Abstract
Only a subset of women with human papillomavirus (HPV) infections will become seropositive, and the factors influencing seroconversion are not well-understood. We used a multiplex serology assay in women with mildly abnormal cytology results to examine seroreactivity to oncogenic HPV genotypes. An unbiased subset of women in the atypical squamous cell of undetermined significance /low-grade squamous intraepithelial lesion Triage Study (ALTS) provided blood samples at trial enrollment for serological testing. A Luminex assay based on GST-L1 fusion proteins as antigens was used to test seroreactivity against eight carcinogenic HPV genotypes (16, 18, 31, 33, 35, 45, 52, 58). We analyzed the relationship between seroprevalence in women free of precancer (N=2464) and HPV DNA status, age, sexual behavior, and other HPV-related risk factors. The overall seroprevalence was 24.5% for HPV16 L1 and ~ 20% for 18L1 and 31L1. Among women free of precancer, seroprevalence peaked in women less than 29 years and decreased with age. Type-specific seroprevalence was associated with baseline DNA detection for HPV16 (OR= 1.36, 95%CI: 1.04–1.79) and HPV18 (OR= 2.31, 95%CI: 1.61–3.32), as well as for HPV52 and HPV58. Correlates of sexual exposure were associated with increased seroprevalence across most genotypes. Women who were current or former smokers were less likely to be seropositive for all eight of the tested oncogenic genotypes. The multiplex assay showed associations between seroprevalence and known risk factors for HPV infection across nearly all tested HPV genotypes but associations between DNA- and serostatus were weak, suggesting possible misclassification of the participants’ HPV serostatus.
Keywords: Human papillomavirus, seroepidemiology, antibodies
INTRODUCTION
Human papillomavirus (HPV) infections are a necessary cause of cervical cancer.1, 2 Women with persistent infections with one of thirteen different types of HPV (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) are at increased risk of developing precancer that may ultimately progress to malignancy.3 HPV 16 and HPV 18 account for the majority (~70%) of the cervical cancer burden worldwide.1 The prevalence of HPV infection is high among young women in the years following sexual debut but the majority of infections, even those caused by carcinogenic HPV genotypes, will resolve within two years.3–5
A subset of women with HPV infections will become seropositive, and antibody responses can take at least 18 months to develop.6, 7 The mechanisms that influence whether a woman mounts a detectable antibody response to HPV (seroconvert) are currently unclear; HPV infects epithelia that have limited interaction with the immunological environment and the virus has several immune-evading strategies.8–10 Some studies have indicated that age and correlates of sexual behavior are associated with HPV seroprevalence11–16, suggesting seroconversion may be influenced partly by cumulative exposure to HPV, in terms both of chance of exposure and repeated exposures over time. Concurrent infection with multiple HPV genotypes occurs frequently, and the distribution of HPV genotype frequencies varies across histological and cytological diagnoses.17 Most studies of seropositivity to HPV antigens have examined antibody response to the HPV L1 antigen, a component of the viral capsid, for only a small number of HPV genotypes. Natural antibody titers may be important in preventing future HPV infection18, 19 and might be a useful measurement of previous HPV exposure.
There is currently no standardized assay for detection of HPV antibodies. VLP ELISA assays are most commonly used and measure total concentration of antibody that binds to laboratory-synthesized virus-like particles (VLPs) using optical density measurements20. Multiplex HPV serology assays that can measure multiple antigens at the same time have been developed and tested with both VLP antigen targets and glutathione s-transferase fusion protein targets.21, 22 The goal of this study was to use a Luminex-based serology assay with GST-fusion protein antigen targets measuring serum responses against eight carcinogenic HPV types to examine factors associated with seropositivity in the ASCUS-LSIL Triage Study.
MATERIALS AND METHODS
The ASCUS/LSIL Triage Study for Cervical Cancer (ALTS)
The ASCUS/LSIL Triage Study for Cervical Cancer (ALTS) was a multicenter, randomized clinical trial conducted from 1996–2000 by the National Cancer Institute to compare three clinical management strategies for women referred with a community Pap smear cytological interpretation of ‘atypical squamous cells of undetermined significance’ (ASCUS, N=3488) or ‘low-grade squamous epithelial lesion’ (LSIL, N=1572) cytology. At trial enrollment, women were randomized into one of three different management strategy arms and were followed every six months for a period of two years. Women in the immediate colposcopy (IC) arm were randomized to receive a colposcopy ideally within three weeks of their enrollment visit. Participants in the HPV triage arm (HPV) were referred for colposcopy if 1) HPV-positive at enrollment or 2) if they were missing their HPV result. Women in the conservative management arm (CM) were only referred to colposcopy in the case of a cytological diagnosis of high-grade squamous intraepithelial lesion (HSIL) or carcinoma. Randomization arm was not relevant to our analysis as we use data from the enrollment visit only and exclude women with a diagnosis of CIN2 or greater.
ALTS procedures have previously been described in detail23 and are summarized below in brief: women with a cytologic diagnosis of ASCUS or LSIL were referred to ALTS from four U.S. clinical centers. If enrolled, participants completed a questionnaire and underwent a pelvic examination. Cervical specimens for cytological evaluation and human papillomavirus testing were collected, and digital photographs of the cervix were taken (cervicography), and there was a voluntary blood draw at the enrollment visit only. Women were eligible for inclusion in the trial if they had a cytological interpretation of ASCUS or LSIL on a Pap test within the previous six months of enrollment, and were excluded if they had a history of hysterectomy, ablative or excisional therapy to the cervix, were less than 18 years of age, or were currently pregnant. Follow-up visits for all trial arms included a pelvic exam, collection of cervical cells for cytological testing and HPV screening, and cervicography.
Study Population
This analysis includes 2464 women drawn from the 5,060 women in ALTS. Women from all trial arms were included in the analysis if they were free of pre-cancer (< cervical intraepithelial neoplasia grade 2 or CIN2) at trial baseline and if they had an available serum specimen (55 % of participants) for serological testing. Serum availability was not associated with baseline histological diagnosis or worst two-year histological outcome, HPV status, age at trial enrollment, smoking status, lifetime number of sex partners, race, education level, or age at sexual debut.
Assay Methods
This project used a glutathione S-transferase fusion protein multiplex serology assay to measure seroreactivity to human papillomavirus antigens.21 The HPV major capsid (L1) proteins for eight different oncogenic HPV genotypes (16, 18, 31, 33, 35, 45, 52, and 58) were expressed as fusion proteins with N-terminal GST and a C-terminal epitope tag.24 Glutathione crosslinked to casein serves as a capture protein for the GST, and was bound to fluorescence-labeled polystyrene beads (SeroMap, Luminex, Austin, TX). Bead sets were individually loaded with different GST-X-tag fusion proteins, with each antigen loaded onto a bead set with a specific color. Serum specimens were diluted to 1:100 and incubated with a bead mixture. Antibodies that bound to beads were stained using biotinylated anti-human-Ig and streptavidin-R-phycoerythrin. The bead mixtures were analyzed in a Luminex 100 system which quantifies the fluorescence intensity associated with the antibodies bound to the viral antigens on each bead. Results are reported as median fluorescence intensity (MFI) of a minimum of 100 beads analyzed per bead set/antigen. The MFI of empty GST-tag-loaded beads was used to define the background fluorescence level, and specific reactivity (net MFI) for a given HPV protein was calculated by subtracting the background level from the MFI of the antigen-loaded beads21. Seropositivity threshold MFI levels were defined using data from 125 women who reported no lifetime sex partners in a study in Busan, South Korea;25 the positivity threshold was set at three standard deviations above the mean level of MFI reactivity in these women, excluding positive outliers.
Statistical Analysis
We performed a cross-sectional analysis of data from the enrollment visit of ALTS. We described the seroprevalence and seroepidemiology across age groups of HPV L1 antigens in the ALTS population for eight high-risk HPV genotypes and used standard contingency table methods, chi-squared tests, and t-tests to evaluate differences between subgroups. We compared genotype-specific seroprevalence in relation to HPV DNA genotype prevalence, and described characteristics of seropositive and seronegative women grouped by HPV DNA status (+/−).
Chi-square testing, logistic regression analyses and multivariate generalized estimating equation regression were used to assess the association between a number of selected demographic, behavioral, and biological factors and HPV serology measures. Variables that showed a Chi-square association with seroprevalence with a significance value of p <0.2 were then used in univariate and multivariate logistic regression models to identify which factors remained statistically significantly associated with seropositivity. Multivariate models of factors associated with seropositivity for each genotype were explored using both forward and backward variable selection methods. We also used univariate and multivariate logistic regression analyses to evaluate factors associated with having a seropositive result for more than one HPV genotype among seropositive women. Analyses were conducted using STATA11 and R.
RESULTS
Characteristics of type-specific HPV DNA and L1 serology concordance and discordance
Population characteristics are described both at the at the woman-level in Table 1 and at the infection level in Supplemental Table S1; concordance between DNA positivity and seropositivity can only be examined on the genotype-specific infection level as women are often infected with multiple types of HPV and the concordance status can be different for each type. Table 1 shows data on the woman level and does not take type-specific concordance or multiple infections into account. In our study population there were 1) 590 (23.9%) women who were DNA positive and seropositive for at least one of the eight tested HPV genotypes (DNA+/Sero+), 2) 472 (19.1%) instances of seropositivity without DNA positivity (DNA−/Sero+), 3) 667 (27.1%) instances of DNA positivity without seropositivity for all tested genotypes (DNA+/Sero−), and 4) 735 (29.8%) of women tested were DNA-negative and seronegative for all eight tested genotypes (DNA−/Sero−). Women who were DNA+ were younger compared to the other groups; 62% of DNA+/Sero+ results and 62% of DNA+/Sero− results occurred in women under 25, while only 36% of DNA−/Sero− results and 36.4% of the DNA−/Sero+ results were in the under 25 group (p<0.001).
Table 1.
Women positive or negative for any high-risk HPV DNA and any high-risk L1 serology, not taking genotype concordance into account (N=2464)
| Women-level positivity |
hrdna−/hrsero− |
hrdna+/hrsero− |
hrdna−/hrsero+ |
hrdna+/hrsero+ |
Chi2 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | 735 | 667 | 472 | 590 | ||||||
| Age at enrollment | ||||||||||
| <25 | 1,235 | 267 | 36.3% | 415 | 62.2% | 172 | 36.4% | 381 | 64.6% | p<0.001 |
| 25–29 | 389 | 107 | 14.6% | 97 | 14.5% | 83 | 17.6% | 102 | 17.3% | |
| 30–44 | 656 | 255 | 34.7% | 140 | 21.0% | 168 | 35.6% | 93 | 15.8% | |
| 45+ | 184 | 106 | 14.4% | 15 | 2.2% | 49 | 10.4% | 14 | 2.4% | |
| Race | ||||||||||
| White/Hispanic | 99 | 29 | 3.9% | 34 | 5.1% | 17 | 3.6% | 19 | 3.2% | p<0.001 |
| White/Non-Hispanic | 1,503 | 518 | 70.5% | 420 | 63.0% | 266 | 56.4% | 299 | 50.7% | |
| Black | 739 | 154 | 21.0% | 176 | 26.4% | 170 | 36.0% | 239 | 40.5% | |
| Asian/Pacific Islander | 57 | 17 | 2.3% | 15 | 2.2% | 10 | 2.1% | 15 | 2.5% | |
| American Indian or Alaskan Native | 59 | 16 | 2.2% | 19 | 2.8% | 9 | 1.9% | 15 | 2.5% | |
| Education (highest completed) | ||||||||||
| <High School | 424 | 110 | 15.0% | 122 | 18.3% | 80 | 16.9% | 112 | 19.0% | p<0.001 |
| High School | 1,641 | 467 | 63.5% | 451 | 67.6% | 313 | 66.3% | 410 | 69.5% | |
| College | 279 | 104 | 14.1% | 70 | 10.5% | 51 | 10.8% | 54 | 9.2% | |
| Graduate School | 120 | 54 | 7.3% | 24 | 3.6% | 28 | 5.9% | 14 | 2.4% | |
| Smoking Status | ||||||||||
| Never | 1,315 | 389 | 52.9% | 312 | 46.8% | 268 | 56.8% | 346 | 58.6% | p<0.001 |
| Former | 308 | 118 | 16.1% | 60 | 9.0% | 74 | 15.7% | 56 | 9.5% | |
| Current | 839 | 227 | 30.9% | 295 | 44.2% | 130 | 27.5% | 187 | 31.7% | |
| Lifetime sex partners | ||||||||||
| 0–1 | 239 | 131 | 17.8% | 35 | 5.2% | 45 | 9.5% | 28 | 4.7% | p<0.001 |
| 2–3 | 499 | 162 | 22.0% | 137 | 20.5% | 97 | 20.6% | 103 | 17.5% | |
| 4+ | 1,726 | 442 | 60.1% | 495 | 74.2% | 330 | 69.9% | 459 | 77.8% | |
| Age at sexual debut with male partner | ||||||||||
| Never | 6 | 4 | 0.5% | 1 | 0.1% | 1 | 0.2% | 0 | 0.0% | p<0.001 |
| <16 | 833 | 201 | 27.3% | 255 | 38.2% | 147 | 31.1% | 230 | 39.0% | |
| 16–20 | 1,457 | 449 | 61.1% | 384 | 57.6% | 281 | 59.5% | 343 | 58.1% | |
| 21–26 | 154 | 78 | 10.6% | 21 | 3.1% | 40 | 8.5% | 15 | 2.5% | |
| 26+ | 14 | 3 | 0.4% | 6 | 0.9% | 3 | 0.6% | 2 | 0.3% | |
| History of STD | ||||||||||
| No | 1,411 | 506 | 68.8% | 376 | 56.4% | 265 | 56.1% | 264 | 44.7% | p<0.001 |
| Yes | 1,053 | 229 | 31.2% | 291 | 43.6% | 207 | 43.9% | 326 | 55.3% | |
A similar age distribution pattern was observed on the infection level when genotype concordance and discordance between DNA status and serostatus was taken into account (Supplementary Table S1). 74% of positive concordant infections (DNA+/Sero+) were found in women under age 25, compared to 48% of the observed concordant negative infections (DNA−/Sero−).
Seroprevalence and DNA prevalence across age groups
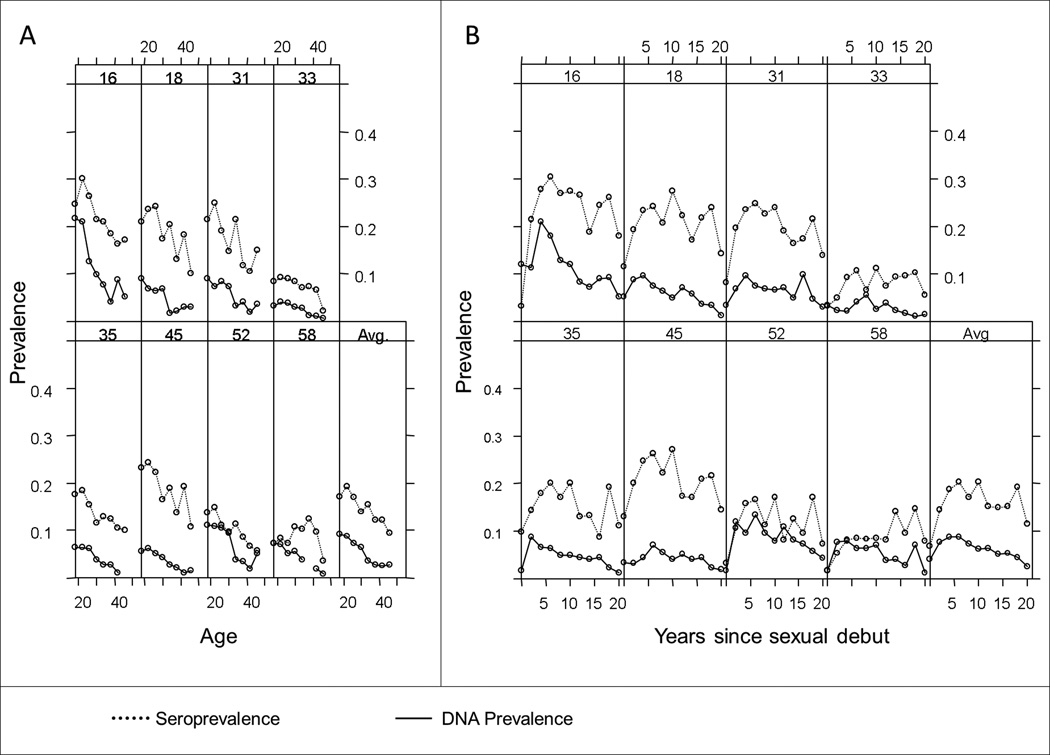
Most genotypes showed a similar pattern of seroprevalence and DNA prevalence across age at enrollment (Figure 1A): a peak seroprevalence between ages 20–25, with high-risk HPV DNA prevalence appearing to peak at an age earlier than 18. DNA prevalence declined and appeared to plateau around age 35, while no plateau was observed for our distribution of seroprevalence. When analyzed by years since reported sexual debut (Figure 1B), DNA prevalence peaked 1 to 5 years after debut, and seroprevalence for most genotypes peaked between 4 and 8 years after debut. While the absolute prevalence differed across genotypes, the patterns appeared to be largely similar aside from HPV types 52 and 58. HPV16 also showed the highest DNA and seroprevalence among the HR-HPV types, reflective of its known high prevalence in the general population.
Figure 1.
Seroprevalence is associated with baseline DNA prevalence and correlates of sexual activity
A multivariate GEE regression conditioned on genotype indicated associations between DNA positivity, age, and seropositivity (Table 2), with DNA positivity increasing the odds of seropositivity (OR 1.49, 95% CI 1.29–1.73) and increasingly older age decreasing the odds of seropositivity. Baseline DNA prevalence was univariately associated with baseline seroprevalence for 4 tested HPV types (Table 3). HPV16 and 18 showed ORs of 1.36 (95% CI 1.04– 1.79) and 2.31 (95% CI 1.61–3.32) respectively. After age stratification, these associations persisted only in women under the age of 25. Older age at sexual debut (17 and up) was associated with decreased risk of baseline seroprevalence for all HPV genotypes except HPV58 (Supplemental Table 2). In univariate analyses, increasing numbers of lifetime sex partners showed an association with increased risk of baseline seropositivity, and older age at enrollment and older age at sexual debut were associated with decreasing risk of seropositivity (Table 4).
Table 2.
Multivariate generalized estimating equations regression conditioned on genotype to examine association between DNA positivity, age, and L1 seropositivity at ALTS baseline in 2464 women referred for mildly abnormal or equivocal cervical cytology.*
| HPV L1 seropositivity Odds Ratio (95% Conf. Interval) |
|
|---|---|
| HPV DNA positivity | 1.49 (1.29–1.73) |
| Age at study enrollment | |
| <25 | Ref. |
| 25–29 | 1.02 (0.92–1.13) |
| 30–44 | 0.74 (0.67–0.82) |
| 45+ | 0.45 (0.36–0.56) |
Statistically significant results (p value <0.05) shown in bold type.
Table 3.
Logistic regression analysis of the association between baseline HPV DNA prevalence and baseline L1 seropositivity of the same genotype in 2464 women in ALTS, stratified by age group at enrollment.
| Genotype | All ages (N= 2462) | <25 (n=1235) | 25–29 (n=389) | 30–34 (n=656) | 45+ (n=184) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | P>z | OR | P>z | OR | P>z | OR | P>z | OR | P>z | |
| HPV16 | 1.36 | 0.024 | 1.53 | 0.01 | 0.72 | 0.42 | 0.69 | 0.42 | 2.27 | 0.26 |
| HPV18 | 2.31 | <0.001 | 2.27 | <0.001 | 2.03 | 0.16 | 1.38 | 0.58 | 8.89 | 0.13 |
| HPV31 | 1.26 | 0.211 | 1.45 | 0.13 | 1.11 | 0.82 | 0.55 | 0.33 | 1.74 | 0.63 |
| HPV33 | 0.74 | 0.549 | 0.56 | 0.42 | 1.65 | 0.52 | . | . | ||
| HPV35 | 1.47 | 0.083 | 1.37 | 0.27 | 2.07 | 0.12 | . | . | ||
| HPV45 | 1.40 | 0.124 | 1.43 | 0.21 | 1.98 | 0.17 | 0.32 | 0.27 | . | . |
| HPV52 | 1.84 | <0.001 | 2.20 | <0.001 | 1.14 | 0.82 | 1.09 | 0.87 | 2.93 | 0.34 |
| HPV58 | 2.22 | <0.001 | 2.27 | 0.01 | 2.01 | 0.38 | 2.90 | 0.03 | . | . |
Statistically significant results (p value <0.05) shown in bold type.
Table 4.
Univariate logistic regression analyses of the association between age at trial enrollment, age at sexual debut, and reported lifetime number of sex partners with baseline HPV DNA positivity and baseline HPV L1 seropositivity for HPV16 and HPV18.
| DNA | Serology | |||||||
|---|---|---|---|---|---|---|---|---|
| HPV16 | HPV18 | HPV16 | HPV18 | |||||
| Age | OR | P>z | OR | P>z | OR | P>z | OR | P>z |
| <25 | ref. | |||||||
| 25–29 | 0.59 | 0.01 | 0.54 | 0.02 | 1.07 | 0.60 | 1.11 | 0.44 |
| 30–44 | 0.35 | <0.001 | 0.32 | <0.001 | 0.69 | <0.001 | 0.74 | 0.01 |
| 45+ | 0.30 | <0.001 | 0.12 | <0.001 | 0.56 | 0.01 | 0.42 | <0.001 |
| Age at Sexual Debut | ||||||||
| <14 | ref. | |||||||
| 14 | 0.87 | 0.51 | 1.48 | 0.13 | 0.95 | 0.74 | 0.79 | 0.15 |
| 15–16 | 0.96 | 0.80 | 0.71 | 0.16 | 0.93 | 0.56 | 0.85 | 0.21 |
| 17–20 | 0.70 | 0.07 | 0.66 | 0.12 | 0.56 | <0.001 | 0.59 | <0.001 |
| 21+ | 0.22 | 0.01 | 0.49 | 0.25 | 0.42 | 0.01 | 0.36 | <0.001 |
| Lifetime sex partners | ||||||||
| 0 partners | . | |||||||
| 1 partner | ref | ref | ||||||
| 2–3 partners | 1.66 | 0.10 | 2.78 | 0.06 | 2.43 | <0.001 | 2.95 | <0.001 |
| >4 | 2.18 | 0.01 | 4.01 | 0.01 | 3.18 | <0.001 | 3.51 | <0.001 |
Statistically significant results (p value <0.05) shown in bold type.
In multivariate models, number of lifetime sex partners was associated with increased risk of seropositivity for all genotypes except HPV35 and HPV58. The association observed between seropositivity and age at enrollment or age at sexual debut only remained statistically significant for some genotypes, though one of the two age-related variables remained statistically significantly associated with seropositivity for all genotypes except HPV58. Our multivariate modeling also indicated that history of sexually transmitted disease was associated with increased odds of seroprevalence across all genotypes, and smoking remained consistently associated with decreased odds of seroprevalence across genotypes (Supplemental Table S2).
Smoking is associated with decreased HPV seroprevalence
Women who were current or former smokers were less likely to be seropositive for all eight of the tested oncogenic genotypes, and this association persisted in multivariate models adjusting for age at enrollment, time since sexual debut, and number of sex partners (Table 5). Current smokers showed increased odds of HPV16 DNA detection at baseline compared to women who reported never smoking (Table 5), which is consistent with the observation in Table 1 that the group of women who were DNA-positive for one of the eight genotypes but were seronegative for all eight genotypes included the largest proportion of current smokers and the lowest proportion of never smokers.
Table 5.
Logistic regression analyses of the associations between smoking, baseline seropositivity, and baseline DNA prevalence.*
| Smoking Statusa | Seroprevalence OR (95% CI) |
P>z | DNA prevalence OR (95% CI) |
P>z | |
|---|---|---|---|---|---|
| HPV16 L1 | |||||
| Never | Ref. | ||||
| Former | 0.72 (0.53– 0.98) | 0.04 | 1.32 (0.87– 2.02) | 0.19 | |
| Current | 0.54 (0.44– 0.68) | <0.001 | 1.62 (1.23– 2.15) | 0.001 | |
| HPV18 L1 | |||||
| Never | Ref. | ||||
| Former | 0.62 (0.44– 0.87) | 0.01 | 0.49 (0.22– 1.09) | 0.08 | |
| Current | 0.52 (0.41– 0.66) | <0.001 | 1.24 (0.85– 1.82) | 0.26 | |
| HPV31 L1 | |||||
| Never | Ref. | ||||
| Former | 0.63 (0.45– 0.89) | 0.008 | 1.71 (1.05– 2.79) | 0.03 | |
| Current | 0.55 (0.46– 0.69) | <0.001 | 1.12 (0.76– 1.64) | 0.57 | |
| HPV33 L1 | |||||
| Never | Ref. | ||||
| Former | 0.72 (0.44– 1.18) | 0.2 | 0.56 (0.19– 1.61) | 0.28 | |
| Current | 0.59 (0.42– 0.84) | 0.003 | 1.03 (0.60– 1.77) | 0.90 | |
| HPV35 L1 | |||||
| Never | Ref. | ||||
| Former | 0.77 (0.53– 1.11) | 0.16 | 0.63 (0.30– 1.36) | 0.25 | |
| Current | 0.58 (0.44– 0.75) | <0.001 | 0.86 (0.56– 1.32) | 0.49 | |
| HPV45 L1 | |||||
| Never | Ref. | ||||
| Former | 0.69 (0.49– 0.96) | 0.03 | 1.05 (0.52– 2.14) | 0.89 | |
| Current | 0.49 (0.39– 0.62) | <0.001 | 1.31 (0.84– 2.05) | 0.23 | |
| HPV52 L1 | |||||
| Never | Ref. | ||||
| Former | 0.69 (0.46– 1.03) | 0.07 | 0.61 (0.34– 1.07) | 0.08 | |
| Current | 0.37 (0.28– 0.51) | <0.001 | 1.11 (0.80– 1.52) | 0.54 | |
| HPV58 L1 | |||||
| Never | Ref. | ||||
| Former | 0.71 (0.44– 1.13) | 0.15 | 0.72 (0.37– 1.39) | 0.33 | |
| Current | 0.55 (0.39– 0.78) | 0.001 | 0.80 (0.53– 1.21) | 0.29 | |
Multivariate regression model also includes age category, lifetime number of sex partners, number of years sexually active, and HPV DNA status (for serology prevalence) and serostatus (for DNA prevalence). Statistically significant results (p value <0.05) shown in bold type.
Multiple seropositivity
Approximately 25% of our study population was seropositive for more than one of our tested HPV types (Supplemental Table S3). Among seropositive women, the presence of HPV DNA increased the risk of having more than one prevalent HPV seropositive result. Having more than one lifetime sexual partner and a younger age at sexual debut was associated with increased odds of seropositivity for multiple types in seropositive women.
Univariate logistic regression analysis indicated that older seropositive women were less likely to be positive for multiple genotypes compared to seropositive women under 25 (women 45+ OR 0.72 95% CI 0.63–0.95).
DISCUSSION
Substantial gaps remain in the understanding of the natural humoral immune response to HPV. Most existing work on this topic focused on a limited number of HPV genotypes, and with evidence that multiple HPV genotypes are involved in carcinogenesis, exploration of the natural history of a broader range of genotypes is needed. In this study we used a multiplex serology assay and showed associations between seroprevalence and known risk factors for HPV infection across nearly all of the tested HR-HPV genotypes (16, 18, 31, 33, 35, 45, 52, and 58) in a population of women referred for low-grade cytological abnormalities who were free of precancer. Our results indicated that risk factors for HPV infection including younger age and correlates of sexual behavior were associated with increased seroprevalence for nearly all tested HPV genotypes in these women. HPV58 seropositivity did not show associations with most expected risk factors.
Similar DNA prevalence and seroprevalence patterns by age were observed across most of the HPV genotypes tested, with six genotypes displaying a peak seroprevalence between ages 20 and 25 in women referred for equivocal or mildly abnormal cytology. In our analysis, DNA prevalence peaked within 5 years of sexual debut and seroprevalence peaked 4–8 years after debut for most genotypes. This is consistent with the prevalence age distribution observed in our study as the median age of sexual debut was 16, as the DNA prevalence was highest below age 20, while seroprevalence was highest in women aged 20–30. We observed an earlier peak of HPV16 seroprevalence in the ALTS population compared to the observed peak HPV16 seroprevalence measured by VLP ELISA in a population-based study of approximately ten thousand women conducted in Costa Rica;26 HPV16 and 18 seroprevalences peaked in women aged 30–39, a decade post-DNA prevalence peak. This difference may be explained by the overall young age at sexual debut for women in this study and for the oversampling for HPV infection due to women being referred into the study based on equivocal or mildly abnormal cervical cytology. Women were referred to ALTS for low-grade cytological abnormalities which can indicate a current or recent infection with the virus, so participants likely have higher levels of recent HPV exposure than studies that drew population-based samples, which may oversample for HPV.27 HPV seroconversion has been reported to occur up to 18 months after initial HPV infection, but the much-delayed seroprevalence peak observed in studies of HPV seroprevalence could be due to the low risk of seroconversion in general.
Women who were enrolled into the study at ages 30–44 and 45+ showed decreasing odds of DNA prevalence and seroprevalence compared to women enrolled under the age of 25 for nearly all of the oncogenic genotypes in this study, possibly indicating waning of seropositivity over time though antibody decay has not been observed in short-term longitudinal studies of the natural immune response to HPV.28
Concordance between genotype-specific HPV DNA detection and seropositivity was relatively infrequent and occurred most frequently in women under age 25. Low concordance of type-specific positivity between DNA and serologic measures of HPV has been reported in other studies using VLP ELISA as well as multiplex serological assays for multiple genital HPV genotypes including HPV16.28–30 Younger women were more likely to be both DNA positive and seropositive for at least one of the eight high-risk genotypes than women who had seropositive-only detections, presumably because seropositivity persists after the more rapid clearance or immune control of the replicating virus and DNA prevalence was highest in the youngest women in this analysis. A study of HPV DNA positivity and seropositivity concordance for HPV6, 11, 16, 18, and 45 in 323 pregnant women enrolled in the Finnish Family study, which measured seropositivity with the GST-fusion protein multiplex assay used here, did not show strong concordance between DNA positivity and seropositivity either at the same visit or when using seropositivity outcomes from a visit 12 months later to account for the lag time between HPV infection and seroconversion.28 The observed low rates of positive concordance between DNA status and serostatus could result from misclassification due to lower accuracy of the multiplex serology assay compared to the VLP ELISA assay, though experimental validation of the assays suggest similar epitope reactivity and antibody titers for GST-fusion protein targets and VLP targets when used in an ELISA.31 In experimental studies, HPV16 GST-fusion proteins used in ELISA assays have shown a similar sensitivity and specificity to VLP assays24, and direct comparisons between GST-ELISA and GST-multiplex assays have indicated greater sensitivity for the multiplex method than the ELISA method.21 However, there have been no direct comparisons of VLP ELISA and the GST-fusion protein multiplex assay performance in human serum, these studies are necessary to better understand the differences observed between VLP-based and multiplex Luminex assays.
Correlates of sexual behavior were associated with increased odds of seroprevalence for most of the tested high-risk HPV genotypes except for HPV58, including younger age at sexual debut, higher numbers of lifetime sex partners, and history of a sexually transmitted disease. The magnitudes of the associations were similar across genotypes, suggesting that the factors driving seropositivity are similar across types; a limited number of studies have looked at determinants of seroprevalence for multiple high risk genotypes together so these results help to clarify that there do not appear to be significant genotype differences in the relationships between risk factors and seropositivity for seven of these HPV types. However, HPV58 seroprevalence did not show any association with multiple expected sexual risk factors of HPV exposure aside from history of sexually transmitted diseases (STDs); it is unclear whether or not this issue is assay-related as there is little work on HPV58 seroprevalence to which to compare our results. Another study using VLP ELISA detection methods only observed an association between number of sex partners and HPV58 seropositivity when analyses were limited to women older than age 4532 and did not see this effect in their younger women, so it is possible that HPV58 acts differently from the other genotypes included in our study in terms of age and sexual exposure risk. For most genotypes, increasing numbers of sexual partners showed increasing odds of seropositivity, likely reflecting the greater cumulative exposure to HPV and potential for multiple instances of antigenic exposure for the immune system.
Current smokers were less likely to be seropositive for all eight oncogenic genotypes regardless of DNA status and had increased odds of HPV16 DNA detection after controlling for age and sexual behavior variables. Some cross-sectional studies using other assays including VLP ELISA have observed similar negative associations between HPV seroprevalence and smoking for several HPV genotypes (16, 18, 31, 58), while others have not or have shown increased risk of seropositivity associated with smoking11, 15, 32. Decreased risk of seroconversion and decreased persistence of seropositivity for HPV16 and 18 in current smokers compared to non-smokers was observed in a small (N=191) prospective study of young HPV-infected women in Finland,33, which is consistent with the decreased seroprevalence for all eight HPV genotypes we observed in smokers in this study. The mechanism through which cigarette smoking might be associated with HPV antibody response specifically is unknown. However, there is substantial evidence that cigarette smoke exposure decreases the magnitude of T-cell responses and can result in decreased IgG antibody response to various pathogens. Long-term smoking and nicotine exposure have been associated with decreased T-cell and natural killer cell activity and with decreased serum titers of overall IgG antibodies in otherwise healthy individuals, as well as with lower rates of antigen-specific IgG antibodies.34–41 Decreased innate and T-cell responses may help to explain smoking’s observed association with increased incidence and persistence of oncogenic HPV DNA detection in immunocompetent women42, 43 as well as the decreased seroprevalence we observe in this study.
Seropositivity to multiple genotypes was common: nearly a quarter of the women in this analysis were seropositive for more than one of the eight tested genotypes. In women who were seropositive for at least one genotype, while the presence of HPV DNA increased the risk of having more than one prevalent HPV seropositive result, we did not observe a dose-response relationship between the number of HPV genotypes a women was DNA-positive for and risk of multiple seropositivity; this is consistent with the idea that serology results represent cumulative exposure, and may give some indication of what HPV genotypes a women has been exposed to in her lifetime but not necessarily the genotypes she is currently detectably infected with.
A limitation of this study is its cross-sectional design, which prevents examination of prospective seroconversion and seropersistance. We were unable to determine whether the decreased seroprevalence in older age groups results from waning antibody titers or from a cohort effect, nor could we examine whether time between infection and seroconversion differed across genotypes, which might help with understanding of some of the differences observed for HPV58. However, the glutathione S-transferase fusion protein multiplex serology assay showed seroprevalence age distributions for nearly all genotypes that are consistent with current understanding of HPV natural history in the cervix.
In summary, seropositivity as determined by the glutathione S-transferase fusion protein multiplex serology assay was associated with known HPV infection risk factors for seven high-risk oncogenic HPV genotypes (HPV 16, 18, 31, 33, 35, 45, and 52) and resulted in age distribution DNA prevalence and seroprevalence patterns for those genotypes that were similar, though shifted to an earlier age, to those generated by VLP ELISA assays in other studies for HPV16 only. Prevalence age curves indicated that for HPV 16, 18, 31, 33, 35, and 45, peak seroprevalence lags behind peak DNA prevalence, with the highest DNA prevalence observed in women 1–5 years since their sexual debut and the highest seroprevalence observed in women 5–15 years from their sexual debut. While smoking either increased or had no detectable effect on the odds of HPV DNA detection at trial enrollment, current smoking was associated with decreased odds of HPV seroprevalence for all eight genotypes and the association was not affected by controlling for HPV DNA status, age, or reported sexual behavior. There appear to be differences in the magnitude of some observed associations between risk factors and seropositivity compared to studies conducted with other assays for HPV16 and HPV18 as well as low rates of concordance between DNA positivity and seropositivity; this may result from misclassification due to differences in assay sensitivity and specificity, or from the make-up of our study population due to oversampling for recent HPV infections and the limitation to women who were <CIN2 at enrollment.
Supplementary Material
ACKNOWLEDGEMENTS
National Cancer Institute, National Institutes of Health Department of Health and Human Services contracts CN-55153, CN-55154, CN-55155, CN-55156, CN-55157, CN-55158, CN-55159 and CN-55105 provided support for ALTS. Some of the equipment and supplies used in these studies were donated or provided at reduced cost by Digene Corporation, Gaithersburg, MD; Cytyc Corporation, Marlborough, MA; National Testing Laboratories, Fenton, MO; DenVu, Tucson, AZ; TriPath Imaging, Inc, Burlington, NC; and Roche Molecular Systems Inc, Alameda, CA. We thank the ALTS Group Investigators for their help in planning and conducting the trial.
Abbreviations
- HPV
human papillomavirus
- ALTS
ASCUS/LSIL Triage Study for Cervical Cancer
- ASCUS
atypical squamous cells of undetermined significance
- LSIL
low-grade squamous epithelial lesion
- CIN
cervical intraepithelial neoplasia
- GST
gluthatione s-tranferse
- ELISA
enzyme-linked immunosorbent assay
- VLP
virus-like particle
Footnotes
Description: The seroepidemiology of human papillomavirus (HPV) has not been well-characterized for multiple oncogenic genotypes aside from HPV16 and HPV18. Using a Luminex-based multiplex serology assay, we show associations between risk factors for human papillomavirus (HPV) infection and HPV seropositivity for eight oncogenic HPV genotypes in women with mildly abnormal cervical cytology results.
Disclosure: Dr. Castle serves as a paid consultant of BD, Gen-Probe/Hologic, GE Healthcare, and Cepheid; he received a Speaker's honorarium from Roche and he serves as a paid member of a Data and Safety Monitoring Board for HPV Vaccines for Merck. Dr. Waterboer is currently affiliated with F.Hoffman-La Roche Ltd. F.Hoffman-La Roche Ltd. did not have any influence on the manuscript and its content.
References
- 1.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, Ghissassi FE. Carcinogenicity of human papillomaviruses. The Lancet Oncology. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24:S11–S25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 3.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. The Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 4.Koshiol J, Lindsay L, Pimenta JM, Poole C, Jenkins D, Smith JS. Persistent Human Papillomavirus Infection and Cervical Neoplasia: A Systematic Review and Meta-Analysis. American Journal of Epidemiology. 2008;168:123–137. doi: 10.1093/aje/kwn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koshiol JE, Schroeder JC, Jamieson DJ, Marshall SW, Duerr A, Heilig CM, Shah KV, Klein RS, Cu-Uvin S, Schuman P, Celentano D, Smith JS. Time to clearance of human papillomavirus infection by type and human immunodeficiency virus serostatus. International Journal of Cancer. 2006;119:1623–1629. doi: 10.1002/ijc.22015. [DOI] [PubMed] [Google Scholar]
- 6.Xi LF, Carter JJ, Galloway DA, Kuypers J, Hughes JP, Lee SK, Adam DE, Kiviat NB, Koutsky LA. Acquisition and Natural History of Human Papillomavirus Type 16 Variant Infection among a Cohort of Female University Students. Cancer Epidemiology Biomarkers & Prevention. 2002;11:343–351. [PubMed] [Google Scholar]
- 7.Carter JJ, Koutsky LA, Hughes JP, Lee SK, Kuypers J, Kiviat N, Galloway DA. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis. 2000;181:1911–1919. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 8.Stanley M. Immunobiology of HPV and HPV vaccines. Gynecologic Oncology. 2008;109:S15–S21. doi: 10.1016/j.ygyno.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Hebner CM, Wilson R, Rader J, Bidder M, Laimins LA. Human papillomaviruses target the double-stranded RNA protein kinase pathway. Journal of General Virology. 2006;87:3183–3193. doi: 10.1099/vir.0.82098-0. [DOI] [PubMed] [Google Scholar]
- 10.Mota F, Rayment N, Chong S, Singer A, Chain B. The antigen-presenting environment in normal and human papillomavirus (HPV)-related premalignant cervical epithelium. Clinical & Experimental Immunology. 1999;116:33–40. doi: 10.1046/j.1365-2249.1999.00826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang SS, Schiffman M, Shields TS, Herrero R, Hildesheim A, Bratti MC, Sherman ME, Rodriguez AC, Castle PE, Morales J, Alfaro M, Wright T, et al. Seroprevalence of human papillomavirus-16, -18, -31, and -45 in a population-based cohort of 10[thinsp]000 women in Costa Rica. Br J Cancer. 2003;89:1248–1254. doi: 10.1038/sj.bjc.6601272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heiligenberg M, Michael K, Kramer M, Pawlita M, et al. Seroprevalence and Determinants of Eight High-Risk Human Papillomavirus Types in Homosexual Men, Heterosexual Men, and Women: A Population Based Study in Amsterdam. Sexually Transmitted Diseases. 2010;37:672–680. doi: 10.1097/OLQ.0b013e3181e71069. [DOI] [PubMed] [Google Scholar]
- 13.Porras C, Bennett C, Safaeian M, Coseo S, Rodriguez AC, Gonzalez P, Hutchinson M, Jimenez S, Sherman M, Wacholder S, Solomon D, van Doorn L-J, et al. Determinants of seropositivity among HPV-16/18 DNA positive young women. BMC Infectious Diseases. 2010;10:238. doi: 10.1186/1471-2334-10-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wideroff L, Schiffman M, Hoover R, Tarone RE, Nonnenmacher B, Hubbert N, Kirnbauer R, Greer CE, Lorincz AT, Manos MM, Glass AG, Scott DR, et al. Epidemiologic determinants of seroreactivity to human papillomavirus (HPV) type 16 virus-like particles in cervical HPV-16 DNA-positive and-negative women. J Infect Dis. 1996;174:937–943. doi: 10.1093/infdis/174.5.937. [DOI] [PubMed] [Google Scholar]
- 15.Coseo S, Porras C, Hildesheim A, Rodriguez AC, Schiffman M, Herrero R, Wacholder S, Gonzalez P, Wang SS, Sherman ME, Jimenez S, Solomon D, et al. Seroprevalence and Correlates of Human Papillomavirus 16/18 Seropositivity Among Young Women in Costa Rica. Sexually Transmitted Diseases. 2010;37:706–714. doi: 10.1097/OLQ.0b013e3181e1a2c5. 10.1097/OLQ.0b013e3181e1a2c5. [DOI] [PubMed] [Google Scholar]
- 16.Dillner J, Kallings I, Brihmer C, Sikstrom B, Koskela P, Lehtinen M, Schiller JT, Sapp M, Mardh PA. Seropositivities to human papillomavirus types 16, 18, or 33 capsids and to Chlamydia trachomatis are markers of sexual behavior. J Infect Dis. 1996;173:1394–1398. doi: 10.1093/infdis/173.6.1394. [DOI] [PubMed] [Google Scholar]
- 17.Wentzensen N, Wilson LE, Wheeler CM, Carreon JD, Gravitt PE, Schiffman M, Castle PE. Hierarchical clustering of human papilloma virus genotype patterns in the ASCUS-LSIL triage study. Cancer Res. 70:8578–8586. doi: 10.1158/0008-5472.CAN-10-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Safaeian M, Porras C, Schiffman M, Rodriguez AC, Wacholder S, Gonzalez P, Quint W, van Doorn L-J, Sherman ME, Xhenseval V, Herrero R, Hildesheim A, et al. Epidemiological Study of Anti-HPV16/18 Seropositivity and Subsequent Risk of HPV16 and -18 Infections. Journal of the National Cancer Institute. 2010;102:1653–1662. doi: 10.1093/jnci/djq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wentzensen N, Rodriguez AC, Viscidi R, Herrero R, Hildesheim A, Ghosh A, Morales J, Wacholder S, Guillen D, Alfaro M, Safaeian M, Burk RD, et al. A Competitive Serological Assay Shows Naturally Acquired Immunity to Human Papillomavirus Infections in the Guanacaste Natural History Study. Journal of Infectious Diseases. 2011;204:94–102. doi: 10.1093/infdis/jir209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiffman M, Safaeian M, Wentzensen N. The use of human papillomavirus seroepidemiology to inform vaccine policy. Sex Transm Dis. 2009;36:675–679. doi: 10.1097/OLQ.0b013e3181bce102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waterboer T, Sehr P, Michael KM, Franceschi S, Nieland JD, Joos TO, Templin MF, Pawlita M. Multiplex Human Papillomavirus Serology Based on In Situ-Purified Glutathione S-Transferase Fusion Proteins. Clin Chem. 2005;51:1845–1853. doi: 10.1373/clinchem.2005.052381. [DOI] [PubMed] [Google Scholar]
- 22.Faust H, Knekt P, Forslund O, Dillner J. Validation of multiplexed human papillomavirus serology using pseudovirions bound to heparin-coated beads. Journal of General Virology. 2010;91:1840–1848. doi: 10.1099/vir.0.019349-0. [DOI] [PubMed] [Google Scholar]
- 23.Guido R, Schiffman M, Solomon D, Burke L. Postcolposcopy management strategies for women referred with low-grade squamous intraepithelial lesions or human papillomavirus DNA–positive atypical squamous cells of undetermined significance: A two-year prospective study. American Journal of Obstetrics and Gynecology. 2003;188:1401–1405. doi: 10.1067/mob.2003.456. [DOI] [PubMed] [Google Scholar]
- 24.Sehr P, Müller M, Höpfl R, Widschwendter A, Pawlita M. HPV antibody detection by ELISA with capsid protein L1 fused to glutathione S-transferase. Journal of Virological Methods. 2002;106:61–70. doi: 10.1016/s0166-0934(02)00134-9. [DOI] [PubMed] [Google Scholar]
- 25.Clifford G, Shin H, Oh J, Waterboer T, Ju Y, Vaccarella S, Quint W, Pawlita M, Franceschi S. Serologic response to oncogenic human papillomavirus types in male and female university students in Busan, South Korea. Cancer Epidemiol Biomarkers Prev. 2007;16:1874–1879. doi: 10.1158/1055-9965.EPI-07-0349. [DOI] [PubMed] [Google Scholar]
- 26.Wang SS, Schiffman M, Herrero R, Carreon J, Hildesheim A, Rodriguez AC, Bratti MC, Sherman ME, Morales J, Guillen D, Alfaro M, Clayman B, et al. Determinants of human papillomavirus 16 serological conversion and persistence in a population-based cohort of 10[thinsp]000 women in Costa Rica. Br J Cancer. 2004;91:1269–1274. doi: 10.1038/sj.bjc.6602088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markt SC, Rodriguez AC, Burk RD, Hildesheim A, Herrero R, Wacholder S, Hutchinson M, Schiffman M. Longitudinal Analysis of Carcinogenic Human Papillomavirus Infection and Associated Cytologic Abnormalities in the Guanacaste Natural History Study: Looking Ahead to Cotesting. Journal of Infectious Diseases. 2012;205:498–505. doi: 10.1093/infdis/jir746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paaso AE, Louvanto K, Syrjänen KJ, Waterboer T, Grénman SE, Pawlita M, Syrjänen SM. Lack of type-specific concordance between human papillomavirus (HPV) serology and HPV DNA detection in the uterine cervix and oral mucosa. Journal of General Virology. 2011;92:2034–2046. doi: 10.1099/vir.0.032011-0. [DOI] [PubMed] [Google Scholar]
- 29.Touze A, de Sanjose S, Coursaget P, Almirall MR, Palacio V, Meijer CJ, Kornegay J, Bosch FX. Prevalence of anti-human papillomavirus type 16, 18, 31, and 58 virus-like particles in women in the general population and in prostitutes. J Clin Microbiol. 2001;39:4344–4348. doi: 10.1128/JCM.39.12.4344-4348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skjeldestad FE, Mehta V, Sings HL, Øvreness T, Turpin J, Su L, Boerckel P, Roberts C, Bryan J, Jansen KU, Esser MT, Liaw K-L. Seroprevalence and genital DNA prevalence of HPV types 6, 11, 16 and 18 in a cohort of young Norwegian women: study design and cohort characteristics. Acta Obstetricia et Gynecologica Scandinavica. 2008;87:81–88. doi: 10.1080/00016340701714703. [DOI] [PubMed] [Google Scholar]
- 31.Rizk RZ, Christensen ND, Michael KM, Muller M, Sehr P, Waterboer T, Pawlita M. Reactivity pattern of 92 monoclonal antibodies with 15 human papillomavirus types. J Gen Virol. 2008;89:117–129. doi: 10.1099/vir.0.83145-0. [DOI] [PubMed] [Google Scholar]
- 32.Bedoya AM, Gaviria AM, Baena A, Borrero M, Duarte DF, Combita AL, Castaño J, Grisales H, Sánchez GI. Age-Specific Seroprevalence of Human Papillomavirus 16, 18, 31, and 58 in Women of a Rural Town of Colombia. International Journal of Gynecological Cancer. 2012;22:303–310. doi: 10.1097/IGC.0b013e31823c2469. 10.1097/IGC.0b013e31823c2469. [DOI] [PubMed] [Google Scholar]
- 33.Simen-Kapeu A, Kataja V, Yliskoski M, Syrjänen K, Dillner J, Koskela P, Paavonen J, Lehtinen M. Smoking impairs human papillomavirus (HPV) type 16 and 18 capsids antibody response following natural HPV infection. Scandinavian Journal of Infectious Diseases. 2008;40:745–751. doi: 10.1080/00365540801995360. [DOI] [PubMed] [Google Scholar]
- 34.Ferson M, Edwards A, Lind A, Milton GW, Hersey P. Low natural-killer-cell activity and immunoglobulin levels associated with smoking in human subjects. Int. J. Cancer. 1979;23:603–609. doi: 10.1002/ijc.2910230504. [DOI] [PubMed] [Google Scholar]
- 35.Gunsolley JC, Pandey JP, Quinn SM, Tew J, Schenkein HA. The effect of race, smoking and immunoglobulin allotypes on IgG subclass concentrations. J Periodontal Res. 1997;32:381–387. doi: 10.1111/j.1600-0765.1997.tb00548.x. [DOI] [PubMed] [Google Scholar]
- 36.Tollerud DJ, Brown LM, Blattner WA, Weiss ST, Maloney EM, Kurman CC, Nelson DL, Hoover RN. Racial differences in serum immunoglobulin levels: relationship to cigarette smoking, T-cell subsets, and soluble interleukin-2 receptors. J Clin Lab Anal. 1995;9:37–41. doi: 10.1002/jcla.1860090107. [DOI] [PubMed] [Google Scholar]
- 37.Obeid P, Bercy P. Effects of cigarette smoking on periodontal health: a review. Adv. Ther. 2000;17:230–237. doi: 10.1007/BF02853162. [DOI] [PubMed] [Google Scholar]
- 38.Fischbacher CM, Bhopal R, Blackwell CC, Ingram R, Unwin NC, White M, Alberti KG. IgG is higher in South Asians than Europeans: does infection contribute to ethnic variation in cardiovascular disease? Arterioscler Thromb Vasc Biol. 2003;23:703–704. doi: 10.1161/01.ATV.0000060449.70345.8E. [DOI] [PubMed] [Google Scholar]
- 39.Geng Y, Savage SM, Razani-Boroujerdi S, Sopori ML. Effects of nicotine on the immune response. II. Chronic nicotine treatment induces T-cell anergy. J. Immunol. 1996;156:2384–2390. [PubMed] [Google Scholar]
- 40.Singh SP. Acute and chronic nicotine exposures modulate the immune system through different pathways. Toxicol. Appl. Pharmacol. 2000;164:65–72. doi: 10.1006/taap.2000.8897. [DOI] [PubMed] [Google Scholar]
- 41.Sopori ML, Kozak W, Savage SM, Geng Y, Kluger MJ. Nicotine-induced modulation of T-cell function: implications for inflammation and infection. Adv. Exp. Med. Biol. 1998;437:279–289. doi: 10.1007/978-1-4615-5347-2_31. [DOI] [PubMed] [Google Scholar]
- 42.Koshiol J, Schroeder J, Jamieson DJ, Marshall SW, Duerr A, Heilig CM, Shah KV, Klein RS, Cu-Uvin S, Schuman P, Celentano D, Smith JS. Smoking and time to clearance of human papillomavirus infection in HIV-seropositive and HIV-seronegative women. Am J Epidemiol. 2006;164:176–183. doi: 10.1093/aje/kwj165. [DOI] [PubMed] [Google Scholar]
- 43.Sarian LO, Hammes LS, Longatto-Filho A, Guarisi R, Derchain SF, Roteli-Martins C, Naud P, Erzen M, Branca M, Tatti S, de Matos JC, Gontijo R, et al. Increased risk of oncogenic human papillomavirus infections and incident high-grade cervical intraepithelial neoplasia among smokers: experience from the Latin American screening study. Sex Transm Dis. 2009;36:241–248. doi: 10.1097/OLQ.0b013e3181935a7d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.