Summary
Study of lung xenografts has proven useful to understand the remaining barriers to successful transplantation of other organ xenografts. In this chapter, the history and current status of lung xenotransplantation will be briefly reviewed and two different experimental models, the ex vivo porcine-to-human lung perfusion and the in vivo xenogeneic lung transplantation, will be presented. We will focus on the technical details of these lung xenograft models in sufficient detail, list the needed materials and mention analysis techniques to allow others to adopt them with minimal learning curve.
Keywords: Xenotransplantation, Xenogeneic, Pulmonary xenograft, Lung transplantation, Lung perfusion
1. Introduction
1.1 Perspective
Solid organ xenograft survival and function have improved over the last decade in association with successful generation of genetically modified pigs (1). Development of α1,3-galactosyltransferase knock-out (GalT-KO) pigs has helped to overcome the preformed-antibody-mediated hyperacute rejection (2) that porcine organs exhibit when transplanted into non-human primates (3). Expression of human complement pathway regulatory proteins (hCPRP), such as hCD46, hCD55, or CD59, controlled the activation of complement which occurs usually within a very short time with wild-type or GalT-KO organs, and significantly delays the organ injury. Despite these genetic modifications, pig lungs activate and sequester platelets and neutrophils, and activate the coagulation cascade, when perfused ex vivo with human blood or transplanted into baboons. These phenomena illustrate that significant major hurdles remain to be overcome in order to reach clinical application of lung xenotransplantation. Further, we suggest that the processes causing lung xenograft injury may be important to control in order to obtain improved results with other organ xenografts.
1.2 Historical and scientific background
The first experience with xenogeneic pulmonary organs was reported in 1968 by Bryant et al. (4). In these experiments, an ex vivo apparatus integrating porcine lungs was used to oxygenate human blood, with the idea that they might be used for patients undergoing cardiac surgical procedures. The lungs rapidly exhibited increased pulmonary vascular resistance and pulmonary edema. Since those early days, many groups have conducted multiple studies in cardiac, kidney, and islet xenotransplantation. In contrast only a few groups have evaluated pulmonary xenotransplantation (historical groups; Michler, Machiarini, Davis, Fodor, Pierson/ Azimzadeh). Whether as a cause or a consequence, lung xenograft outcomes remain relatively poor.
One unique feature of the porcine lung xenograft is the presence of pulmonary intravascular macrophages (PIMs), which we (5) and others (6) have shown elaborate thromboxane and vWF multimers, and are associated with severe constriction of the pulmonary vasculature. In addition, lung xenografts are associated with rapid disappearance of platelets and neutrophils from the circulation even when anti-pig antibody and complement activation are potently inhibited. We believe that both of these phenomena are simply more readily apparent for the lung than for other organs due to the relatively high number of PIMs, and the very large and highly vasoreactive endothelial surface area in the lung. Therefore although lungs have played a relatively minor role in the field of xenotransplanation to date, in our estimation lung xenograft studies offer a unique, valuable opportunity to contribute to future progress in the field.
1.3 Animal models
To study the outcome of pulmonary xenografts or drugs and methods that might improve the function of those organs, different animal models have been developed.
1.3.1 Rodent models
The use of rodents as organ donor and recipient offers the possibility to conduct studies in a relatively rapid, high-throughput and cost-effective fashion. Another advantage lies in the reduced amount of pharmacologic or molecular reagents needed to perform mechanistically informative studies when compared to larger animals, and thus lower costs to perform definitive experiments.
Schroeder et al. (7) published a study in 2003 in which lungs from male wild-type mice were perfused ex vivo with human blood. After removal of the heart-lung block, the main pulmonary artery was cannulated with an intravenous catheter. For the experiment, the lungs were ventilated with room air. During the first 15 min, lungs were flushed with 5% human albumin in saline at a continuous flow rate of 0.2 to 0.5 mL per minute. Thereafter, the albumin solution was replaced by the human blood perfusate at unchanged flow conditions. As a control, lungs were also perfused with autologous blood. In vivo orthotopic left lung xenotransplantation models from hamster or guinea pig to rat have also been performed (8, 9, 10, 11). Orthotopic mouse lung allograft models have recently been developed that can take advantage of the multiple genetic modifications to the mouse, including GalT-KO mice that could be used as recipients for theoretically “discordant” Gal-expressing wild-type lungs (12). To the best of our knowledge this model has not been developed for the study lung xenotransplantation.
However, the relevance of these rodent models to the clinically relevant pig-to-human combination is doubtful, in particular because many approaches (genetic modification to the pig) and reagents (e.g., anti-human or anti-pig monoclonal antibodies) that are developed for clinical use do not cross-react with rodents.
1.3.2 Concordant large animal models
Fox-to-dog (13) as well as Macaque monkey-to-baboon (14) orthotopic lung transplantations have been performed in the past, using either no immunosuppression or FK506. Another series of concordant transplantations was conducted by Sadeghi et al. (15) who transplanted cynomolgus monkey heart-lung blocks into baboons. Recipient animals received cyclosporine and steroid-based immunosuppression.
1.3.3 Large animal models of discordant lung xenotransplantation
In 1998, a cross-circulation lung perfusion model was described as a method to deplete anti-GalT antibodies from the blood of the “recipient” animal (16). The baboon underwent thoracotomy and anticoagulation, and an “outflow” cannula was placed in the inferior vena cava. Via a roller pump, the blood was perfused through main pulmonary artery of the porcine lung, drained by gravity into a heated reservoir and returned to the baboon via a venous cannula in the right atrial appendage (Fig. 1).
Fig. 1.
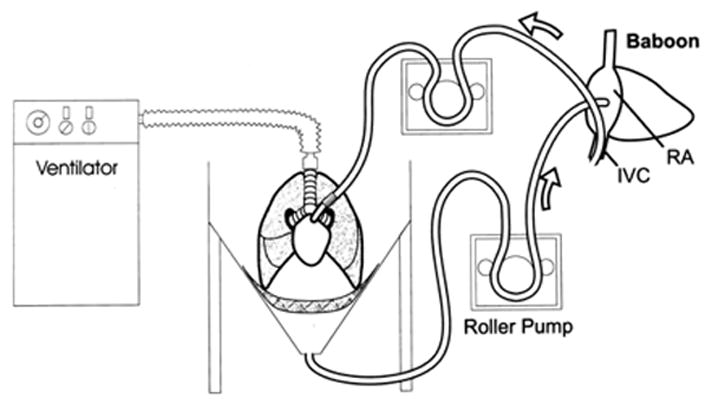
Diagram of extracorporeal lung perfusion circuit used by Daggett et al. (16). Blood is removed from the baboon inferior vena cava and delivered to the swine main pulmonary artery with a roller pump. Blood is drained into a reservoir and returned to the baboon with a second roller pump.
With the same aim to effect immunoabsorption of anti-GalT antibodies, Macchiarini et al. (17) performed experiments in which the recipient animal (goat) was used to cross-circulate the right lung from a GalT-expressing pig prior to receiving the left lung of the same donor animal in an orthotopic transplantation.
Pigs have been identified as the most feasible source of organs for use in humans. Thus pig-to-human and pig-to-primate models are now preferred, because they closely simulate the intended application in the clinic. Importantly, these models permit evaluation of reagents that interact with pivotal pig proteins or with key human and non-human primate molecules. In particular, pig-to-primate orthotopic left lung xenotransplantation and ex vivo perfusion of porcine lungs with fresh human blood are currently the most widely used to study discordant pulmonary xenograft injury.
In the following paragraphs, these two models will be reviewed and explained in detail.
2. Materials
Drugs and medications
Calcium Chloride, 100 mg/mL.
Dopamine HCL Inj., 40 mg/mL.
Heparin, 1000 IU/mL.
Human albumine 5%, 250 mL.
Isoflurane.
Ketamine HCI Inj. 100 mg/mL.
Noradrenalin (Norepinephrine), 1 mg/mL.
Perfadex (Xvivo Perfusion AB, Gothenburg, Sweden).
Propofol (Abbott Laboratories, North Chicago, IL 60064, USA).
Vasopressin Inj. 10 U/0.5 mL.
Xylazine (AnaSed Injection), 20 mg/mL.
8.4% Sodium Bicarbonate Inj.1mEq/mL.
0.9% Sodium Chloride.
Ex vivo lung perfusion circuit and accessories
Glass beaker (ACE Glass Incorporated, Vineland, NJ 08360, USA).
Blood reservoir 1000 ml (ACE Glass Incorporated, Vineland, NJ 08360, USA).
Latex tubing 3/8”.
Y- 3/8” x 3/8” x 3/8” Blood tubing connector.
Straight metal tubing-PA adapter (Custom-made).
Metal rig.
Blood pump (Polyston A/S, Vaerlose, Denmark).
Stirrer (Thermolyne Nuova II, Dubuque, ID 52001, USA).
Reservoir-water heater (American Pharmaseal Company RK 300, Valencia, CA 91355, USA).
Biological atmosphere mixture gas (Airgas, INC, Salem, NH 03079, USA).
Human blood (freshly collected) in blood collection bags containing CPDA-1 (Terumo, Somerset, NJ 08873, USA).
Fresh-frozen human plasma (2 bags per lung side).
Monitoring and anesthesia
Isoflurane vaporizer.
Endotracheal tube (ETT) and mask.
Ventilator.
Veterinary anesthesia ventilator.
Flowmeter.
Pressure monitoring.
Pressure transducer.
Monitoring line.
Syringe pumps.
Biological atmosphere mixture gas in tank.
95% Oxygen, 5% Carbon dioxide in tank.
Sutures, catheters and disposables
Prolene 4–0, 5–0, 6–0, Silk 0 and #1 ties (Ethicon, Somerville NJ 08876, USA).
Vessel loops VL-205 (Key Surgical Inc, Hannover, Germany).
Pediatric venous cannula 12–20 Fr (Medtronic Perfusion Systems, Brooklyn Park, MN 55428, USA).
Swan-Ganz 7.5F catheter (Edwards Lifesciences, Irvine, CA 92614, USA).
Arrow-flex polyurethane sheath catheter (Arrow, Reading, PA 19605, USA).
Angiocatheter 18–22G (Jelco, Medex Medical Ltd, Rossendale, Lancashire, UK).
Tracheal tubes 3.5–6.0 mm
BD Vacutainer (EDTA, Serum, CTAD) (Becton Dickinson, Franklin Lakes, NJ 07417, USA).
Ethyl alcohol 190 Proof, 95%.
Equipment and reagents for analyses and assays
Computer linked to the detectors (Transonic Systems Inc., Model T206, Ithaca, NY, USA) to record flow and pressure continuously.
Automated blood cell analyzer.
Flow cytometer and antibodies for P-selectin and CD41 for measuring platelet activation at the cellular level.
Asserachrome-βTG enzyme-linked immunosorbent assay (ELISA) from Diagnostica Stago (Parsippany, NJ, USA) to assess platelet activation by beta-thromboglobulin (βTG) level formation.
Enzygnost micro F1+2 ELISA (Dade Behring, Marburg, Germany) for measuring thrombin formation.
C3a and/or C5a ELISA (Quidel, San Diego, CA, USA).
Cytokine Luminex arrays (Luminex Corporation, Austin, TX 78727, USA) for measuring cytokines, chemokines and other molecules of interest.
3. Methods (see Note 1)
3.1 Ex vivo porcine-to-human lung perfusion model
Ex vivo xenogeneic perfusion of a porcine lung using human donor blood is the most clinicially relevant and thus important model than can be used to assess the impact of interventions on short-term outcomes (within 6–8 hours). It closely replicates the kinetics, physiology and immunohistochemical findings seen during in vivo pig lung transplantation in a non-human primate. All changes that occur during in vivo hyperacute rejection (HAR), such as increased pulmonary vascular resistance (PVR), loss of transpulmonary blood flow, failure of oxygenation and tracheal or lung edema are observed in both models. Only perfusion of pig lungs with whole human blood offers the possibility to perform mechanistic studies in the clinically relevant species combination and to evaluate interaction of formed blood elements across species. Functional parameters as well as blood and tissue samples can be collected at pre-specified intervals over a specified time range to study the mechanisms of rejection (see Note 2).
It is possible to perfuse one lung, both lungs en bloc, or to divide the pulmonary bloc into left and right lungs and perfuse the “pair” separately. We primarily utilize the paired model because the impact of modifications to one circuit can be assessed in the context of a contemporaneous contralateral reference (“control”) experiment where only the intervention under study differs between the two circuits. This approach allows biologically significant differences to become more readily apparent, especially given biologic variability among pigs, and since uncontrolled variables (such as anti-pig antibody titer) could otherwise exert confounding influences on experimental outcome and complicate study interpretation (see Note 3).
3.1.1 Donor organ procurement
Premedicate the pig with intramuscular ketamine and xylazine and isoflurane by mask (see Note 4).
Intubate through a midline surgical tracheostomy, and an ETT of appropriate size secured with multiple heavy ties (see Note 5).
Ventilate and anesthetize the pig with inhaled isoflurane through the ETT.
Open the chest with a median sternotomy contiguous with the tracheostomy incision.
Obtain vascular access peripherally or by cannulating a vessel in the surgical field with IV tubing (see Note 6).
After heparinization (see Note 7), introduce a large angiocatheter (>14 gauge) or an angulated cannula (aortic or venous cannula, 12–20Fr) into the pulmonary trunk through a Prolene purse-string to introduce the flush solution.
Administer premedication (e.g., prostacyclin).
Ligate the right superior and inferior venae cavae, and left superior vena cava, and cross-clamp the ascending aorta, before administration of the flush solution (see Note 8).
Make an incision in the left atrium, or amputate the left ventricular apex, to allow free drainage of the pulmonary vein effluent.
After administration of the flush solution, inflate the lungs, place a clamp on the trachea or endotracheal tube, and explant the lungs en bloc with the heart, removing the trachea, which for convenience can be done including the tracheostomy site with the ETT in place.
If the experimental design calls for perfusion of both lungs at the same time, secure a return cannula into the left ventricle (it can be, but need not be, introduced across the mitral valve into left atrium), with umbilical tape gently but securely tightened around the LV apex.
Establish pulmonary artery (PA) perfusion via the angulated cannula in the pulmonary trunk through which administers the flush solution, with care to preserve competence of the pulmonic valve during subsequent perfusion.
Alternatively (and more commonly) introduce a flanged metal or plastic cannula through an incision in the right ventricular outflow tract, through the pulmonary valve, and secure with a heavy tie around the main pulmonary artery distal to the pulmonoplegia site. Cannula sizes and dimensions take into account the size of the vessels to be cannulated (which depend on pig size) and the size of tubing used for the perfusion circuit (typically ”(6.4mm) or 3/8” (9.5mm)).
Insert an endotracheal tube into the trachea at a convenient cervical location (safely above the high take-off of the right upper lobe “pig” bronchus), and attach securely with umbilical tapes or a strong silk ligature if the tracheostomy tube from the procurement is not transferred to the perfusion apparatus.
Put the gently inflated lungs (to avoid barotraumas) with the ETT clamped on ice until the blood for the perfusion has been prepared and the experiment can be started.
For the “paired” perfusion system (side-by-side individual right and left lungs), separate surgically the lungs as follows. Because the right main pulmonary artery is relatively short, cannulate it before dividing the left pulmonary artery, securing the right PA cannula with a heavy tie just distal to the main PA bifurcation (see Note 9).
Then, divide the left pulmonary artery just past the main PA bifurcation, usually leaving sufficient length to secure a cannula (e.g. stainless steel cannula) with a simple heavy silk suture (see Note 10). The PA cannulas will later be connected to the inflow perfusion tubing.
Insert a size-matched balloon-cuffed ETT into the cervical trachea as described above. The trachea must be left with the right lung due to origination of the right upper lobe (“pig bronchus”) from the trachea.
Divide the left main bronchus flush with the trachea, and close the resulting tracheal defect with a continuous 4–0 or 5–0 Prolene suture.
Cannulate the left main bronchus directly, securing the endotracheal tube (3.0–6.0 mm) with its tip just proximal to the left main bronchial bifurcation with a 4–0 Prolene suture, taking multiple partial-thickness bites in the outer airway, tieing the suture tightly, and then securing the same ligature over the ET tube (we prefer to use an ET tube with a balloon, to assure secure fixation of the ETT). Thus, inflate the tube balloon gently.
To prevent the ETT from being dislodged, extend the bronchial Prolene suture proximally along the ETT, and additionally tie the suture around the ETT just proximal to the proximal end of the balloon.
For storage, inflate each lung gently (avoiding barotraumas), clamp the ETT, place the lung in a plastic bag, and cool on water or saline ice.
3.1.2 Human blood preparation
The preparation of the human blood used for the perfusion of the porcine lungs can be done in various ways. It is essential to use some freshly collected blood from healthy donors in order that the platelet and neutrophil function simulate in vivo biologic phenomena (see Note 11).
Use regular clinical blood banking collection bags containing CPDA-1 to collect the fresh blood (~450 mL / 1 blood unit). Depending on the size of the pigs and thus the size of the lungs, it needs to be determined how many individual donors are needed (see Note 12).
In the “paired” lung model, apportion the blood between the circuits in approximate proportion to relative pig lung weight (60% right and 40% left can be assumed, in our experience).
To prevent clotting of the blood in the circuit while reconstituting calcium-dependent physiologic mechanisms (the coagulation and complement pathways are both critically dependent on calcium), add heparin (3 IU/mL blood-plasma-mixture) prior to adding calcium chloride (1.6 mg/mL blood-plasma-mixture) to neutralize the CPDA chelating agent.
Add sodium bicarbonate to obtain a target pH of 7.4.
3.1.3 Organ perfusion
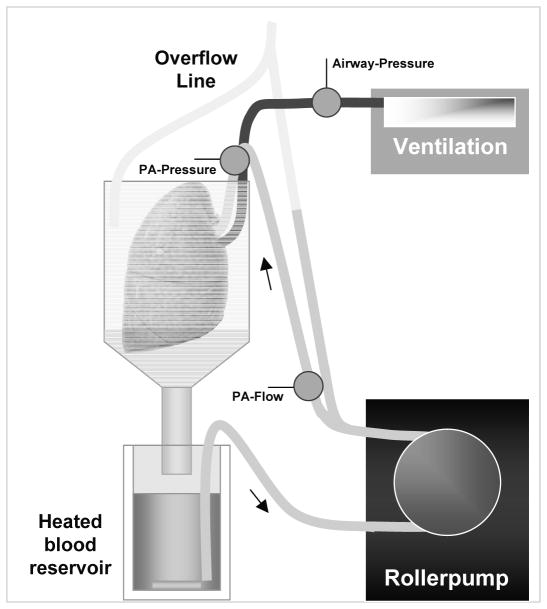
The system described permits perfusion of single lungs or lungs en bloc in a simple, safe, highly reproducible manner (Fig. 2). The perfusion circuit can of course be modified for other purposes (e.g., to study ischemia reperfusion injury), or for ex vivo lung allograft experiments.
Fig. 2.
Diagram of the ex vivo lung perfusion circuit. Blood is pumped via silicon tubing into the pulmonary artery by a roller pump. After the perfusion of the lung, the blood is drained into a heated blood reservoir from which it is recirculated to the lung.
Clean the tubing system, and any other system components that will have contact with the blood, rinse extensively with clean water, disinfect (Ethyl alcohol 95%), and dry overnight, before blood is introduced into the perfusion circuit.
Coat all blood-contacting circuit surfaces with human albumin 5% in 0.9% “normal” saline to minimize blood activation by contact with recycled glass, plastic, and silicon circuit components (see Note 13).
Suspend the pig lung in a funnel over the jacketed glass-beaker that will collect the blood issuing from the pulmonary veins.
Warm the reservoir beaker with a circulating water pump.
Pump blood from the reservoir via the tubing system, and return to the lung via the PA (see Note 14).
Connect an appropriately adjusted volume-controlled ventilator to the endotracheal tube and ventilate the lung with a custom-ordered gas mixture of 21% oxygen and 5–8% carbon dioxide balanced with nitrogen. This concentration of carbon dioxide maintains pCO2 at 35–45 mmHg, and prevents the profound alkalosis that would otherwise occur in the absence of a significant source of tissue metabolism, and thus CO2, in the ex vivo circuit.
To evacuate air from the blood-containing tubing, pour the prepared human blood into the reservoir and start the roller pump.
De-air the circuit and connect the inflow tubing to the pulmonary artery. The flow setting of the pump should be up to 75 mL/kg body weight (BW)/min (see Note 15).
For side-by-side bilateral lung perfusion, devote 60% of blood volume and flow to the right lung, and 40% to the left side, based on the normal proportion of relative lung weight.
Ventilate the lungs at 7 mL/kg BW and adjust ventilator tidal volume according to the lung compliance, seeking to achieve peak airway pressure of 12–15 cm H2O (~8–11 mmHg).
To record physiologic lung function parameters, use pressure transducers to record PA and airway pressures, and a flow probe to continuously monitor the blood flow into the lung in the inflow-tubing just proximal to the pulmonary artery. Calculate the PVR by dividing mean PA pressure by blood flow.
Collect blood samples directly from the pulmonary vein effluent.
Excise lung biopsies behind tissue clamps, leave in place for the duration of the experiment (stapling devices could be used, but add considerable expense).
Trisect each lung biopsy and process for later histological, molecular, and immunohistological analyses.
Assess lung function by blood gas analyses, measuring pO2, pCO2 and pH over time under consistent circumstances. By intermittently switching from the ventilator gas mixture (21% O2) to 100% oxygen for 3–5 min, measure the capacity of lung oxygenation. If preferred for a specific application (and if the cost of the oxygenator can be afforded), a “deoxygenator” (oxygenator swept with 8% CO2 and 10–14% O2 balanced with nitrogen) can be integrated into the circuit (see Note 16), and oxygenation capacity of the lung assessed by change in step-up on pO2 across the lung over time during ventilation with room air.
3.2 In vivo porcine lung transplantation model
As for other xenogeneic organ transplants, the transplantation of a porcine pulmonary graft into a non-human primate, represents the gold standard to test the function, observe blood parameter changes, and determine survival of a transplant in a clinically relevant “translational” xenogeneic model. Even though the ex vivo perfusion model facilitates informative mechanistic studies, it will never offer the possibility to discover perhaps unexpected phenomena that may occur during attempted life-support in a living animal, and allow monitoring of perturbations in the physiology of the recipient. Importantly, the in vivo model is not perturbed by the artificial elements of the circuit (blood-tubing and blood-air interactions, for example), or by unappreciated effects of “pooling” multiple human donors. Although studies in cynomolgus monkeys have been reported (18), most in vivo lung xenotransplant work has been done in baboons, where adult animals are of appropriate size (>10 and preferably >15 kg) to accept left lung grafts from 6–10-kg pigs (16, 19, 20) (see Note 17).
3.2.1 Donor organ procurement
Perform the anesthesia of the donor pig and surgical procurement of the heart-lung bloc as described in the ex vivo lung perfusion section.
For the in vivo lung transplantation, separate the left porcine lung surgically from the heart.
Excise the pulmonary veins as an island from the left atrium.
Because there is often a large distance between the upper and lower left pulmonary veins, imbricate the intervening left atrial wall to reduce the donor vein cuff size to more closely approximate the smaller baboon pulmonary vein cuff.
Divide the PA just proximal to the left main pulmonary artery bifurcation (see Note 18).
Finally, clamp the left main bronchus to ensure that the lung stays inflated and is cut close to the bifurcation of the trachea.
Store the lung on ice until the implantation of the organ.
3.2.2 Preparation of the recipient
Sedate baboon recipients with ketamine (10 mg/kg BW, i.m.).
After the intubation, maintain general anesthesia with isoflurane (1–3%, i.h.).
For initial intravenous access, place an angiocatheter in a superficial extremity vein.
Place an arterial line in the femoral artery for monitoring of hemodynamic parameters (see Note 19).
For later positioning of a Swan-Ganz catheter, prepare the femoral vein and cannulate directly with an inducer sheath.
To prevent blood clotting at the catheters, give heparin as a bolus (70 IU/kg BW, i.v.) and infuse at 200 IU/h. Flush lines with heparinized saline until use.
Open the chest of the animal with a transverse “clamshell” incision at the fourth or fifth intercostal space.
Open both, the pericardium and the pleura with longitudinal incisions.
Introduce a flow-directed PA balloon catheter into the femoral vein, and manually guide across the tricuspid valve (see Note 20) and out the pulmonary outflow tract to the proximal PA.
Position a snared vessel loop around the right pulmonary artery, working between the aorta and SVC. Securing this loop allows reversible selective perfusion of the transplanted left lung, and thus intermittent or continuous rigorous assessment of left lung function.
A flow probe (Transonic Systems Inc, Ithaca, NY) on the ascending aorta allows measurement of the cardiac output (see Note 21).
3.2.3 Explantation of the left native baboon lung
For the explantation of the native lung, displace the heart from the pericardium and into the right chest, avoiding hemodyanamic embarrassment by incising the right inferior pericardium along the diaphragm. To this end, retract a suture tied on the left atrial appendage toward the right, and protect it with a rubber tube (Rummell snare) from rubbing against the contracting heart. Sutures through the pericardium just anterior to the left hilum, similarly protected with rubber tubing and retracted anteriorly and to the right, aid in exposure with minimal hemodynamic compromise.
Working within (particularly helpful!) and outside the pericardium, identify and dissect the left hilar structures.
Ligate left upper and lower pulmonary veins centrally and peripherally with heavy suture (Silk 0 or #1) and divide.
Use a straight vascular clamp to occlude the left PA as close to the mediastinum as possible, and divide the LPA distally just proximal to the left main PA bifurcation.
After dividing the inferior pulmonary ligament, divide the left main bronchus at the hilum and control bronchial vessels with sutures or (for non-survival procedures) with cautery.
Restore ventilation to the right lung by placing a straight clamp on the left main bronchus (see Note 22).
3.2.4 Implantation of the xenogeneic organ
Place the cooled xenogeneic lung in the left pleura of the recipient baboon.
If necessary, tailor the donor or recipient bronchus, pulmonary artery, and vein to enable anastomosis of the structures and accommodate common size discrepancies.
First, anastomose donor and recipient bronchus with a continuous suture (4/0 or 5/0 Prolene). It is also possible to use an interrupted 4/0 Prolene suture if intermediate-term survival with bronchial healing is desired.
Because the donor lung has intrinsically stiffer than the baboon lung, the clamps on the bronchus can now be removed without significant interference in completing the vascular anastamoses.
For the end-to-end anastomosis of the pulmonary artery, use a continuous Prolene 5/0 or 6/0 suture.
For the later venting, keep the suture open and not tied and knotted yet (see Note 23).
To simplify the connection of the pulmonary veins, free up the recipient pulmonary vein and left atrium from the pericardium posteriorly to allow placement of a clamp proximally on the left atrium.
Remove ligatures on the pulmonary veins, preserving maximal recipient cuff diameter, and incise the atrial tissue between the upper and lower recipient vein orifices to obtain one large foramen.
Connect the donor and recipient left atrial cuffs with a Prolene 4/0 or 5/0.
To restore perfusion to the transplanted lung, open the clamp on the pulmonary veins and assure hemostasis of the venous anastomosis.
Open slowly the proximal clamp on the pulmonary artery to evacuate air from the PA.
Tie the suture after venting the suture, fully establishing blood flow to the xenograft.
3.2.5 Longitudinal graft assessment
After transplantation of the xenogeneic organ, it is essential to control the hemodynamic parameters and stabilize the recipient. Inotropic medication is almost always required during graft implantation (see Note 24). In 2007, Nguyen et al. (19) used the described in vivo model to perfuse a porcine lung in a baboon for up to 255 min. At the same laboratory recent experiments have shown that it is possible to keep the transplanted recipient animal alive under general anesthesia for 24 h (unpublished data). Others have done so for up to 3–5 days (21). During this time, it is possible to collect not only tissue samples from the xenogeneic lung, but to also record ventilation parameters, systemic, pulmonary arterial and central venous pressures. Flow probes on the ascending aorta and on the left pulmonary artery allow to record cardiac output and flow to the transplanted lung respectively. By pulling gently on the snared vessel loop on the right pulmonary artery, it is possible to partially or completely occlude this vessel and thereby adjust recipient dependence on the pulmonary xenograft (see Note 25). During this single lung perfusion period, pulmonary vascular resistance, cardiac output, systemic arterial pressure and systemic arterial oxygen saturation indicate whether the transplanted left lung is able to support life.
Since rejection of the xenogeneic pulmonary graft usually happens within hours and is often associated with lung edema and oxygenation failure, animals are so far being kept under general anesthesia to avoid stress and discomfort of the animals. If future experiments show an improvement of the graft survival and function it is in principal feasible to close the chest and recover the animal from the operation.
3.3 Analyses and assays
To compare the function and the outcome of different xenogeneic lung transplantations or lung perfusions, an easy measurable parameter is the time after which the organ is rejected or loses its function. To determine this time point, markers as the loss of blood flow though the lung, an increased vascular resistance, failure of blood oxygenation, occurrence of tracheal edema or the loss of perfusate due to lung edema should be taken in consideration. To understand the biology of organs in a xenogeneic surrounding, it is essential to analyze the results concerning functional and hemodynamic, hematologic, immunologic and immunohistologic changes. The previously described models offer the possibility to have access to all of these parameters by recording pressures and flows and collecting tissue and blood samples in the run of the experiment.
3.3.1 Functional and hemodynamic analyses
Measure the function of the organ by the pulmonary vascular resistance (PVR) (see Note 26) and by the ability of the lung to oxygenate the blood. Especially within the first 30 min of perfusion and also before lung demise, an increase in PVR can be observed. If possible, record those parameters continuously or in short intervals during this time. The best approach is to record flow and pressure continuously on a computer linked to the detectors.
Measure the oxygenation of the blood by analyzing the pulmonary vein blood gases after a short time (3–5 min) on 100% FiO2, and record as the amount of step-up in pO2 relative to pulmonary vein pO2 on 21% FiO2. If a deoxygenator is incorporated in the circuit, this maneuver and calculation becomes unnecessary to measure lung gas transport function intermittently during the experiment.
3.3.2 Hematologic analyses
With the xenogeneic perfusion of an organ multiple hematological changes can be observed in the perfusing blood.
After enabling blood flow through the organ, initially collect blood samples at very short intervals (5–10 min) as sequestration and activation of cells occurs immediately; thereafter pre-determined intervals of 30–60 min are usually sufficient to track trends over time, and compare results between groups treated in various ways.
For cell counts, enumerate white blood cells, including neutrophils, monocytes, and platelets by standard automated techniques in perfusate/blood samples (see Note 27).
For measuring platelet activation at the cellular level, a very useful parameter is the cell surface expression of P-selectin (CD62P) determined by flow cytometry. To measure CD62P expression on platelets, stain blood samples by monoclonal antibodies specific for CD41 (as a marker for platelets) and CD62P (expressed by activated platelets) according to the instructions of the manufacturer. Identify platelets and platelet aggregates by size and by the presence of CD41 staining, and analyze later for expression of CD62P. Express results as the percentage of CD62P-positive cells among CD41-positive cells (see Note 28).
To measure platelet activation and coagulation cascade in archived plasma samples, assess platelet activation by beta-thromboglobulin (βTG) level formation using a commercial ELISA (Asserachrome-βTG). Measure thrombin formation as the production of prothrombin fragments 1+2 (F1+2) generated by the cleavage of prothrombin in thrombin by ELISA (see Note 29).
Determine the activation of the complement cascade by measuring C3a or C5a levels in plasma samples collected in EDTA blood tubes and stored in EDTA at −70°C (C3a ELISA; Quidel, San Diego, CA).
3.3.3 Cytokine measurements
As cytokines are signaling molecules, secreted by numerous cells of the immune system, the measurement and level of cytokines can be used to analyze the strength of immune activation and immune response. The analyses may include interleukin 1B (IL-1B), IL-6, IL-8, interferon gamma (IFNγ), TNF inflammatory cytokines, chemokines, soluble CD40L and sCD62P (platelet activation). New high throughput technologies (Luminex, arrays) efficiently measure multiple targets simultaneously in a small volume of plasma (22).
3.3.4 Histology and immunohistology
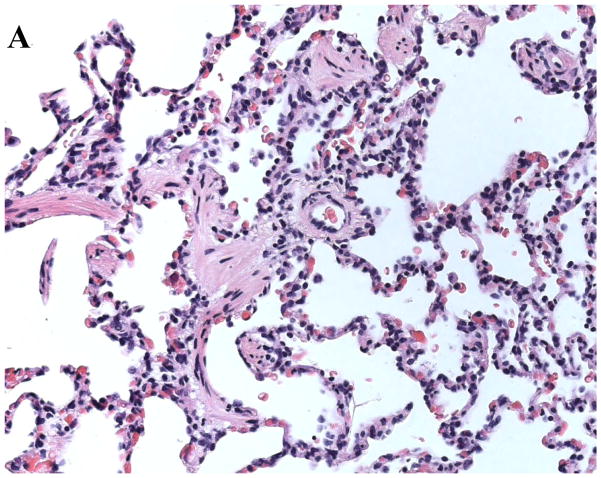
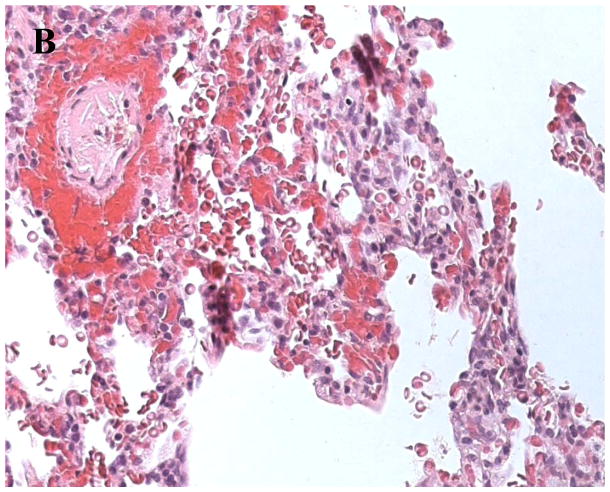
At various time points throughout the experiments tissue samples from the xenogeneic organ should be collected to prepare histological slides. A standard staining to visualize cell infiltration of the tissue and vessel thrombosis is the hematoxylin and eosin stain (H&E). Figure 3a shows lung tissue, collected from a GalT-KO.hCD39 transgeneic lung after four hours of perfusion with human whole blood. Histology shows an intact lung tissue without significant pathological abnormalities. In contrast, figure 3b demonstrates findings seen in a hyperacutely rejected organ. This lung tissue from a wild type pig that was perfused with human blood for only 12 minutes shows a loss of integrity of alveolar endothelial wall structures, hemorrhage and vessel thrombosis. Further immunohistochemical (IHC) staining includes visualization of human monocytes (CD14), neutrophils (myeloperoxidase), T-cells (CD3, CD4, CD8) or B-cells (CD20), total (CD41) and activated (P-selectin) platelets, tissue factor, IgG and IgM, complement pathway products (C4d, C5b-9), vWF (to identify endothelium), and others. Do IHC on snap-frozen lung tissue insufflated with OCT diluted with saline (~1:1) into the airways through a small-gauge angiocatheter (22–24G) and syringe, to reduce edge artifact.
Fig. 3.
A Histological analysis of a GalTKO.hCD39 transgeneic pig lung after 4 hours of xenogeneic perfusion. Histology shows an intact pulmonary tissue with only very little hemorrhage and cell infiltration. B. Example of a hyperacutely rejected lung. The slide shows a thrombosed vessel and massive hemorrhage in lung tissue from a wild type pig after 12 minutes of xenogeneic perfusion.
3.3.5 PCR/gene expression analyses
The rejection of an organ does not only affect its function and tissue structure, it also modulates the up- and down regulation of genes (23). Snap-frozen tissue samples collected at various time points during the experiment can be used to measure gene expression by real-time RT-PCR or cDNA microarray analysis (24). By designing PCR primers and probes that recognize pig or human sequences only, it is possible to monitor changes in gene expression within lung tissue parenchymal cells or within human infiltrating cells.
Footnotes
All transplant experiments require surgical and anesthesia instruments, equipment, and expertise for the procurement of the organ and, if performed, for the performance and monitoring of the transplant. Involvement of a surgical team trained in transplant and/or cardiothoracic surgical techniques is essential to successfully conduct xenotransplant research.
It is advisable to have very short intervals (1–2 min) for the blood and tissue sampling as well as for the recording of the functional parameters at the beginning of the perfusion. Especially during this period, blood (cell sequestration) and functional changes (increase in PVR) can be frequently observed.
The perfusion system (e.g. flow rate and cannula sizes) and other logistics (e.g. the human blood volume) can be adapted to perfuse lungs of pigs with a bodyweight of 2 to 50 kg or more.
We use a combination of ketamine (10–15 mg/kg IM) plus xylazine (0.5–1 mg/kg IM) for the anesthesia induction. Anesthesia is maintained with isoflurane (0.5–4.0% inhaled).
Ethicon silk 1-0 ties (or equal) can be used. It is possible to suspend the lungs from those ties.
Especially in small pigs it can be difficult to get IV access on a peripheral vessel (e.g. ear vein). In that case it is possible to place an angiocatheter in the internal jugular vein through the midline surgical tracheostomy.
We give a heparin dose of 500 IU/kg.
We use perfadex (50 mL/kg BW).
If the right PA is not long enough to insert a cannula to allow perfusion of the upper right lobe, it is instead possible to secure the cannula to the main pulmonary artery and then close the hole where the left PA was dissected with suture. It is essential to assure that the closure suture does not obstruct the vessel as this would significantly reduce the blood flow to the lung and increase the measured PA pressure. We use a Prolene 5-0 double layer mattress/simple continuous suture.
As mentioned for the ties that secure the ETT to the airway, we recommend using the ties on the PA catheter/connector as well to hang the lungs. This allows to reduce the weight of the lung on a single anatomic structure and also prevents to vessel (PA) from kinking.
Examples in the literature using non-diluted blood (25, 26) or blood diluted with blood-type-matched human plasma (27) for xenogeneic lung perfusions have been conducted. Unpublished work shows that dilution of blood with electrolyte solutions or use of refrigerated or overnight-stored blood significantly attenuates the pace of hyperacute heart or lung rejection.
For pigs <20kg, we utilize two units (2 individual donors, each contributing 450 mL) of blood expanded with four 240 mL individual donor “units” of thawed “recovered” or “fresh-frozen” plasma. One fresh human donor and two plasma “units” are usually sufficient for an “en bloc” experiment.
While some activation of complement and adhesion of formed blood elements is demonstrable in our circuit, this effect is readily controlled for by measuring these parameters after a consistent (20 min) interval of perfusate circulation through the circuit. Blood perfused for longer intervals or in the context of iso- or allo- lung perfusion exhibit little additional alteration over relevant study intervals (unpublished observations).
Our circuit design incorporates an overflow line that allows the blood to continue to mix and circulate while bypassing the lung when high pulmonary vascular resistances occurs, to avoid PA pressures above 45 cm H20 (equivalent to 4.4 kP or 33 mmHg) and avoid stasis in the tubing. This peak PA pressure is chosen to simulate the maximum pressure that we presume a normal right ventricle could overcome for a sustained period in vivo.
In our experiments we have seen that the PA flow can show a wide range of values. Certainly, the genetics of the donor pig (e.g. wild type or GalT-KO pig), but also other parameters (ischemia reperfusion) have influence on the blood flow and contribute to the variability in pulmonary vascular resistances in different experiments.
The oxygenator adds substantial expense, and is a source of significant blood activation.
It is important to size-match donor and recipient for not only physiologic but also surgical reasons. The main bronchus of the pig has usually a wider lumen than the primate bronchus. To facilitate the bronchus anastomosis, it is thus important to use a donor pig which has a 20–40% lower bodyweight than the recipient baboon.
A pig PA 50% larger than the baboon left main PA should be anticipated and managed using standard surgical tailoring approaches.
We prefer inserting an arterial line to both of the femoral arteries. This procedure allows us to collect arterial blood without loosing the real-time invasive blood pressure measurement for the time of the drawing which can be crutial in animals with unstable or critical condition.
We suggest forwarding the PA balloon catheter after the opening of the chest as the right placement of the catheter can be difficult to achieve without the manual guidance across the tricuspid valve.
Due to the movement of the heart, it is often not easy to find a position on the ascending aorta where a stable flow signal can be recorded by the flow probe. We found that ultrasound gel (for non sterile operations) improves the signal in most cases.
Although, only the right lung is ventilated during the implantation of the left lung, it is most likely not necessary to change the settings of the ventilation machine. During the single-lung ventilation, arterial blood gases should be collected to assure an appropriate oxygenation of the recipient.
We typically place a clamp on the donor PA to facilitate alignment, position it adjacent to the recipient LPA, and avoid tension on or stricture of the anastamosis during its performance.
In our experiments we use dopamine (2–20 μg/kg/min), noradrenalin (2–20 μg/kg/min) and vasopressin (0.2–1.0 IU/min). Additionally, we also routinely use inhaled nitric oxide to minimize pulmonary vascular resistance during graft implantation and subsequent assessment; the utility of this approach has not been rigorously assessed.
We recommend to perform this maneuver only after a sufficient reperfusion time and to occlude the vessel only partly at first. As mentioned before, the transplanted lung shows initially a higher vascular resistance and a lower compliance. By completely occluding the right PA shortly after the implantation, this could lead to a cardiac decompensation with acute right heart failure.
We estimate the PVR by dividing the pulmonary arterial pressure by the pulmonary flow.
Samples are collected in ethylenediaminetetraacetic acid (EDTA) anti-coagulated blood tubes.
The blood can be fixed immediately after collection to avoid non-specific platelet activation during sample processing and to allow simultaneously antibody staining of all time-points at the same time. Additional markers (such as glycoprotein V or activation of GPIIbIIIa receptor) can also be analyzed in a similar way.
Importantly, plasma samples used in coagulation studies must be collected in CTAD tubes and processed according to the manufacter’s instructions.
References
- 1.Pierson RN, 3rd, Dorling A, Ayares D, et al. Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 2009;16:263–280. doi: 10.1111/j.1399-3089.2009.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schroeder C, Allan JS, Nguyen BN, et al. Hyperacute rejection is attenuated in GalT knockout swine lungs perfused ex vivo with human blood. Transplant Proc. 2005;37:512–513. doi: 10.1016/j.transproceed.2004.12.133. [DOI] [PubMed] [Google Scholar]
- 3.Hisashi Y, Yamada K, Kuwaki K, et al. Rejection of Cardiac Xenografts Transplanted from alpha1,3-Galactosyltransferase Gene-Knockout (GalT-KO) Pigs to Baboons. Am J Transplant. 2008;8:2516–2526. doi: 10.1111/j.1600-6143.2008.02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryant L, Eiseman B, Avery M. Studies of the porcine lung as an oxygenator for human blood. J Thorac Cardiovasc Surg. 1968;55:255–263. [Google Scholar]
- 5.Collins BJ, Blum MG, Parker RE, et al. Thromboxane mediates pulmonary hypertension and lung inflammation during hyperacute lung rejection. J Appl Physiol. 2001;90:2257–2268. doi: 10.1152/jappl.2001.90.6.2257. [DOI] [PubMed] [Google Scholar]
- 6.Cantu E, Balsara KR, Li B, et al. Prolonged function of macrophage, von Willebrand factor-deficient porcine pulmonary xenografts. Am J Transplant. 2007;7:66–75. doi: 10.1111/j.1600-6143.2006.01603.x. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder C, Wu GS, Price E, et al. Hyperacute rejection of mouse lung by human blood: characterization of the model and the role of complement. Transplantation. 2003;76:755–760. doi: 10.1097/01.TP.0000069836.91593.09. [DOI] [PubMed] [Google Scholar]
- 8.Komatsu K, Youm W, Konishi H, et al. Prolonged survival of hamster-to-rat pulmonary xenografts by tacrolimus (FK506) and cyclophosphamide. J Heart Lung Transplant. 1996;15:722–727. [PubMed] [Google Scholar]
- 9.Miyata Y, Ohdan H, Yoshioka S, et al. Relationship of xenogeneic microchimerism to graft outcome in hamster-to-rat lung xenotransplantation. J Heart Lung Transplant. 1998;17:233–240. [PubMed] [Google Scholar]
- 10.Nonaka M, Kadokura M, Kataoka D, et al. Hyperacute xenorejection of guinea pig-to-rat lung transplantation can be attenuated by blood which has perfused another xenograft. Ann Thorac Cardiovasc Surg. 2000;6:146–150. [PubMed] [Google Scholar]
- 11.De Perrot M, Keshavjee S, Tabata T, et al. A simplified model for en bloc double lung xenotransplantation from hamster to rat. J Heart Lung Transplant. 2002;21:286–289. doi: 10.1016/s1053-2498(01)00338-2. [DOI] [PubMed] [Google Scholar]
- 12.Yang YG, deGoma E, Ohdan H, et al. Tolerization of anti-Galalpha1-3Gal natural antibody-forming B cells by induction of mixed chimerism. J Exp Med. 1998;187:1335–42. doi: 10.1084/jem.187.8.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veith FJ, Richards KU, Hagstrom JWC, Montefusco CM. Intrafamilial lung xenogragft from fox to dog. J Thorac Cardiovasc Surg. 1981;81:546–552. [PubMed] [Google Scholar]
- 14.Takeda M, Kawauchi M, Nakajima J, Furuse A. Methorexate in rescue therapy for xenotransplanted lung in primes. (Abstract po-46) Third International Congress of Xenotransplantation; Boston. 1995.1995. [Google Scholar]
- 15.Sadeghi AM, Laks H, Drinkwater DC, et al. Heart-lung xenotransplantation in primates. J Heart Lung Transplant. 1991;10:442–447. [PubMed] [Google Scholar]
- 16.Daggett CW, Yeatman M, Lodge AJ, et al. Total respiratory support from swine lungs in primate recipients. J Thorac Cardiovasc Surg. 1998;115:19–27. doi: 10.1016/s0022-5223(98)70438-6. [DOI] [PubMed] [Google Scholar]
- 17.Macchiarini P, Oriol R, Azimzadeh A, et al. Characterization of a pig-to-goat orthotopic lung xenotransplantation model to study beyond hyperacute rejection. J Thorac Cardiovasc Surg. 1999;118:805–814. doi: 10.1016/s0022-5223(99)70049-8. [DOI] [PubMed] [Google Scholar]
- 18.Blum M, Chang AC, Collins BJ, et al. The effect of nitric oxide and thromboxane blockade on pulmonary vascular resistance in a pig-to-primate lung transplant model. Fourth International Congress for Xenotransplantation; Nantes, France. September 1997.1997. [Google Scholar]
- 19.Nguyen BN, Azimzadeh AM, Zhang T, et al. Life-supporting function of genetically modified swine lungs in baboons. J Thorac Cardiovasc Surg. 2007;133:1354–1363. doi: 10.1016/j.jtcvs.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 20.Yeatman M, Daggett CW, Parker W, et al. Complement-mediated pulmonary xenograft injury - studies in swine-to-primate orthotopic single lung transplant models. Transplantation. 1998;65:1084–1093. doi: 10.1097/00007890-199804270-00013. [DOI] [PubMed] [Google Scholar]
- 21.Cantu E, Parker W, Platt JL, Duane Davis R. Pulmonary xenotransplantation: rapidly progressing into the unknown. Am J Transplant. 2004;4(Suppl 6):25–35. doi: 10.1111/j.1600-6135.2004.0342.x. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen BN, Azimzadeh AM, Schroeder C, et al. Absence Of Gal Epitope Prolongs Survival Of Swine Lungs In An Ex Vivo Model Of Hyperacute Rejection. Xenotransplantation. 2011 doi: 10.1111/j.1399-3089.2011.00633.x. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azimzadeh AM, Dawson H, Nguyen BN, et al. aGal antigen modulates the expression of pro-inflammatory genes in hyperacute lung rejection. 8th International Xenotransplantation Congress; Goteborg, Sweden. Sept 2005.2005. [Google Scholar]
- 24.Knosalla C, Yazawa K, Behdad A, et al. Renal and cardiac endothelial heterogeneity impact acute vascular rejection in pig-to-baboon xenotransplantation. Am J Transplant. 2009;9:1006–1016. doi: 10.1111/j.1600-6143.2009.02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macchiarini P, Mazmanian GM, Oriol R, Rieben Robert, Dartevelle Philippe, et al. Ex vivo lung model of pig-to-human hyperacute xenograft rejection. J Thorac Cardiovasc Surg. 1997;114:315–325. doi: 10.1016/S0022-5223(97)70175-2. [DOI] [PubMed] [Google Scholar]
- 26.Kim HK, Kim JE, Wi HC, et al. Aurintricarboxylic acid inhibits endothelial activation, complement activation, and von Willebrand factor secretion in vitro and attenuates hyperacute rejection in an ex vivo model of pig-to-human pulmonary xenotransplantation. Xenotransplantation. 2008;15:246–256. doi: 10.1111/j.1399-3089.2008.00481.x. [DOI] [PubMed] [Google Scholar]
- 27.Schroeder C, Allan J, Nguyen BN, et al. Xenogenic Ex Vivo Perfusion of Lungs From Galt K/O Pigs: Initial Results. Transplantation. 2004;78 (suppl 2):20. [Google Scholar]