ABSTRACT
BACKGROUND
Elimination of wasteful diagnostic testing will improve value for the United States health care system.
OBJECTIVE
Design and implement a multimodal intervention to improve evidence-based ordering of cardiac biomarkers for the diagnosis of acute coronary syndrome (ACS).
DESIGN
Interrupted times series.
SUBJECTS
A total of 60,494 adult inpatient admissions from January 2009 through July 2011 (pre-intervention) and 24,341 admissions from November 2011 through October 2012 (post-intervention) at an academic medical center in Baltimore, Maryland.
INTERVENTION
Multimodal intervention introduced August through October 2011 that included dissemination of an institutional guideline and changes to the computerized provider order entry system.
MAIN MEASURES
The primary outcome was percentage of patients with guideline-concordant ordering of cardiac biomarkers, defined as three or fewer troponin tests and zero CK-MB tests in patients without a diagnosis of ACS. Secondary outcomes included counts of tests ordered per patient, incidence of diagnosis of ACS, and estimated change in charges for cardiac biomarker tests in the post-intervention period.
KEY RESULTS
Twelve months following the intervention, we estimated that guideline-concordant ordering of cardiac biomarkers increased from 57.1 % to 95.5 %, an absolute increase of 38.4 % (95 % CI, 36.4 % to 40.4 %). We estimated that the intervention led to a 66 % reduction in the number of tests ordered, and a $1.25 million decrease in charges over the first year. At 12 months, there was an estimated absolute increase in incidence of primary diagnosis of ACS of 0.3 % (95 % CI, 0.0 % to 0.5 %) compared with the expected baseline rate.
CONCLUSIONS
We implemented a multimodal intervention that significantly increased guideline-concordant ordering of cardiac biomarker testing, leading to substantial reductions in tests ordered without impacting diagnostic yield. A trial of this approach at other institutions and for other diagnostic tests is warranted and if successful, would represent a framework for eliminating wasteful diagnostic testing.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-014-2919-5) contains supplementary material, which is available to authorized users.
KEY WORDS: wasteful diagnostic testing, acute coronary syndrome, cardiac biomarkers
INTRODUCTION
Several estimates suggest that 30 cents of every health care dollar spent in the United States do not improve health outcomes.1–3 An estimated $250 to $325 billion dollars are spent annually on unnecessary, duplicative, and/or inappropriate diagnostic tests.2 Identifying specific wasteful tests and treatments and developing interventions to reduce overutilization are critical initiatives for combating the rising cost of health care in the United States.
Cardiac biomarkers, such as creatine kinase (CK, total and MB-fraction) and troponin, are frequently ordered in the emergency department (ED) and inpatient units to evaluate patients suspected of having an acute coronary syndrome (ACS). In 2010, more than 17 million patients visiting an ED in the United States received cardiac biomarker testing.4 Guidelines developed in conjunction with the American Heart Association specify that troponin, evaluated no more than three times, is the preferred biomarker for diagnosis of ACS, and total CK and CK-MB should only be used if troponin is not available at the corresponding testing laboratory.5 An analysis of more than 11,000 patients presenting to an ED found that there were zero instances where ACS was detected from a positive CK-MB when the troponin was negative.6 Despite these guidelines, a recent survey of emergency medicine physicians at 98 US hospitals found that 85 % of institutions combine troponin with additional biomarkers such as CK-MB.7
A chart review of patients admitted to an internal medicine service at our institution revealed that providers ordered troponin, CK and CK-MB as a panel, with more than 20 % of patients receiving more than three of each of these tests. We estimated a potential annual reduction of more than 50,000 tests and $1 million in charges if providers ordered troponin alone, no more than three times for the diagnosis of ACS.8 In this study, we aimed to design and implement a multimodal intervention grounded in behavioral theory to align cardiac biomarker ordering with guidelines at our institution.
METHODS
Study Design and Setting
We used an interrupted times series design over the time period January 2009 through October 2012 to assess the impact of an intervention on cardiac biomarker ordering at Johns Hopkins Bayview Medical Center (JHBMC). JHBMC is a 555-bed academic medical center in Baltimore, MD. Details on the setting and provider makeup are included in Online Appendix 1.
Description of Intervention
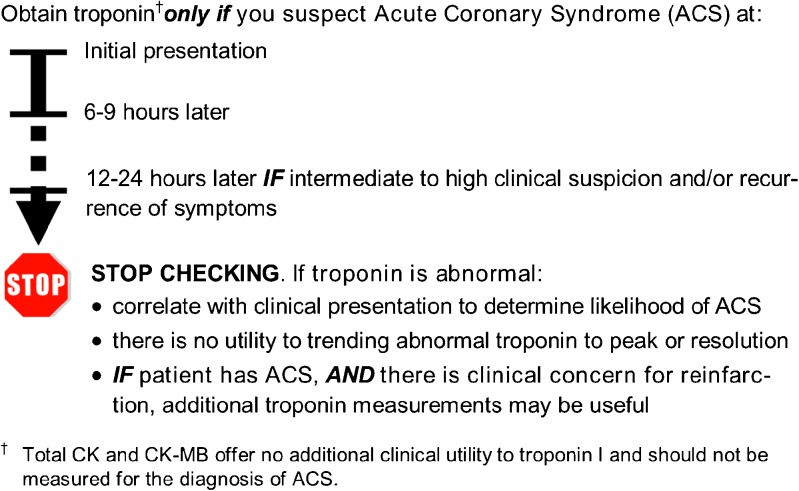
In consultation with cardiologists at our institution, we developed an institutional guideline to specify appropriate ordering of cardiac biomarkers for the diagnosis of acute coronary syndrome (ACS). This guideline was consistent with the 2007 National Academy of Clinical Biochemistry laboratory medicine practice guideline.5 The guideline suggests ordering troponin alone, without CK or CK-MB, in patients with suspected ACS. Furthermore, the guideline specifies that troponin should be assessed no more than three times, appropriately spaced over 18–24 hours, noting that there is no clinical utility to further testing or trending elevated troponin to peak or resolution in the absence of a diagnosis of ACS.
We designed a multimodal intervention based on the classification scheme of factors that influence behaviors from the PRECEDE model of behavioral change9: predisposing factors including knowledge and attitudes; enabling factors that facilitate or prevent behavior; and reinforcing factors that reward or reinforce the desired behavior change. A systematic review of interventions to modify diagnostic test ordering behavior found that interventions based on more than one category were more successful.10
We targeted predisposing factors with informational sessions to high-utilizing internal medicine and emergency department providers to outline the initiative and guideline in August and September 2011. We also disseminated a pocket-sized quick reference card summarizing the recommended ordering algorithm to all hospital providers (Fig. 1).
Figure 1.
Quick reference guide for ordering cardiac biomarkers for diagnosis of ACS.
We targeted enabling factors by changing the computerized provider order entry system in October 2011 (Meditech, Westwood, MA). We removed orders for CK and CK-MB from all standardized order sets, including several admission and routine daily order sets. We removed troponin orders from all order sets except two that are used for evaluation of new symptoms concerning for ACS. We created a duplicate order pop-up warning when a troponin level was ordered sooner than 6 hours after another troponin level. Likewise, we created pop-up warnings when a provider attempted to order CK-MB or CK. For CK-MB, the warning read: “According to national guidelines, troponin is the preferred biomarker for detecting myocardial injury. CK-MB is only appropriate when troponin levels are unavailable.” For CK, the warning read: “Creatine phosphokinase (CK) should not be ordered for evaluation of acute coronary syndrome as it offers no additional benefit beyond troponin alone.” In response to these pop-up warnings, providers could choose to either erase the order or override the warning. Finally, with the exception of ED triage order sets, we eliminated orders for nurses to initiate cardiac biomarker testing, and educated nursing staff and providers that orders for cardiac biomarkers should be ordered directly by providers. Online Appendix 1 contains further details on intervention development and implementation.
Data Source and Patient Selection
We obtained data from a hospital administrative database containing patient characteristics, provider orders, and clinical and billing data. For any missing or nonsense data, we consulted the electronic medical record. We included all adult patients (≥ 18 years) admitted to Johns Hopkins Bayview Medical Center from 1 January 2009 through 31 October 2012. Patients admitted on observation status (< 24 hours) to internal medicine and short stay surgical services were included. We excluded patients with a length of stay > 30 days, given the increased possibility of repeated clinical episodes warranting evaluation of ACS. Two patients were excluded because their birth dates were not available. The Johns Hopkins Medicine institutional review board (IRB) determined that the project met institutional criteria as a quality improvement initiative and was exempt from IRB review. To protect patient privacy, we created a data set with identifying information limited to patients’ age in years at admission and month of admission. We obtained a waiver of HIPAA privacy authorization through the Johns Hopkins Medicine IRB.
Measures
The primary outcome was the percentage of patients per month with guideline-concordant ordering of cardiac biomarkers, defined as zero CK-MB tests and three or fewer troponin tests. Patients with a primary diagnosis of ACS were excluded from primary outcome assessment, as our guideline acknowledged the utility of additional testing in these patients. Secondary outcomes were the mean tests per patient per month for troponin, CK-MB, and CK, as well as monthly incidence of ACS using ICD-9 codes 410.x or 411.x. We examined both the primary ICD-9 diagnosis code, the code reflecting the principal reason for hospitalization, and all secondary ICD-9 codes for the hospitalization.
As a post-hoc analysis, we obtained counts of times warning messages were displayed for CK and CK-MB orders in each month of the post-intervention period for patients admitted to adult inpatient units. Given differences in reporting systems, inclusion and exclusion criteria were not applied to this post hoc analysis (Results reported in Online Appendix 2).
Data Analysis
We divided the study time frame into three periods: pre-intervention, January 2009 through July 2011; intervention roll-out, August 2011 through October 2011; and, post-intervention, November 2011 through October 2012. We controlled for age and gender by standardizing to January 2009, categorizing age as 18–44 years, 45–64 years, and ≥ 65 years. Segmented linear regression analysis11 was used to test for changes in each of the primary and secondary outcomes as a result of the intervention. Regression models included a term for baseline level, a term for baseline time trend, and terms to estimate the changes in level and trend beginning with the first post-intervention month (November 2011). We selected January 2009 as the start date for the pre-intervention period, as it provided 31 months of repeated outcomes measurements spanning multiple years, thus improving our ability to identify any existing secular trends compared with a shorter period. We excluded the three months of the intervention roll-out period (August 2011 through October 2011) from regression analyses. We controlled for autocorrelation by assuming a first-order autoregressive process. Regression models were populated using backward elimination with criteria of p < 0.20 to retain terms in the model. We used two-tailed tests with α = 0.05 to determine if changes in level and/or trend were significant. The regression results were used to estimate the absolute effect of the intervention and 95 % confidence interval for each outcome at 12 months using multivariate delta methods.12 Given differences in use of cardiac biomarker testing, we stratified by admission service to internal medicine services versus other services in addition to reporting data on all admissions. We estimated the changes in troponin, CK, and CK-MB tests ordered in the post-intervention period by comparing actual utilization with that predicted from the pre-intervention trends from the regression analyses. We estimated the changes in charges using the average charge per test obtained from administrative billing data during the post-intervention period ($51.90 for troponin, $12.33 for CK, and $30.80 for CK-MB). These charges represent negotiated all-payer payment rates under the Health Services Cost Review Commission in Maryland.13 We used SAS version 9.3 for all analyses (SAS Institute Inc., Cary, NC).
RESULTS
There were 60,494 patients admitted in the 31 months of the pre-intervention period (mean 1,951 patients per month) and 24,341 patients admitted in the 12 months of the post-intervention period (mean 2,028 patients per month). The baseline characteristics of the pre-intervention and post-intervention patients were similar (Table 1). The mean age was slightly higher in the post-intervention group (54.5 years versus 53.9 years; p < 0.001). Of the patients, 46.8 % were male in each group (p = 0.96). A higher percentage of patients were admitted to internal medicine services in the post-intervention period compared with the pre-intervention period (51.8 % versus 50.0 %; p < 0.001).
Table 1.
Characteristics of Patients in the Pre-Intervention and Post-Intervention Periods
| Characteristic | Pre-intervention* | Post-intervention† | p value |
|---|---|---|---|
| N | 60,494 | 24,341 | n/a |
| Mean age, years (SD) | 53.9 (19.1) | 54.5 (18.7) | < 0.001 |
| Age group (%) | < 0.001 | ||
| 18-44 years | 32.8 | 30.5 | |
| 45-64 years | 36.8 | 39.2 | |
| ≥ 65 years | 30.4 | 30.3 | |
| Male (%) | 46.8 | 46.8 | 0.96 |
| Admission service (%) | < 0.001 | ||
| Internal medicine‡ | 50.0 | 51.8 | |
| Other§ | 50.0 | 48.2 |
* Pre-intervention period January 2009 through July 2011
† Post-intervention period November 2011 through October 2012
‡ Internal medicine services included general medicine, cardiology, pulmonary, and renal
§ Other services included general surgery, orthopedics, gynecology, obstetrics, psychiatry, neurology, neurosurgery, urology, vascular surgery, trauma, otolaryngology, burn surgery, plastic surgery, chemical dependency
Impact on Guideline-Concordant Cardiac Biomarker Test Ordering
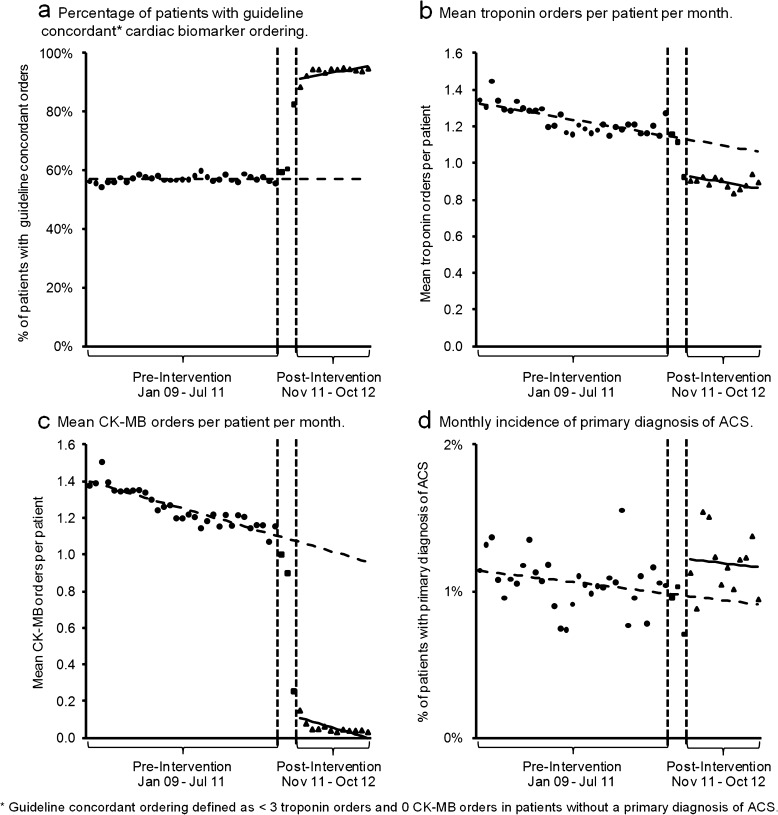
Following introduction of the multimodal intervention, there was an absolute increase of 34.0 % (95 % CI 31.9 % to 36.0 %) in the proportion of patients receiving guideline-concordant testing, along with an increasing trend of 0.4 % per month (95 % CI 0.1 % to 0.6 %; Table 2). Twelve months following the start of the intervention, the percentage of patients receiving guideline-concordant cardiac biomarker testing was estimated to be 95.5 %, an absolute increase of 38.4 % (95 % CI 36.4 % to 40.4 %) from the expected baseline result (Fig. 2a).
Table 2.
Results from segmented Linear Regression Models for Each Outcome for All Patients and Stratified by Admission to Internal Medicine Versus other Services
| Parameter estimate (95 % CI) | All services | Internal medicine services* | Other services† |
|---|---|---|---|
| Guideline concordant cardiac biomarker ordering | |||
| Level in January 2009 | 57.1 % (56.4 % to 57.8 %) | 23.0 % (21.6 % to 24.3 %) | 87.8 % (87.5 % to 88.2 %) |
| Baseline trend | N/A (p > 0.20) | 0.1 % per month (0.0 % to 0.2 %) | N/A (p > 0.20) |
| Post-intervention level change | 34.0 % (31.9 % to 36.0 %) | 60.0 % (57.3 % to 62.7 %) | 9.1 % (8.4 % to 9.7 %) |
| Post-intervention trend change | 0.4 % per month (0.1 % to 0.6 %) | 0.4 % per month (0.1 % to 0.7 %) | N/A (p > 0.20) |
| Troponin tests ordered per patient | |||
| Level in January 2009 | 1.33 (1.30 to 1.36) | 2.39 (2.34 to 2.45) | 0.32 (0.30 to 0.34) |
| Baseline trend | −0.006 per month (−0.004 to −0.008) | −0.01 per month (−0.02 to −0.01) | −0.001 per month (−0.002 to −0.000) |
| Post-intervention level change | −0.20 (−0.25 to −0.15) | −0.39 (−0.50 to −0.28) | −0.05 (−0.08 to −0.02) |
| Post-intervention trend change | N/A (p > 0.20) | 0.009 per month (−0.003 to 0.021) | N/A (p > 0.20) |
| CK-MB tests ordered per patient | |||
| Level in January 2009 | 1.41 (1.38 to 1.44) | 2.56 (2.51 to 2.61) | 0.31 (0.29 to 0.33) |
| Baseline trend | −0.01 per month (−0.01 to −0.01) | −0.02 per month (−0.02 to −0.02) | −0.002 per month (−0.003 to −0.001) |
| Post-intervention level change | −0.96 (−1.01 to −0.91) | −1.76 (−1.86 to −1.66) | −0.21 (−0.24 to −0.17) |
| Post-intervention trend change | N/A (p > 0.20) | 0.01 per month (−0.00 to 0.02) | N/A (p > 0.20) |
| Primary diagnosis of ACS | |||
| Level in January 2009 | 1.14 % (1.01 % to 1.28 %) | 2.34 % (2.06 % to 2.61 %) | 0.007 % (−0.006 % to 0.020 %) |
| Baseline trend | −0.005 % per month (−0.0012 % to 0.002 %) |
−0.011 % per month (−0.026 % to 0.004 %) | N/A (p > 0.20) |
| Post-intervention level change | 0.25 % (0.03 % to 0.47 % | 0.49 % per month (0.04 % to 0.95 %) | N/A (p > 0.20) |
| Post-intervention trend change | N/A (p > 0.20) | N/A (p > 0.20) | 0.005 % per month (0.001 % to 0.008 %) |
* Internal medicine services included general medicine, cardiology, pulmonary, and renal
† Other services included general surgery, orthopedics, gynecology, obstetrics, psychiatry, neurology, neurosurgery, urology, vascular surgery, trauma, otolaryngology, burn surgery, plastic surgery, chemical dependency
Figure 2.
Age-standardized and gender–standardized rates of primary and secondary outcomes for the pre-intervention, intervention roll-out, and post-intervention periods. Trend lines from the segmented linear regression models for the pre-intervention and post-intervention periods are depicted.
Impact on Tests Ordered Per Patient
In the first post-intervention month, there was a level change of −0.20 troponin tests per patient (95 % CI, −0.25 to −0.15), without a significant change in the downward baseline month-month trend (Table 2). At 12 months after the intervention, the estimated number of troponin tests ordered per patient was 0.86, an absolute change of −0.20 troponin tests per patient (95 % CI, −0.25 to −0.15) compared to the predicted result if the baseline trend had continued (Fig. 2b).
In the first post-intervention month, providers ordered −0.96 fewer CK-MB tests per patient (95 % CI, −1.01 to −0.91), with no change in the downward baseline trend in the post-intervention period (Table 2). In the twelfth post-intervention month, the estimated number of CK-MB tests per patient was 0.00, an absolute change of −0.96 CK-MB tests per patient (95 % CI, −1.00 to −0.92) compared to the predicted result (Fig. 2c).
Impact on Incidence of ACS Diagnosis
In the first post-intervention month, there was an increase in incidence of ACS of 0.3 % per month (95 % CI, 0.0 % to 0.5 %), with no change in the baseline trend (Table 2). In month 12 of the post-intervention period, the estimated incidence of primary diagnosis of ACS was 1.2 %, an absolute increase of 0.3 % (95 % CI, 0.0 % to 0.5 %; Fig. 2d). The estimated incidence of primary or secondary diagnosis of ACS in month 12 of the post-intervention period was 6.1 %, an absolute increase of 1.4 % (95 % CI, 0.3 % to 2.5 %).
Results Stratified by Admission Service
Improvements in guideline-concordant biomarker test ordering and reductions in ordering of each cardiac biomarker were found for patients admitted to internal medicine services as well as other services. The changes were more marked in patients admitted to internal medicine services; however, baseline levels of guideline-concordance were lower and test ordering higher for patients on internal medicine compared with other services (Table 2).
Impact on Patient Charges
Compared to baseline trends from the pre-intervention period, tests ordered in the post-intervention year were reduced by 4,369 (16 % decrease), 24,134 (87 % decrease), and 23,376 (95 % decrease), for troponin, CK, and CK-MB respectively. The total reduction in tests ordered was 51,914 (66 % decrease). This translated into a reduction in charges of $1.25 million for the three tests combined, a 50 % reduction, in the post-intervention year (Table 3).
Table 3.
Predicted* Versus Actual Number of Tests and Charges for Cardiac Biomarker Testing in the Post-Intervention Year
| Test | Predicted | Actual | Difference |
|---|---|---|---|
| Number of tests ordered | |||
| Troponin | 26,634 | 22,265 | −4,369 (−16 %) |
| CK | 27,867 | 3,703 | −24,134 (−87 %) |
| CK-MB | 24,660 | 1,279 | −23,376 (−95 %) |
| Total | 79,161 | 27,247 | −51,914 (−66 %) |
| Charges (thousands) | |||
| Troponin | $1,382 | $1,156 | -$226 (−16 %) |
| CK | $344 | $45.7 | -$298 (−87 %) |
| CK-MB | $760 | $39.4 | -$720 (−95 %) |
| Total | $2,486 | $1,241 | -$1,245 (−50 %) |
* Predicted number of tests ordered based on forecast mean tests per patient assuming the baseline pre-intervention trend continued in the post-intervention period
DISCUSSION
We designed and implemented a multimodal intervention that achieved significant, substantial, and durable improvement in alignment of cardiac biomarker test ordering at our institution with national guidelines. These changes persisted throughout the post-intervention 12-month follow-up period. The ordering of CK/CK-MB was virtually eliminated, and troponin testing was reduced by 16 %. Over one year, we estimate the intervention led to reductions of more than 51,000 tests and $1.25 million in charges. Rather than decreasing our ability to diagnose ACS, the interrupted times series analysis detected a small, but statistically significant increase in the primary diagnosis of ACS and primary or secondary diagnosis of ACS in the post-intervention period.
Changing physician ordering behavior can be extremely challenging, and neither clinical practice guidelines14 nor clinical decision support tools15 alone have lived up to their promise of improving care quality. We believe that the combination of provider education and changes to the electronic ordering system were effective in aligning physician ordering behavior with evidence-based guidelines. Given the concurrence of these interventions, it is not possible to identify the relative impact of each. We observed a transient increase in frequency of order warning messages coinciding with housestaff turnover in July 2012 without a change in ordering outcomes, suggesting that these messages may have had some impact on the results. However, we cannot know if providers would have ignored the messages had provider education not occurred.
The results were robust over a 12-month follow-up period. As detailed in the Online Appendix, over this timeframe there was an expansion in the number of hospitalist providers and in the annual turnover of housestaff membership that occurred in July 2012. We did not adjust the results for temporal changes to provider makeup. We recognize that these changes may make sustained behavioral change more challenging, but we did not observe any meaningful impact on intervention outcomes at these times.
Our results compare favorably with three prior studies that have reported interventions to reduce overutilization of cardiac biomarker testing. Notably, our study was the only to use an interrupted times series design to take into account baseline trends. Meng et al. developed and implemented an algorithm at three Canadian tertiary hospitals that required prior authorization for ordering more than three troponin tests. In a 6-month pre-post analysis, they observed a 30 % reduction in troponin test ordering and associated labor costs, with no significant change in the incidence of myocardial infarction.16 Kumwilisak et al. developed a consensus-based guideline for appropriate laboratory test ordering in a surgical intensive care unit (SICU) and implemented the guideline via “repeated staff education.”. They found a 23 % reduction in cardiac biomarker ordering at 6 months post-intervention, with no differences in survival rate, SICU length of stay, or SICU readmissions.17 Finally, Calderon-Margarit et al. report a combined educational and administrative intervention targeting diagnostic lab tests, in which CK-MB was abolished and frequency of troponin ordering was limited. A 12-month pre-post analysis identified an 8 % reduction in troponin, with no difference in in-hospital mortality rates and 30-day readmission rates.18
Our study has several limitations. First, we used a quasi-experimental design with no control group. However, we used an interrupted time series approach that identified significant changes in outcome levels at the time of our intervention, which protects against most alternative explanations. Second, our intervention was limited to a single institution, which may limit generalizability to other institutions. However, a recent survey identified that 85 % of hospital emergency departments in the United States continue to use CK and CK-MB in addition to troponin for diagnostic evaluation of patients with chest pain.7 Chest pain accounts for approximately 9 % of all ED visits in patients over the age of 15 years in the United States.19 Thus, there is likely a significant opportunity to export this intervention to other institutions. Finally, our study is limited in its ability to detect harm. Our intervention was designed to discourage overuse of testing, and we attempted to measure the potential for underuse of testing by comparing rates of ACS diagnosis pre-intervention and post-intervention. Although the incidence of primary diagnosis of ACS did not decrease, we cannot exclude the possibility that some patients in the post-intervention period were not tested and failed to receive an appropriate diagnosis, were misclassified in the administrative data, or were subsequently diagnosed at another institution.
We believe our intervention has broad implications for physician ordering behavior in the United States and the potential impact of physicians to reduce wasteful health care spending. At our single institution, our intervention reduced patient charges by $1.25 million in one year, mostly from eliminating the use of CK/CK-MB. If practice patterns at other hospitals mirror ours, the potential charge reduction would be several billion dollars per year nationally. Furthermore, reduced cardiac biomarker testing results in fewer false positive test results, which may lead to decreased ordering of downstream cardiac tests such as noninvasive stress testing, echocardiography, and invasive procedures such as cardiac catheterization and percutaneous coronary intervention (PCI).20
We provide an example of a successful intervention that led providers to order “less” in the realm of excess cardiac biomarker testing that does not improve diagnostic yield, and receive “more” in terms of potential savings to payers, providers, and patients, along with mitigation of potential downstream harm and cost associated with spurious tests results. Given this success, we believe this approach warrants trial at other institutions and with other diagnostic tests that may be inappropriately ordered. If reproducible, this approach would represent a key tactic in combating spiraling health care costs and improving the value of care delivered to patients.
Electronic supplementary material
(DOCX 28 kb)
(DOCX 32 kb)
Acknowledgements
Contributors
We would like to thank Dr. David Hellmann, M.D. for his leadership, guidance, and enthusiastic support of this project. We thank Fang Zhang, PhD, for his guidance in designing and executing the statistical analysis. We thank Angel Sampedro, BS for his assistance in acquiring the data.
Funders
This work was supported by a Putting the Charter into Practice grant from the American Board of Internal Medicine Foundation. Dr. Larochelle’s work was supported by HRSA (T32 HP10251, T32 HP12706), the Ryoichi Sasakawa, Fellowship Fund, and the Harvard Pilgrim Health Care Institute. The funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; or in the preparation, review, and approval of the manuscript.
Prior Presentation
Preliminary 3 month results presented at The Society of General Internal Medicine Annual Meeting, Orlando, FL, May 12, 2012.
Conflicts of Interest
The authors declare that they do not have a conflict of interest.
REFERENCES
- 1.Delaune J, Everett W. Waste and inefficiency in the U.S. health care system. NEHI; 2008. www.nehi.net/writable/publication_files/file/waste_clinical_care_report_final.pdf. Accessed April 11, 2014.
- 2.Kelley R. Where can $700 billion in waste be cut annually from the U.S. healthcare system? Thomson Reuters; 2009. www.ncrponline.org/PDFs/2009/Thomson_Reuters_White_Paper_on_Healthcare_Waste.pdf. Accessed April 11, 2014.
- 3.Wennberg J, Brownlee S, Fisher E, Skinner J, Weinstein J. Improving quality and curbing health care spending. The Dartmouth Institute for Health Policy and Clinical Practice; 2008. www.dartmouthatlas.org/downloads/reports/agenda_for_change.pdf. Accessed March 20, 2013 [PubMed]
- 4.National Hospital Ambulatory Medical Care Survey 2010 emergency department summary tables. www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2010_ed_web_tables.pdf. Accessed April 11, 2014.
- 5.Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, et al. National academy of clinical biochemistry laboratory medicine practice guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115(13):e356–e375. doi: 10.1161/CIRCULATIONAHA.107.182882. [DOI] [PubMed] [Google Scholar]
- 6.Volz KA, McGillicuddy DC, Horowitz GL, Sanchez LD. Creatine kinase-MB does not add additional benefit to a negative troponin in the evaluation of chest pain. Am J Emerg Med. 2012;30(1):188–190. doi: 10.1016/j.ajem.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Parker R, Suter R. Survey examines EDs’ use of cardiac markers. ACEP News. September 2009. Accessed April 11, 2014.
- 8.Larochelle M, Trost J. Developing a program to rein in overuse of diagnostic testing in the inpatient setting. JGIM. 2011;26(S1):S566–S567. [Google Scholar]
- 9.Green LW, Kreuter MW. Health program planning: An educational and ecological approach. 4. New York, NY: McGraw-Hill Higher Education; 2005. [Google Scholar]
- 10.Solomon DH, Hashimoto H, Daltroy L, Liang MH. Techniques to improve physicians’ use of diagnostic tests: A new conceptual framework. JAMA. 1998;280(23):2020–2027. doi: 10.1001/jama.280.23.2020. [DOI] [PubMed] [Google Scholar]
- 11.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang F, Wagner AK, Soumerai SB, Ross-Degnan D. Methods for estimating confidence intervals in interrupted time series analyses of health interventions. J Clin Epidemiol. 2009;62(2):143–148. doi: 10.1016/j.jclinepi.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray R. Setting hospital rates to control costs and boost quality: The Maryland experience. Health Aff (Millwood) 2009;28(5):1395–1405. doi: 10.1377/hlthaff.28.5.1395. [DOI] [PubMed] [Google Scholar]
- 14.Casey DE., Jr Why don't physicians (and patients) consistently follow clinical practice guidelines? JAMA Int Med. 2013;173(17):1581–1583. doi: 10.1001/jamainternmed.2013.7672. [DOI] [PubMed] [Google Scholar]
- 15.Black AD, Car J, Pagliari C, et al. The impact of eHealth on the quality and safety of health care: A systematic overview. PLoS Med. 2011;8(1):e1000387. doi: 10.1371/journal.pmed.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng QH, Zhu S, Booth C, Stevens L, Bertsch B, Qureshi M, et al. Impact of the cardiac troponin testing algorithm on excessive and inappropriate troponin test requests. Am J Clin Pathol. 2006;126(2):195–199. doi: 10.1309/GK9BFAB1Y5LNBWU1. [DOI] [PubMed] [Google Scholar]
- 17.Kumwilaisak K, Noto A, Schmidt UH, Beck CI, Crimi C, Lewandrowski K, et al. Effect of laboratory testing guidelines on the utilization of tests and order entries in a surgical intensive care unit. Crit Care Med. 2008;36(11):2993–2999. doi: 10.1097/CCM.0b013e31818b3a9d. [DOI] [PubMed] [Google Scholar]
- 18.Calderon-Margalit R, Mor-Yosef S, Mayer M, Adler B, Shapira SC. An administrative intervention to improve the utilization of laboratory tests within a university hospital. Int J Qual Health Care. 2005;17(3):243–248. doi: 10.1093/intqhc/mzi025. [DOI] [PubMed] [Google Scholar]
- 19.Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United states, 1999–2008. NCHS Data Brief. 2010;No. 43:April 24, 2013. [PubMed]
- 20.Blich M, Sebbag A, Attias J, Aronson D, Markiewicz W. Cardiac troponin I elevation in hospitalized patients without acute coronary syndromes. Am J Cardiol. 2008;101(10):1384–1388. doi: 10.1016/j.amjcard.2008.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 28 kb)
(DOCX 32 kb)