years, providing an overall average follow-up time of 8.8 years (4.9 years during active trial treatment and 3.9 years following trial cessation). Post-trial deaths following first in-trial PAD events were ascertained using administrative databases, as described below. Post-trial morbidity (non-fatal hospitalization) data were not available for the 5,558 Veterans’ Affairs (VA) participants in ALLHAT, including 281 VA participants with in-trial PAD; therefore, post-PAD non-fatal events are described only through the end of the active trial. In addition, database access was not available for Canadian participants including the eight Canadian participants with in-trial PAD. Finally, post-trial data on medications, BP, outpatient morbidity and treatment, and laboratory data were not collected via administrative databases.17
Mortality Outcomes
During the in-trial phase, cause of death was determined by investigators. Mortality data were available through national administrative databases for the entire cohort during both in-trial and post-trial periods, except for participants enrolled in Canada, as noted above. Both the National Death Index (NDI) and Social Security Administration databases were used to collect all-cause mortality. Death certificates were obtained for those deaths discovered through the administrative databases and used to confirm patient identification; the NDIPlus provided ICD-10 codes with causes of death. Additional details of the mortality outcome have been published.17
Statistical Methods
Data were analyzed according to each participant’s randomized treatment assignments (intent-to-treat analysis). Baseline characteristics and intermediate outcomes were compared across three treatment groups using the Z-test for continuous covariates and contingency table analysis for categorical data. Cumulative event rates were calculated using the Kaplan-Meier method. Hazard ratios and 95 % confidence intervals (CIs) were obtained from the Cox proportional hazards regression model.22 The follow-up period includes both randomized trial (mean follow-up duration 4.9 years) and subsequent extension period follow-up (3.9 years). The proportional hazards regression model incorporated the participant’s entire trial experience to evaluate differences between cumulative event curves and to obtain two-sided P values. Heterogeneity of effects in subgroups was examined by testing for treatment-covariate interaction with the Cox proportional hazards regression model, using P < 0.05. Multivariate Cox regression models were employed to examine differences in risk across randomized treatment comparisons, unadjusted and while controlling for age, race, ethnicity, gender, previous treatment for hypertension, pulse pressure, heart rate, current or former smoking, type II diabetes, evidence of coronary heart disease, aspirin use, history of CABG, history of myocardial infarction or stroke, and body mass index (BMI). A P-value <0.05 was used to indicate statistical significance for results. Post-PAD mortality rates were calculated using the Kaplan-Meier method. However, given the many main, subgroup, and interaction analyses performed, statistical significance at the 0.05 level should be interpreted cautiously.
RESULTS
Of 32,804 participants eligible for long-term mortality follow-up (33,357, less 553 Canadian participants), 830 (2.5 %) participants experienced clinically severe PAD (i.e., requiring hospitalizations or outpatient revascularization procedures) during the active follow-up phase of ALLHAT: 402, or 2.7 % of the chlorthalidone group, 198, or 2.2 % of the amlodipine group, and 230, or 2.6 % of the lisinopril group. Mean time to detection of PAD was 2.6 years for the chlorthalidone and lisinopril groups and 2.8 years for the amlodipine group. Among the 830 participants, 520 (63 %) died: 64.2 % in the chlorthalidone group, 60.1 % in the amlodipine group, and 62.2 % in the lisinopril group. Nearly half (241, 46 %) of the 520 deaths occurred in-trial while 279 (54 %) occurred post-trial (Fig. 1).
Figure 1.
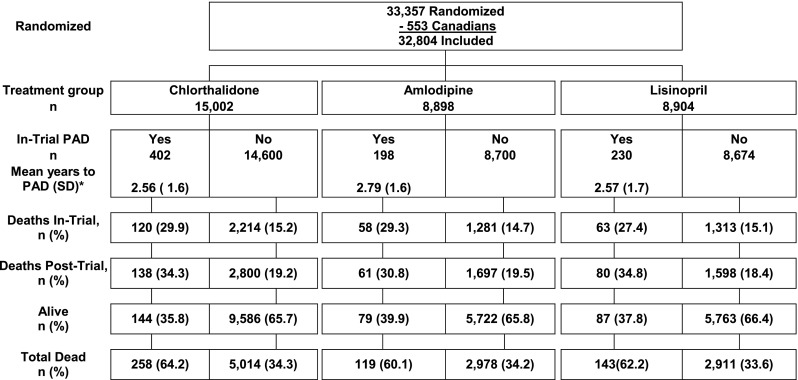
Consort diagram for participants who developed hospitalized or treated PAD (revascularization procedure or hospitalized) during the randomized phase of ALLHAT and were eligible for long-term mortality follow-up. *Wilcoxon test for the equality of survival function probability values are: amlodipine vs. chlorthalidone P = 0.099; lisinopril vs. chlorthalidone P = 0.915.
History of PAD at entry into ALLHAT was not directly ascertained. Baseline characteristics by assigned treatment group of those who experienced clinically severe PAD during the study are presented in Table 1. Those on chlorthalidone were more likely to have type 2 diabetes than those on lisinopril (P = 0.011) and less likely to take aspirin than those on amlodipine (P = 0.029). The chlorthalidone group had a lower total (P = 0.004) and borderline lower LDL-cholesterol (P = 0.051) than the amlodipine group and a lower total cholesterol than the lisinopril group (P = 0.033). Compared with those assigned to amlodipine and lisinopril, those on chlorthalidone tended to be slightly older, to have higher mean fasting glucose levels, were less likely to be female, and were less likely to have other major cardiovascular disease. Percent of PAD patients who had ever smoked was slightly lower in the amlodipine group compared to the chlorthalidone and lisinopril groups (76.3, 78.3, and 78.3 %, respectively). There was no difference in either the amlodipine vs. chlorthalidone or lisinopril vs. chlorthalidone comparisons in current (P = 0.234 and P = 0.986, respectively) or former (P = 0.977 and P = 0.974, respectively) smokers compared to those never having smoked.
Table 1.
Baseline Characteristics by Treatment Group for Those with Diagnosed PAD* During ALLHAT
| N (%) unless otherwise specified | Treatment group | P | |||
|---|---|---|---|---|---|
| Chlorthalidone | Amlodipine | Lisinopril | |||
| 402 | 198 | 230 | A vs. C | L vs. C | |
| Age, years, mean (SD) | 68.5 (6.9) | 68.9 (7.5) | 68.8 (7.5) | 0.513 | 0.598 |
| 55–64 | 115 (28.6) | 63 (31.8) | 74 (32.2) | 0.418 | 0.346 |
| 65+ | 287 (71.4) | 135 (68.2) | 156 (67.8) | ||
| Women | 137 (34.1) | 75 (37.9) | 87 (37.8) | 0.360 | 0.343 |
| Black | 142 (35.3) | 68 (34.3) | 77 (33.5) | 0.813 | 0.639 |
| Hispanic | 32 (8.0) | 15 (7.6) | 23 (10.0) | 0.869 | 0.381 |
| Education, years, mean (SD) | 11.2 (3.7) | 10.8 (4.0) | 11.3 (3.4) | 0.151 | 0.792 |
| Type II diabetes | 221 (58.8) | 100 (53.5) | 104 (47.9) | 0.231 | 0.011 |
| ASCVD | 252 (62.7) | 132 (66.7) | 160 (69.6) | 0.340 | 0.081 |
| History of CHD | 149 (37.6) | 83 (42.1) | 80 (34.9) | 0.290 | 0.501 |
| Hx CABG | 115 (28.6) | 59 (29.8) | 76 (33.0) | 0.762 | 0.243 |
| Hx MI or stroke | 126 (31.3) | 67 (33.8) | 67 (29.1) | 0.538 | 0.561 |
| OASCVD | 135 (33.6) | 72 (36.4) | 90 (39.1) | 0.500 | 0.169 |
| ST/T wave | 29 (7.3) | 21 (10.7) | 25 (11.0) | 0.165 | 0.122 |
| Smoking† | |||||
| Current | 138 (34.3) | 56 (28.3) | 79 (34.4) | 0.234 | 0.986 |
| Former | 177 (44.0) | 95 (48.0) | 101 (43.9) | 0.977 | 0.974 |
| Never | 87 (21.6) | 47 (23.7) | 50 (21.7) | ||
| Medication use, n (%) | |||||
| Aspirin | 168 (41.8) | 105 (53.0) | 107 (46.5) | 0.029 | 0.232 |
| Antihypertensive medication‡ | 356 (88.6) | 180 (90.9) | 211 (91.7) | 0.380 | 0.205 |
| Estrogen (%♀) | 19 (13.9) | 11 (14.7) | 14 (16.1) | 0.873 | 0.647 |
| Heart rate, mean (SD) | 73.7 (11.7) | 73.3 (10.6) | 74.3 (10.9) | 0.701 | 0.542 |
| BMI, mean (SD) | 28.4 (5.7) | 28.1 (5.9) | 27.6 (5.2) | 0.542 | 0.097 |
| LVH by ECG (Minnesota code) | 25 (7.0) | 10 (5.9) | 12 (6.0) | 0.646 | 0.644 |
| Cholesterol, mg/dl, mean (SD)§ | |||||
| Total | 216.3 (50.2) | 230.4(62.3) | 225.6 (52.5) | 0.004 | 0.033 |
| LDL | 135.5 (38.7) | 142.7 (41.7) | 141.3 (39.9) | 0.051 | 0.097 |
| Triglycerides | 197.5 (183.6) | 216.9 (259.9) | 209.7 (239.0) | 0.366 | 0.530 |
| HDL | 43.2(13.7) | 44.1 (13.7) | 44.4 (14.1) | 0.421 | 0.279 |
| Fasting glucose, mg/dl, mean (SD)§ | 140.0 (72.6) | 131.9 (61.3) | 132.4 (63.5) | 0.250 | 0.251 |
| eGFR, ml/min per 1.73 m2, mean (SD) | 73.0 (23.0) | 75.3 (22.0) | 73.2 (24.2) | 0.252 | 0.916 |
| Blood pressure, mmHg, mean (SD) | |||||
| Systolic, total | 148 (16) | 149 (16) | 147 (16) | 0.577 | 0.539 |
| Diastolic, total | 81 (10) | 81 (11) | 81 (11) | 0.830 | 0.848 |
| Systolic, treated at baseline | 147 (16) | 148 (16) | 146 (16) | 0.345 | 0.629 |
| Diastolic, treated at baseline | 81 (10) | 81 (11) | 81 (11) | 0.673 | 0.601 |
| Pulse pressure | 67 (15) | 68 (16) | 66 (17) | 0.458 | 0.609 |
Abbreviations: A amlodipine; BMI body mass index; C chlorthalidone; CABG coronary artery bypass graft; CHD coronary heart disease; eGFR estimated glomerular filtration rate by simplified four-variable Modification of Diet in Renal Disease Study formula; HTN hypertension; L lisinopril; LVH left ventricular hypertrophy; MI myocardial infarction; (O)ASCVD (other) atherosclerotic cardiovascular disease; SD standard deviation
*Refers to documented hospitalized PAD or lower extremity PAD revascularization procedure
†Current and former smoking compared with never smoker at baseline
‡Names or classifications of specific pre-ALLHAT antihypertensive medications used prior to enrollment were not collected at baseline
§Reduced N(C/A/L): cholesterol = 384/187/217; LDL = 348/173/195; Triglycerides = 299/144/180; HDL = 384/187/216; glucose = 291/142/173; eGFR = 388/188/216
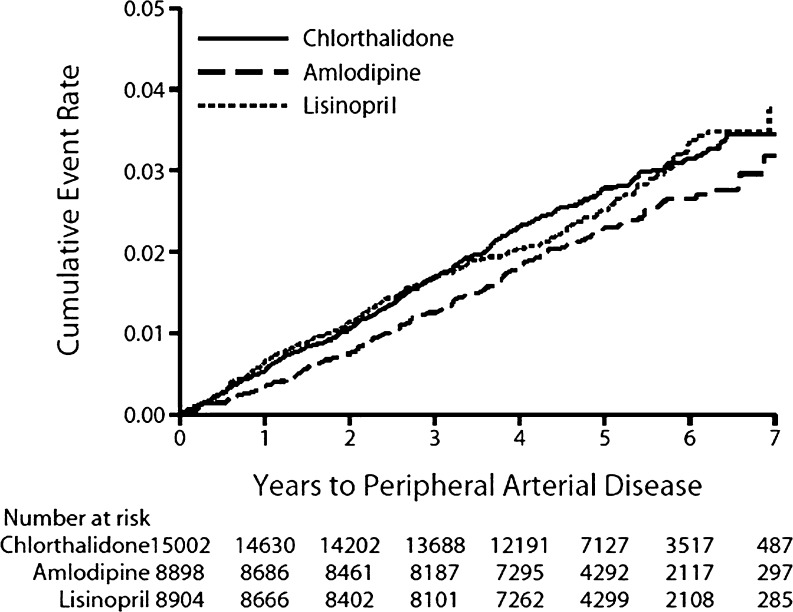
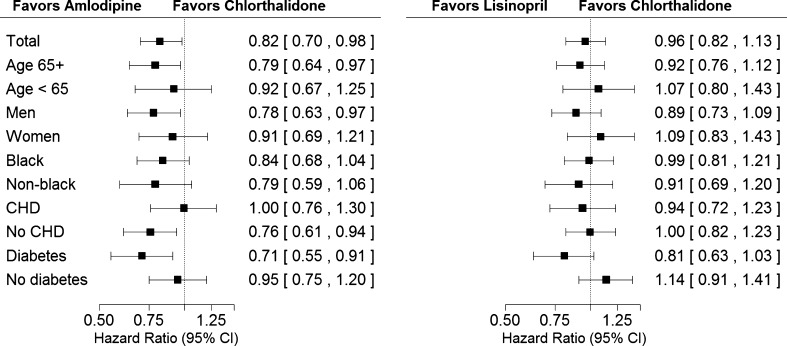
In-trial medication status and mean BPs by randomized treatment group for those with and without in-trial PAD events are shown in the online appendix. Kaplan-Meier plots and Cox proportional hazard ratios by treatment group for time to first post-baseline PAD event are shown in Figure 2. Following adjustment for baseline characteristics, participants assigned to amlodipine and lisinopril showed similar risks compared with those assigned to chlorthalidone (HR, 0.86; 95 % CI, 0.72–1.03, and HR, 0.98; 95 % CI, 0.83–1.17, respectively) for first clinically advanced PAD event. Similarly, there was no difference in post-PAD mortality in either comparison (Table 2). When looking at pre-specified subgroups plus the (post-hoc) history of coronary heart disease subgroup for those with in-trial PAD, there were no significant subgroup-treatment interactions between amlodipine and chlorthalidone or lisinopril and chlorthalidone (Fig. 3).
Figure 2.
Years to peripheral arterial disease.
Table 2.
PAD and Post-PAD Mortality by Treatment Group
| Chlorthalidone | Amlodipine | Lisinopril | Amlodipine vs. chlorthalidone | Lisinopril vs. chlorthalidone | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of events | 6-Year rate per 100 persons (SE) | Number of events | 6-Year rate per 100 persons (SE) | Number of events | 6-Year rate per 100 persons (SE) | HR (95 % CI) | P | HR (95 % CI) | P | |
| PAD | 402 | 3.2 (0.2) | 198 | 2.7 (0.2) | 230 | 3.4 (0.3) | Univariate: | |||
| 0.82 (0.70–0.98) | 0.027 | 0.96 (0.82–1.13) | 0.651 | |||||||
| Multivariate*: | ||||||||||
| 0.86 (0.72–1.03) | 0.099 | 0.98 (0.83–1.17) | 0.847 | |||||||
| 10-Year rate per 100 persons (SE) | 10-Year rate per 100 persons (SE) | 10-Year rate per 100 persons (SE) | ||||||||
| Post-PAD mortality | 258 | 74.2 (3.0) | 119 | 74.5 (5.8) | 143 | 73.2 (4.7) | Univariate: | |||
| 0.92 (0.74–1.15) | 0.454 | 0.95 (0.77–1.18) | 0.651 | |||||||
| Multivariate*: | ||||||||||
| 0.96 (0.76–1.23) | 0.762 | 1.01 (0.80–1.27) | 0.918 | |||||||
*Adjusted for baseline variables of age, race, ethnicity, gender, heart rate, pulse pressure, aspirin use, history of atherosclerotic cardiovascular disease, history of coronary heart disease, history of diabetes, current smoking, ever smoking, BMI, and estimated glomerular filtration rate
Figure 3.
Peripheral arterial disease by treatment group comparisons and subgroups. No significant treatment × subgroup interactions were identified. Interaction P values are as follows: Amlodipine vs. chlorthalidone: age, P = 0.421; gender, P = 0.385; race, P = 0.737; history of CHD, P = 0.120; history of diabetes, P = 0.093. Lisinopril vs. chlorthalidone: age, P = 0.408; gender, P = 0.239; race, P = 0.603; history of CHD, P = 0.704; history of diabetes, P = 0.040. Peripheral arterial disease refers to in-trial hospitalization or lower extremity revascularization procedure.
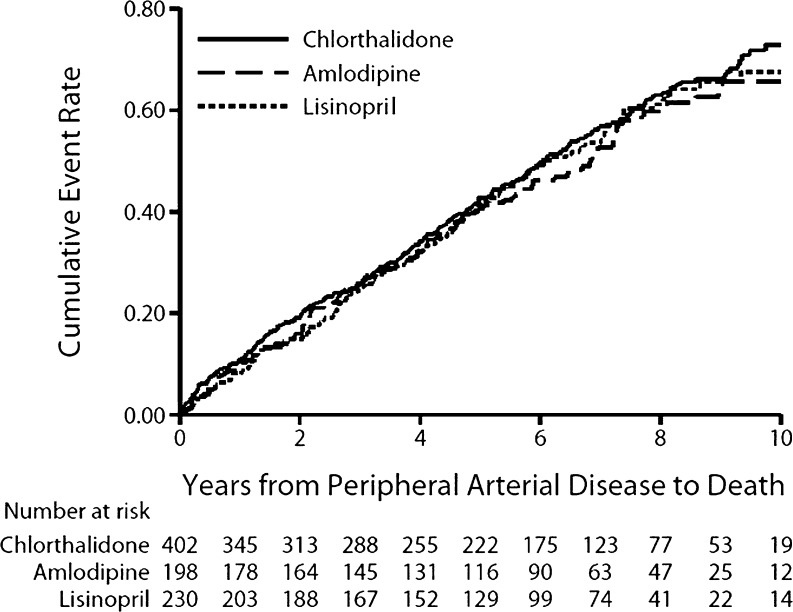
In-trial pre- and post-PAD nonfatal events and post-PAD total and cause-specific mortality are provided in Table 3. Both preceding and following PAD events, there were no differences in incident myocardial infarctions, strokes, heart failure, or coronary revascularization procedures in either the amlodipine vs. chlorthalidone or lisinopril vs. chlorthalidone comparisons. Similarly, total mortality among those with PAD did not differ in either comparison. There were also no treatment group differences regarding cause-specific mortality. Overall mortality rates at 1, 5, and 10 years post-PAD were 9.6, 41.9, and 69.8 %, respectively (Fig. 4).
Table 3.
Pre- and Post-PAD* Nonfatal and Fatal Events
| N (%) | In-trial PAD* event | Amlodipine vs. chlorthalidone | Lisinopril vs. chlorthalidone | ||||
|---|---|---|---|---|---|---|---|
| Chlorthalidone N = 402 | Amlodipine N = 198 | Lisinopril N = 230 | HR (95 % CI) | P value | HR (95 % CI) | P value | |
| In-trial nonfatal events | |||||||
| Pre-PAD events | |||||||
| MI | 25 (6) | 12 (6) | 14 (6) | 0.95 (0.48–1.89) | 0.885 | 1.00 (0.52–1.92) | 0.993 |
| Stroke | 17 (4) | 11 (6) | 4 (2) | 1.31 (0.61–2.79) | 0.489 | 0.41 (0.14–1.22) | 0.110 |
| Cardiac revascularization | 40 (10) | 19 (10) | 26 (11) | 0.94 (0.55–1.62) | 0.827 | 1.15 (0.70–1.89) | 0.574 |
| Heart failure | 23 (6) | 10 (5) | 16 (7) | 0.87 (0.42–1.83) | 0.720 | 1.25 (0.66–2.36) | 0.500 |
| Post-PAD events | |||||||
| MI | 48 (12) | 20 (10) | 21 (9) | 0.82 (0.48–1.40) | 0.473 | 0.74 (0.44–1.25) | 0.264 |
| Stroke | 24 (6) | 10 (5) | 13 (6) | 0.86 (0.41–1.79) | 0.684 | 0.94 (0.48–1.86) | 0.869 |
| Cardiac revascularization | 32 (8) | 22 (11) | 23 (10) | 1.39 (0.81–2.39) | 0.233 | 1.25 (0.73–2.13) | 0.422 |
| Heart failure | 39 (10) | 25 (13) | 25 (11) | 1.32 (0.79–2.18) | 0.286 | 1.08 (0.65–1.80) | 0.770 |
| Total mortality | 258 (64) | 119 (60) | 143 (62) | 0.92 (0.74–1.15) | 0.454 | 0.95 (0.77–1.18) | 0.651 |
| Cause-specific mortality†: | |||||||
| CVD | 132 (51) | 55 (46) | 70 (49) | 0.83 (0.60–1.14) | 0.252 | 0.85 (0.63–1.16) | 0.304 |
| MI | 27 (20) | 9 (16) | 15 (21) | 0.62 (0.28–1.38) | 0.242 | 0.96 (0.50–1.85) | 0.903 |
| Stroke | 13 (10) | 10 (18) | 11 (16) | 1.47 (0.64–3.36) | 0.359 | 1.47 (0.66–3.27) | 0.350 |
| Non-CVD | 120 (47) | 60 (50) | 68 (48) | 1.00 (0.73–1.38) | 0.996 | 1.04 (0.77–1.41) | 0.802 |
*Refers to documented hospitalized PAD or lower extremity PAD revascularization procedure
†Fifteen deaths with unknown cause
Figure 4.
Post-peripheral arterial disease all-cause mortality by treatment group.
DISCUSSION
The final results of the randomized phase of ALLHAT, published in 2002,16 showed no significant difference in the prespecified PAD endpoint (treated medically as an outpatient, hospitalized, or revascularized PAD) between those assigned to amlodipine compared to their counterparts in the chlorthalidone group (though there was a trend toward less PAD in the amlodipine group: HR, 0.87; 95 % CI, 0.75–1.01) nor between lisinopril and chlorthalidone (HR, 1.04; 95 % CI, 0.90–1.19).16 In the current analysis, using a more rigorous endpoint of documented new or recurrent PAD hospitalization or revascularization, i.e., clinically advanced PAD, than previously used, we again show no difference in risk of treatment with amlodipine or lisinopril compared with chlorthalidone (HR, 0.86; 95 % CI, 0.72–1.03 and HR, 0.98; 95 % CI, 0.83–1.17, respectively). Post-PAD nonfatal and fatal events were similar across treatment groups.
The choice of antihypertensive therapy must reflect the best scientific evidence and take into account all known benefits and risks of the antihypertensive drugs. Toward that end, active-controlled comparative trials are required. Optimal antihypertensive treatment to prevent new onset or recurrent PAD remains undetermined, especially given the need to take into account all outcomes associated with a given treatment. Evidence regarding use of beta-blockers in hypertensive patients with PAD is limited; active-controlled clinical trials have yet to prove their superiority. Questions not yet fully addressed have arisen regarding possible compromised blood flow to the extremities while on beta-blockers. Though non-selective beta-blockers have traditionally been contraindicated in patients with PAD, newer beta-blockers such as the non-selective beta blocker carvedilol and the β1-selective nebivolol, both of which have vasodilating effects, have shown promise.23,24 The International Verapamil-SR/Tradandolapril Study (INVEST), in a post-hoc analysis of hypertensive participants with coronary heart disease and concomitant PAD, reported no significant difference in primary outcome (all-cause death, non-fatal stroke, or non-fatal myocardial infarction) between participants assigned to a calcium antagonist-based treatment strategy when compared with those assigned to a beta-blocker-based strategy.25 The potential vasoprotective effects of ACE-inhibitors on systemic vasculature, including inhibition of multiple steps in atherogenesis, have been described.26,27 Placebo-controlled trials have reported benefits of the ACE-inhibitor ramipril over placebo in reducing clinical outcomes in persons with clinical and subclinical PAD,28,29 but conclusive comparative effectiveness clinical trial data are lacking.30 Practice guidelines for treatment of at-risk hypertensive patients with PAD suggest using ACE-inhibitors and/or beta-blockers, but are based largely on placebo-controlled trials.31 A definitive case has not been convincingly made for using any of these antihypertensive classes—calcium channel blockers, ACE-inhibitors, or beta-blockers—in hypertensive patients at risk of developing PAD.
Several limitations must be considered in interpreting this report. First, history of PAD at baseline was not specifically collected; thus, the actual PAD incidence cannot be determined. However, randomization presumably allowed for equitable distribution across treatment groups of participants with and without pre-enrollment PAD. Formal screening for PAD at follow-up visits was not performed; thus, asymptomatic PAD was likely missed. In addition, post-trial morbidity, medication data, and laboratory values were not available for analyses; thus, possible links between the PAD events described here and post-trial mortality may have been missed. Definitions and diagnostic criteria for PAD differ among studies, complicating comparisons between different reports. Gregg and colleagues, using NHANES data, defined PAD according to precise ankle-brachial index results obtained under protocol-specific guidelines;32 ALLHAT defined the PAD outcome based on symptomatic disease requiring presentation to the physician or hospital and/or the performance of lower extremity arterial revascularization procedures or amputations. In an attempt to establish a more rigorous definition, we limited PAD in this study to lower extremity disease that could be verified: those events requiring hospitalization or revascularization, both of which provided hospital or operative documentation for central review (albeit further decreasing the sample size somewhat with exclusion of non-hospitalized, non-revascularized subjects). Indeed, the small sample size, and the resultant lack of power, may at least partially contribute to the absence of significant interactions in multiple subgroup analyses that were performed to assess the impact of three antihypertensive treatments on new PAD events. As in other trials, secondary outcomes including PAD were not presented to an adjudications committee for diagnostic validation, though the physician-led central review of documentation established a level of confidence in the validity of events. In addition, the large sample size and randomized, double-blind design of ALLHAT provides reassurance of equity in event reporting across the study treatment groups.19 Further, early and fortuitous planning of post-trial follow-up of ALLHAT participants provided outcome data by which we could assess long-term sequelae of in-trial events among participants who experienced in-trial PAD.17,33
PAD is a marker for high cardiovascular risk, including risk of mortality.34,35 In these analyses PAD is associated with high post-event mortality: 70 % over 10 years of follow-up. Reduction of major modifiable risk factors, including hypertension, is critical, but the optimal antihypertensive therapy, when considering multiple cardiovascular disease outcomes, remains undetermined.6 ALLHAT data provide several lessons. First, once PAD, sufficiently advanced as to require hospitalization or revascularization, develops, it is associated with a high rate of subsequent cardiovascular disease morbidity and all-cause mortality regardless of the type of antihypertensive treatment, thus underscoring the need for cardiovascular disease prevention. Second, decisions as to choice of antihypertensive treatment in high-risk hypertensive patients at risk of PAD must consider all evidence of clinical outcomes associated with that treatment. Our findings here do not dispute previously reported ALLHAT findings that neither the calcium-channel blocker amlodipine nor the ACE-inhibitor lisinopril is superior to the diuretic chlorthalidone in preventing coronary heart disease or other major cardiovascular outcomes. Rather, they reinforce the compelling need for comparative clinical outcome trials similar to ALLHAT to assess optimal drug combinations and to address optimal prevention of PAD in the context of other major cardiovascular events.
Electronic supplementary material
(PDF 533 kb)
ACKNOWLEDGMENTS
The authors thank Dr. Ellen Breckenridge, The University of Texas School of Public Health, for editorial assistance in the preparation of this manuscript.
Funding
This research was supported by contracts NO1-HC-35130 and HHSN268201100036C from the National Heart, Lung, and Blood Institute. The ALLHAT investigators acknowledge contributions of study medications supplied by Pfizer, Inc. (amlodipine and doxazosin), AstraZeneca (atenolol and lisinopril), and Bristol-Myers Squibb (pravastatin) and financial support provided by Pfizer, Inc.
Prior Presentations
None
Conflict of Interest
Dr. Basil has received honoraria from Daiichi Sankyo and Takeda.
Dr. Probstfield has received research support from GlaxcoSmithKline and Sanofi Aventis.
Dr. Rahman has received honoraria from Boehringer Ingelheim.
Drs. Baraniuk, Dart, Davis, Ellsworth, Fendley, Habib, Piller, Simpson, and Whelton have no financial interests to disclose.
Footnotes
Clinical Trial Registration
For a complete list of members of the ALLHAT Collaborative Research Group, see JAMA 2002;288:2981–2997.
REFERENCES
- 1.Murabito JM, D’Agostino RB, Silbershatz H, Wilson WF. Intermittent claudication. A risk profile from the Framingham Heart Study. Circulation. 1997;96:44–49. doi: 10.1161/01.CIR.96.1.44. [DOI] [PubMed] [Google Scholar]
- 2.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–743. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 3.Bozkurt AK, Tasci I, Tabak O, Gumus M, Kaplan Y. Peripheral artery disease assessed by ankle-brachial index in patients with established cardiovascular disease or at least one risk factor for atherothrombosis–CAREFUL study: a national, multi-center, cross-sectional observational study. BMC Cardiovasc Disord. 2011;11:4. doi: 10.1186/1471-2261-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meijer WT, Grobbee DE, Hunink MGM, Hofman A, Hoes AW. Determinants of peripheral arterial disease in the elderly: the Rotterdam study. Arch Intern Med. 2000;160:2934–2938. doi: 10.1001/archinte.160.19.2934. [DOI] [PubMed] [Google Scholar]
- 5.Criqui MH, Denenberg JO. The generalized nature of atherosclerosis: how peripheral arterial disease may predict adverse events from coronary artery disease. Vasc Med. 1998;3:241–245. doi: 10.1177/1358836X9800300311. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the Medicare population. Vasc Med. 2008;13:209–215. doi: 10.1177/1358863X08089277. [DOI] [PubMed] [Google Scholar]
- 7.Antonopoulos S, Kokkoris S, Stasini F, et al. High prevalence of subclinical peripheral artery disease in Greek hospitalized patients. Eur J Intern Med. 2005;16:187–191. doi: 10.1016/j.ejim.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Cacoub PP, Abola MT, Baumgartner I, et al. REACH Registry Investigators. Cardiovascular risk factor control and outcomes in peripheral artery disease patients in the REduction of Atherothrombosis for Continued Health (REACH) registry. Atherosclerosis. 2009;204:e86–e92. doi: 10.1016/j.atherosclerosis.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Clement DL, De Buyzere ML, Duprez DA. Hypertension in peripheral arterial disease. Curr Pharm Des. 2004;10:3615–3620. doi: 10.2174/1381612043382819. [DOI] [PubMed] [Google Scholar]
- 10.De Buyzere ML, Clement DL. Management of hypertension in peripheral arterial disease. Prog Cardiovasc Dis. 2008;50:238–263. doi: 10.1016/j.pcad.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Singer DR, Kite A. Management of hypertension in peripheral arterial disease: does the choice of drugs matter? Eur J Vasc Endovasc Surg. 2008;35:701–708. doi: 10.1016/j.ejvs.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Lauer MS. Comparative effectiveness research: the view from the NHLBI. J Am Coll Cardiol. 2009;53:1084–1086. doi: 10.1016/j.jacc.2008.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matchar DB, McCrory DC, Orlando LA, et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med. 2008;148:16–29. doi: 10.7326/0003-4819-148-1-200801010-00189. [DOI] [PubMed] [Google Scholar]
- 14.Sciarretta S, Palano F, Tocci G, Baldini R, Volpe M. Antihypertensive treatment and development of heart failure in hypertension: a Bayesian network meta-analysis of studies in patients with hypertension and high cardiovascular risk. Arch Intern Med. 2011;171:384–394. doi: 10.1001/archinternmed.2010.427. [DOI] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National Heart, Lung, and Blood Institute, National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 16.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive And Lipid-Lowering Treatment To Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 17.Cushman WC, Davis BR, Pressel SL, ALLHAT Collaborative Research Group et al. Mortality and morbidity during and after the Antihypertensive And Lipid-Lowering Treatment To Prevent Heart Attack Trial. J Clin Hypertens (Greenwich) 2012;14:20–31. doi: 10.1111/j.1751-7176.2011.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The fifth report of the Joint National Committee On Detection Evaluation, and treatment of high blood pressure (JNC V) Arch Int Med. 1993;153:154–183. doi: 10.1001/archinte.1993.00410020010002. [DOI] [PubMed] [Google Scholar]
- 19.Davis BR, Cutler JA, Gordon DJ, et al. Rationale and design for the Antihypertensive And Lipid-Lowering Treatment To Prevent Heart Attack Trial (ALLHAT) Am J Hypertens. 1996;9:342–360. doi: 10.1016/0895-7061(96)00037-4. [DOI] [PubMed] [Google Scholar]
- 20.Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group Diuretic versus alpha-blocker as first-step antihypertensive therapy: final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Hypertension. 2003;42:239–246. doi: 10.1161/01.HYP.0000086521.95630.5A. [DOI] [PubMed] [Google Scholar]
- 21.Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Protocol. Available at: https://allhat.sph.uth.tmc.edu/Forms/protocol.pdf. Accessed April 28, 2014
- 22.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Regression. 2. New York, NY: Springer; 2003. [Google Scholar]
- 23.Stafylas PC, Sarafidis PA. Carvedilol in hypertension treatment. Vasc Health Risk Manag. 2008;4:23–30. doi: 10.2147/vhrm.2008.04.01.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espinola-Klein C, Weisser G, Jagodzinski A, et al. ß-Blockers in patients with intermittent claudication and arterial hypertension: results from the nebivolol or metoprolol in arterial occlusive disease trial. Hypertension. 2011;58:148–154. doi: 10.1161/HYPERTENSIONAHA.110.169169. [DOI] [PubMed] [Google Scholar]
- 25.Bavry AA, Anderson RD, Gong Y, et al. Outcomes among hypertensive patients with concomitant peripheral and coronary artery disease: findings from the INternational VErapamil-SR/Trandolapril STudy. Hypertension. 2010;55:48–53. doi: 10.1161/HYPERTENSIONAHA.109.142240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch AT, Duprez D. The potential role of angiotensin-converting enzyme inhibition in peripheral arterial disease. Vasc Med. 2003;8:273–278. doi: 10.1191/1358863x03vm502oa. [DOI] [PubMed] [Google Scholar]
- 27.Coppola G, Romano G, Corrado E, Grisanti RM, Novo S. Peripheral artery disease: potential role of ACE-inhibitor therapy. Vasc Health Risk Manag. 2008;4:1179–1187. doi: 10.2147/vhrm.s3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostergren J, Sleight P, Dagenais G, et al. HOPE study investigators. Impact of ramipril in patients with evidence of clinical or subclinical peripheral arterial disease. Eur Heart J. 2004;25:17–24. doi: 10.1016/j.ehj.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 29.Ahimastos AA, Lawler A, Reid CM, Blombery PA, Kingwell BA. Brief communication: ramipril markedly improves walking ability in patients with peripheral arterial disease: a randomized trial. Ann Intern Med. 2006;144:660–664. doi: 10.7326/0003-4819-144-9-200605020-00009. [DOI] [PubMed] [Google Scholar]
- 30.Gey DC, Lesho EP, Manngold J. Management of peripheral arterial disease. Am Fam Physician. 2004;69:525–532. [PubMed] [Google Scholar]
- 31.Hirsch AT, Haskal ZJ, Hertzer NR, American Association for Vascular Surgery, Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, ACC/AHA Task Force on Practice Guidelines, American Association of Cardiovascular and Pulmonary Rehabilitation, National Heart, Lung, and Blood Institute, Society for Vascular Nursing, TransAtlantic Inter-Society Consensus, Vascular Disease Foundation et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary. J Am Coll Cardiol. 2006;47:1239–1312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 32.Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, Burt V, Curtin L, Engelgau M, Geiss L. 1999–2000 National Health And Nutrition Examination Survey. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999–2000 National Health And Nutrition Examination Survey. Diabetes Care. 2004;27:1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 33.Extension Protocol: Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Available at: https://allhat.sph.uth.tmc.edu/Forms/ExtensionProtocol.pdf. Accessed April 28, 2014
- 34.Cimminiello C, Borghi C, Kownator S, PANDORA Study Investigators et al. Prevalence of peripheral arterial disease in patients at non-high cardiovascular risk Rationale and design of the PANDORA study. BMC Cardiovasc Disord. 2010;10:35. doi: 10.1186/1471-2261-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawson DL, Hiatt WR, Creager MA, Hirsch AT. Peripheral arterial disease: medical care and prevention of complications. Prev Cardiol. 2002;5:119–130. doi: 10.1111/j.1520-037X.2002.00558.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 533 kb)