Abstract
Traumatic brain injury (TBI) is significant, from a public health standpoint, because it is a major cause of the morbidity and mortality of young people. Cerebral edema after a TBI, if untreated, can lead to devastating damage of the remaining tissue. The current therapies of severe TBI (sTBI), as outlined by the Brain Trauma Foundation, are often ineffective, thus a new method for the treatment of sTBI is necessary. Herein, the reduction of cerebral edema, after TBI, using an osmotic transport device (OTD) was evaluated. Controlled cortical impact (CCI) was performed on adult female CD-1 mice, and cerebral edema was allowed to form for 3 h, followed by 2 h of treatment. The treatment groups were craniectomy only, craniectomy with a hydrogel, OTD without bovine serum albumin (BSA), and OTD. After CCI, brain water content was significantly higher for animals treated with a craniectomy only, craniectomy with a hydrogel, and OTD without BSA, compared to that of control animals. However, when TBI animals were treated with an OTD, brain water content was not significantly higher than that of controls. Further, brain water content of TBI animals treated with an OTD was significantly reduced, compared to that of untreated TBI animals, TBI animals treated with a craniectomy and a hydrogel, and TBI animals treated with an OTD without BSA. Here, we demonstrate the successful reduction of cerebral edema, as determined by brain water content, after TBI using an OTD. These results demonstrate proof of principle for direct water extraction from edematous brain tissue by direct osmotherapy using an OTD.
Key words: : cerebral edema, direct osmotherapy, hollow fiber-hydrogel device, osmotic transport device, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is the foremost cause of morbidity and mortality in persons under 45 years of age worldwide and accounts for a larger number of casualties in combat in Iraq and Afghanistan than in any other recent U.S. war.1,2 In the United States, approximately 200,000 victims of TBI need hospitalization annually, and approximately 52,000 U.S. deaths per year result from TBI.1 TBI can be divided into two phases: primary and secondary injury. Primary injury is caused by the direct external mechanical force, whereas secondary injury refers to a cascade of delayed deleterious physiological events that may last from hours to days. Secondary injury plays a major role in the morbidity and mortality resulting from TBI and is characterized by ischemia, blood–brain barrier (BBB) rupture, and cerebral edema.3
Cerebral edema is an increase in brain tissue water content. The two major types are vasogenic and cytotoxic (cellular) edema. Vasogenic edema, characterized by the disruption of the BBB, is the accumulation of water in the extracellular space. Subsequent to BBB disruption, blood components accumulate in the brain tissue, causing an influx of water, which results in swelling of the tissue. In contrast, cytotoxic edema involves intracellular accumulation of water and cell swelling.1,3
TBI is characterized by mixed cytotoxic and vasogenic edema mechanisms contributing to overall cerebral edema. After TBI, glial cells swell as a result of changes in the extracellular pH and concentrations of ions, including potassium, sodium, and chloride.3 The resulting cytotoxic edema combines with the vasogenic edema caused by direct BBB injury. Reduced blood flow to the affected brain area (cerebral ischemia) leads to further ion shifts, exacerbating cytotoxic edema. A vicious cycle involving components of both types of edema can proceed until the brain swells uncontrollably, resulting in permanent brain damage or death.
In recent years, with the advances in diagnostic imaging, the Brain Trauma Foundation (BTF) guidelines for the treatment and surgical intervention of severe TBI (sTBI) have been refined.4 In particular, these guidelines state that the management of sTBI requires a combinatorial approach of surgical and therapeutic treatments, including osmotherapy, barbiturates, ventriculostomy, and decompressive craniectomy. Osmotherapy includes the use of systemic circulation of either mannitol or hypertonic saline solutions to relieve cerebral edema. Barbiturates are thought to reduce intracranial pressure (ICP) through metabolic suppression.5 Ventriculostomy consists of cerebrospinal fluid (CSF) drainage by a ventricular catheter. In the most severe cases of TBI, decompressive craniectomy is performed to relieve increased ICP resulting from cerebral edema.
Although these treatments are suggested by the BTF guidelines, even combinations of these therapies may have limited success in treating sTBI.6–8 Recent studies suggest that alternative treatments/therapies are needed for managing sTBI and improving outcome while preventing the limitations of the current therapies.
Although osmotherapy is usually effective at acutely reducing ICP, its disadvantages include clinical variability, temporary duration of effect, and potential deleterious systemic consequences, such as dehydration and electrolyte imbalances.9,10 In addition, even maximum concentrations of the osmolytes can be ineffective for treating sTBI.9,11–13 Similar to osmotherapy, barbiturates can have a short duration of ICP reduction, followed by minimal effect on ICP after the initial suppression.5 Further, it remains unknown whether barbiturates improve neurological outcome.5,14,15
When used alone for treating TBI, ventriculostomy alone is often ineffective given that the total CSF volume (approximately 150 mL for adults) is only approximately 10% of brain volume.
Surgical treatment with decompressive craniectomy is more for the reduction of elevated ICP and to prevent transtentorial herniation, rather than to directly treat cerebral edema. External brain herniation, which follows a craniectomy, can lead to venous compression and ischemia and, consequently, cause further secondary injury. Further, the recent results of the DECRA trial suggest that treatment with a decompressive craniectomy for diffuse TBI may not significantly improve outcome.8
A new method that removes water in a direct manner from brain tissue would potentially circumvent some of the limitations of current therapies. The ideal method would be a medical device that is capable of removing water from brain tissue in a controlled fashion, would be a topical treatment that conforms to the surface of the brain, and not harm the underlying tissue. A device that meets these requirements is an osmotic transport device (OTD), such as a hollow fiber-hydrogel device (HFHD). An OTD consists of a hollow-fiber membrane module embedded in a hydratable material for water extraction by osmotically driven flux. Use of an OTD for treatment of edema allows for a novel method of treating cerebral edema: direct osmotherapy. Direct osmotherapy is the removal of water from edematous tissue by direct contact. For cerebral edema, direct osmotherapy requires that the brain tissue be exposed (i.e., by a craniectomy or burr hole).
An OTD in the form of an HFHD has been recently used for enhancing the survival of mice with severe cerebral edema induced by water intoxication.16 The HFHD, one type of an OTD, is capable of removing water from the tissue by direct osmotherapy. In the present study, the reduction of edema for sTBI is compared for treatment with a craniectomy and direct osmotherapy by an OTD. The mechanisms of water removal from brain tissue are considered for these two treatments, and a discussion is provided on the components of the optimal OTD.
Methods
Osmotic transport device
The specifics of the OTD (Fig. 1) are similar to those of the HFHD (described elsewhere).16
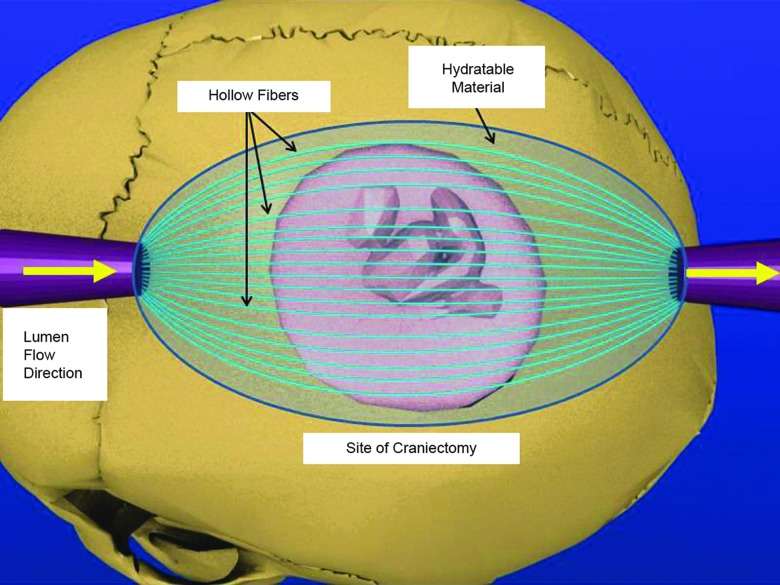
FIG. 1.
Schematic of direct osmotherapy by an osmotic transport device (OTD). After craniectomy and opening of the dura, the OTD is placed with a hydratable material onto the exposed brain surface. Flexible hollow fibers are placed to maximize the surface area treated. An aqueous solution containing an impermeable osmotic agent is pumped across the injured surface area through the semipermeable hollow fiber membrane lumen. The hollow fiber membrane is selected such that it rejects the osmotic agent, but allows easy passage of ions and water. The lumen solution induces an osmotic pressure driving force for water removal. The rate of pumping is controlled to allow fluid from the tissue to flow up to the membrane device as a result of the chemical potential gradient of water. A hydratable material with significantly large permeability is used to maintain membrane-tissue contact and to cover the entirety of the exposed brain tissue. Color image is available online at www.liebertpub.com/neu
Briefly, the OTD consists of hollow fibers embedded in a moldable hydratable material, such as a hydrogel or dura substitute material. The hydratable material is in direct contact with, and conforms to, the exposed injured tissue, following a craniectomy, to maximize the treatment area. A lumen solution is flowed through the hollow fibers at a flow rate so that the Reynolds number is between 50 and 100. The lumen solution consists of an osmotic agent, such as proteins or polymers, in an aqueous salt solution. The osmotic agent is chosen for its ability to: 1) induce osmotic pressure greater than the transmembrane pressure of the hollow fiber; 2) allow for a range of osmotic pressures to be achieved by varying its concentration; and 3) be impermeable to the hollow fiber pores.
In this study, the lumen solution was 350 g/L of bovine serum albumin (BSA) in artificial cerebrospinal fluid (aCSF; pH 7.4). BSA was the chosen osmotic agent because its concentration-dependent osmotic pressure range is well known17,18 and its concentration-dependent viscosity is manageable.19 The BSA is also impermeable to the hollow fibers used (regenerated cellulose, 13 kDa molecular weight cutoff [MWCO], 132294; Spectra/Por®; Spectrum Laboratories, Los Angeles, CA). aCSF, chosen in order to aid in ion balance, was prepared as previously described.20 The hydratable material used was an agar hydrogel (0.3% agar in aCSF). The treatment area of the cerebral cortex was 13.9±0.5 mm2 (mean±standard error of the mean [SEM]).
Animals
All experiments were conducted under protocols (A-2010-0018) approved by the University of California Riverside Institutional Animal Care and Use Committee. Adult female CD-1 mice were used in all experiments.
The six experimental groups were: 1) no injury (control); 2) no treatment (TBI only); 3) TBI plus treatment with a craniectomy only; 4) TBI plus treatment with a craniectomy and a hydrogel placed on the exposed tissue; 5) TBI plus treatment using an OTD without BSA (only aCSF, no osmotic agent); and 6) TBI plus direct osmotherapy by an OTD.
Surgical technique and controlled cortical impact model
Before induction of cerebral edema by controlled cortical impact (CCI), animals were anesthetized with an 80-mg/kg ketamine, 10-mg/kg xylazine mixture (0.09% of body weight). After determining an adequate plane of anesthesia through the loss of paw pinch reflex, surgical procedures began. Reflex activity was monitored continuously throughout the entire study and supplemental doses of half of the initial dose were administered as needed.
Anesthetized animals were placed into a standard rodent stereotactic frame. A mid-line skin incision was made and reflected. A right-sided craniectomy was performed (anterior border: coronal suture; posterior border: lambdoid suture; medial border: mid-line; lateral border: temporalis attachment).
sTBI was induced by CCI. A 3-mm impactor tip was discharged at a 20-degree angle with a velocity of 5.0 m/s with a 200-ms dwell time and an impact depth of 1.0 mm. After TBI was induced, the skull was replaced for 3 h to allow for the formation of edema. Cyanoacrylate gel (Plastics One, Roanake, VA) was used to adhere the bone flap to the skull, closing the craniectomy. For the TBI-only group, the skull remained for an additional 2 h (total of 5 h).
After the formation of edema (3 h), treatment began and lasted for 2 h. For all treatment groups, the bone flap was removed after 3 h and the dura was carefully and atraumatically opened with microdissection. After dura removal, treatment began and was either a craniectomy only, a craniectomy with a hydrogel, an OTD without BSA, or an OTD.
Reduction of cerebral edema was determined by analyzing brain water content. After the treatment procedure, brains were dissected out postmortem, separated into four pieces (right-left cerebral hemispheres and right-left cerebellum pieces), and subjected to wet-dry weight comparisons to determine percent water content, as previously described.21,22 Intergroup comparisons of brain tissue water content were done using one-way analysis of variance and post-hoc Bonferroni's tests. Brain tissue water content is presented as mean±SEM. During the monitoring period, animals that expired were not used for brain water content data analysis.
Results
Brain water content analysis
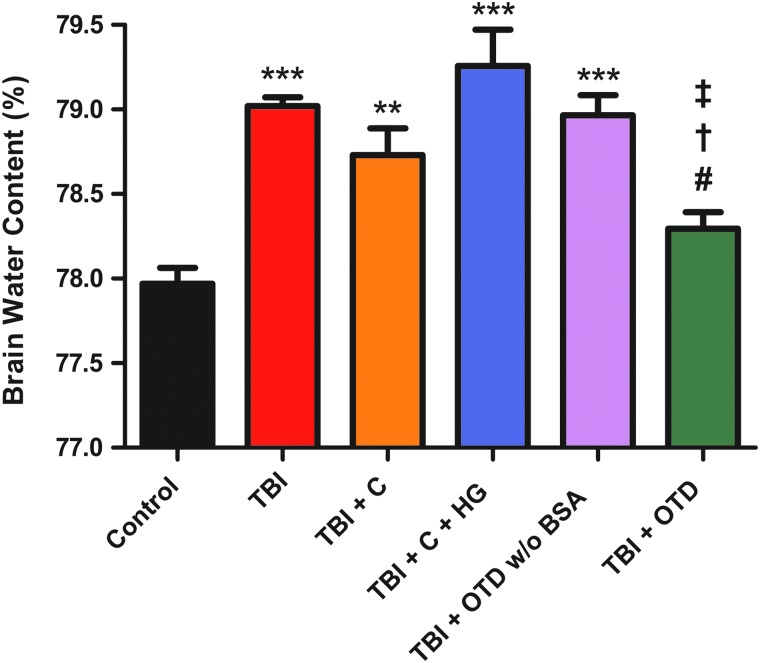
Brain water content of the right and left cerebral hemispheres was analyzed (Table 1). Right cerebral hemisphere brain water content of untreated TBI animals (TBI, 79.02±0.051%; n=5) was significantly higher than that of control animals (control, 77.97±0.094%; n=7; p<0.001). Right cerebral hemisphere brain water content of TBI animals treated with a craniectomy (TBI+C, 78.73±0.159%; n=5) was significantly higher than that of control animals (p<0.01). Right cerebral hemisphere brain water content of TBI animals treated with a craniectomy and a hydrogel (TBI+C+HG, 79.29±0.215%; n=5) was significantly higher than that of control animals (p<0.001). Right cerebral hemisphere brain water content of TBI animals treated using an OTD without BSA (TBI+OTD w/o BSA, 78.97±0.119%; n=5) was significantly higher than that of control animals (p<0.001; Fig. 2).
Table 1.
Brain Water Content
| Brain water content (%) | |||
|---|---|---|---|
| Groups | Left cerebral hemisphere | Right cerebral hemisphere | Cerebellum |
| Control | 77.79±0.130 | 77.97±0.094 | 74.90±0.168 |
| TBI | 77.93±0.082 | 79.02±0.051 | 74.56±0.452 |
| TBI+C | 78.08±0.112 | 78.73±0.159 | 75.47±0.100 |
| TBI+C+HG | 78.03±0.199 | 79.26±0.215 | 75.42±0.197 |
| TBI+OTD w/o BSA | 78.14±0.1632 | 78.97±0.119 | 75.19±0.223 |
| TBI+OTD | 77.78±0.087 | 78.29±0.099 | 75.41±0.223 |
Brain water content of left cerebral hemisphere tissue and right cerebral hemisphere tissue. The experimental groups are untreated, uninjured control animals (control), TBI animals receiving no treatment (TBI), TBI animals treated with craniectomy only (TBI+C), TBI animals treated with a craniectomy and hydrogel (TBI+C+HG), TBI animals treated using an OTD without BSA (TBI+OTD w/o BSA), and TBI animals treated using an OTD (TBI+OTD).
TBI, traumatic brain injury; C, craniectomy; HG, hydrogel; OTD, osmotic transport device; BSA, bovine serum albumin.
FIG. 2.
Brain water content. Right cerebral hemisphere brain water content (%) is shown for untreated, uninjured control animals (control), injured animals receiving no treatment (TBI), injured animals treated with craniectomy only (TBI+C), injured animals treated with a craniectomy and a hydrogel (TBI+C+HG), injured animals treated using an OTD without BSA (TBI+OTD w/o BSA), and injured animals treated using an OTD (TBI+OTD). Right cerebral hemisphere brain water content was significantly higher for untreated TBI animals (***p<0.001 vs. control), animals treated with a craniectomy only (**p<0.01 vs. control), animals treated with a craniectomy and a hydrogel (***p<0.001 vs. control), and animals treated using an OTD without BSA (***p<0.001 vs. control), compared to control animals. No significance was observed between right cerebral hemisphere brain water content of control animals and that of animals treated using an OTD (p>0.05). Statistical significance was also observed for right cerebral hemisphere brain water content between untreated TBI animals and animals treated using an OTD (#p<0.05), for right cerebral hemisphere brain water content between TBI animals treated with a craniectomy and a hydrogel and animals treated with an OTD (†p<0.001), and for right cerebral hemisphere brain water content between TBI animals treated with an OTD without BSA and animals treated with an OTD (‡p<0.05). No significance was observed between any of the other groups. TBI, traumatic brain injury; C, craniectomoy; HG, hydrogel; OTD, osmotic transport device; BSA, bovine serum albumin. Color image is available online at www.liebertpub.com/neu
TBI animals treated with an OTD had significantly lower right cerebral hemisphere brain water content (TBI+OTD, 78.29±0.099%; n=5), compared to that of untreated TBI animals (p<0.05). Similarly, right cerebral hemisphere brain water content of TBI animals treated with an OTD was significantly lower than that of the TBI animals treated with a craniectomy and a hydrogel (p<0.001) and TBI animals treated with an OTD without BSA (p<0.05). Finally, right cerebral hemisphere brain water content of TBI animals treated with an OTD was not significantly different, compared to that of control animals (p>0.05).
For all treatment groups, no difference was observed for brain water content of the contralateral cerebral hemisphere (left cerebral hemisphere), compared to that of control animals (p>0.05). Similarly, no difference was observed for brain water content of the cerebellum between all groups (Table 1).
Discussion
Here, we have utilized an OTD to reduce cerebral edema after TBI with direct osmotherapy. First, in our model of CCI-induced post-traumatic edema, we observed a significant increase in brain water content 5 h after TBI. Second, craniectomy alone did not reduce brain water content after TBI. Third, an OTD with BSA, but not either gel alone or OTD without BSA, significantly reduced brain water content after TBI to a level indistinguishable from control (nonimpacted) animals.
Although there has been conflicting evidence on the efficacy of craniectomy for the treatment of cerebral edema,8 decompressive craniectomy is still performed on the most severe cases of brain swelling. Decompressive craniectomy treats elevated ICP to prevent transtentorial herniation, rather than directly treating cerebral edema. An OTD has been shown to directly treat edema.16,23 In this study, we examined the efficacy of craniectomy and an OTD for the reduction of edema after CCI in a mouse model. Animals treated with a craniectomy only did not have lower brain water content than untreated TBI animals given that craniectomy primarily treats increased ICP resulting from swelling. However, because an OTD directly treats edema, animals treated with an OTD had significantly lower brain water content than untreated TBI animals. The combination of a craniectomy and an OTD is more advantageous than a craniectomy only because the combined therapy will also directly reduce edema with direct osmotherapy.
Together with our previous data,16,23 we now have an improved understanding of direct osmotherapy by an OTD. We have shown that individual OTD components are unable to reduce edema. Craniectomy plus hydrogel (Fig. 2; TBI+C+HG vs. TBI+OTD), craniectomy plus hollow fibers (containing an osmotic agent, no hydratable material),23 and craniectomy plus OTD without an osmotic agent (Fig. 2; TBI+OTD w/o BSA vs. TBI+OTD) are all incapable of lowering brain water content. However, when all components of an OTD are used, edema can be directly treated. The roles of the hydratable material are to maintain a liquid-liquid interface between the edematous tissue and hollow fiber membranes and structurally support the hollow fiber membranes. The osmotic agent in the lumen solution provides the driving force for water removal from, whereas the aCSF in the lumen solution maintains ionic homeostasis between the OTD and tissue. The role of the hollow fiber membrane is to allow unhindered transport of ions, metabolites, and water between the lumen solution and the injured tissue while completely rejecting the osmotic agent from transporting into the tissue (which maintains the driving force). The role of the craniectomy is to expose the injured tissue for direct contact with the OTD and simultaneously reduce ICP. Herein, our data indicate that all components of the OTD are necessary for treatment of cerebral edema and our current data significantly expand upon our earlier publication on this device16 in identifying the likely mechanism of the OTD in water extraction.
Mechanisms of water removal from brain tissue
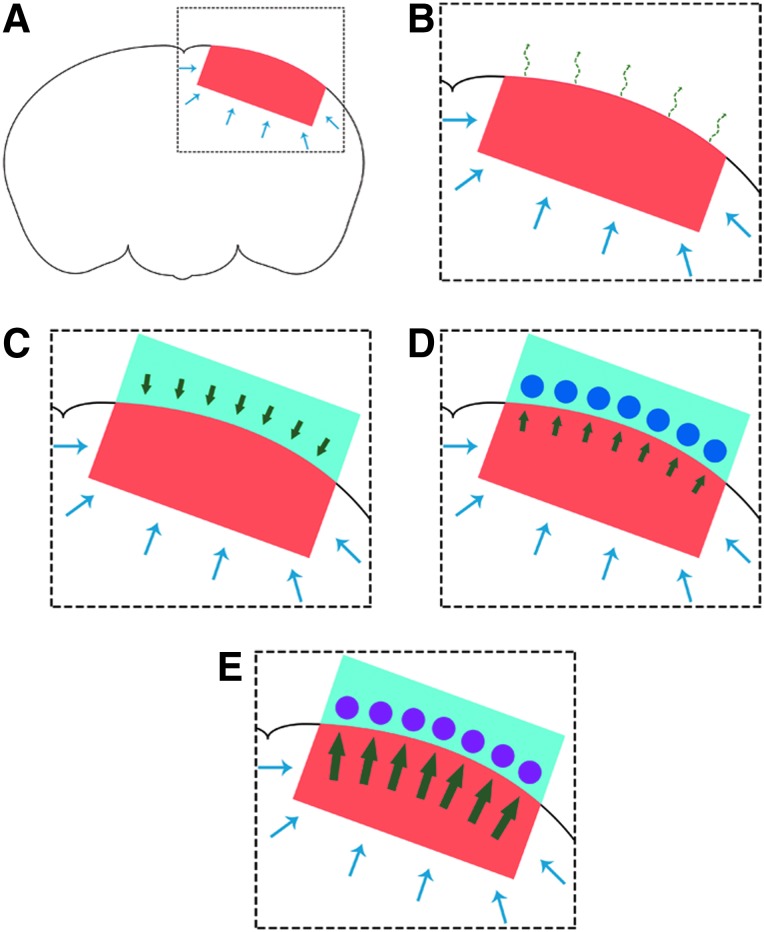
Any treatment aimed at treating cerebral edema must be capable of removing water from the injured tissue in order to prevent further (secondary) damage. Here, possible mechanisms for removal of water from the studied treatment groups is examined (Fig. 3). The transport of water into the injured tissue (red) after a TBI is shown by the blue arrows (Fig. 3A). For the treatments described in this work, the transport of water in response to the injury remains unaffected.
FIG. 3.
Water transport and removal in the craniectomy, craniectomy and hydrogel, OTD without BSA, and direct osmotherapy treatments. (A) Schematic of the mouse brain and the injury site after TBI (from CCI) is presented with the injury site and penetration into the tissue shown in red. The water transport into the injured tissue is shown by the blue arrows. (B) Schematic of the injured mouse brain showing water transport and removal with a craniectomy. The possible mechanism of indirect water removal by a craniectomy is evaporation (dashed curved green arrows), which can be approximated by Henry's law.24 (C) Schematic of the injured mouse brain showing water transport with only the hydrogel (light blue) placed on the tissue after a craniectomy. As a result of the chemical potential gradient of water, water flows (by diffusion) from the hydrogel into the brain tissue (green arrows). The water diffusion can be approximated by Fick's law.24 (D) Schematic of the injured mouse brain showing water transport and removal by an OTD with no osmotic agent in the lumen solution. The aCSF lumen solution allows for the water flux to be in the direction (green arrows) of the hollow fibers (blue circles). The water flux is approximated by Fick's law.24 (E) Schematic of the injured mouse brain showing water transport and removal by direct osmotherapy with an OTD. The osmotic agent allows for the chemical potential gradient of water to be towards the OTD. Osmotically driven flow25 causes the water to be transported (green arrows) into the hollow fibers (pink circles) for removal from the injured tissue. Direct osmotherapy with an OTD has the capability for enhanced water removal, compared to diffusive flux (increased magnitude of the green arrows compared to panel D). Color image is available online at www.liebertpub.com/neu
If the injury is treated with a craniectomy, the water is indirectly removed. While a craniectomy is primarily for reducing the ICP, water may be removed by evaporation from the surface or the brain (dashed curved green arrows; Fig. 3B). This mechanism of water removal is driven by the amount of the water in the tissue and the partial pressure of the water in the air that the tissue is exposed to. This can be approximated by Henry's law.24
When a hydrogel (light blue) is applied to the exposed tissue after a craniectomy, water is transported into the tissue as a result of the chemical potential of water in the hydrogel, compared to its chemical potential in the brain tissue (Fig. 3C). For the majority of hydrogels, the gradient of the chemical potential of water is toward the brain tissue and thus the diffusion of water proceeds into the tissue until equilibrium is established (chemical potential of water in the hydrogel is equal to the chemical potential of water in the tissue). The green arrows display the diffusive water flux into the brain tissue from the hydrogel. The flux can be approximated by Fick's law, assuming that the diffusion coefficient of the water is independent of its concentration.24
However, when hollow fibers are placed within the hydrogel and an aCSF lumen solution (no osmotic agent) is passed through the hollow fibers (blue circles), there is a flux of water from the tissue into the hydrogel, then into the hollow fibers, and the water is ultimately transported away from the injured tissue (Fig. 3D). The chemical potential of water in the aCSF solution is such that the gradient of water diffusion is shifted toward the hollow fibers. The flux can also be approximated by Fick's law.24 However, in this case, the flux, driven by the gradient of the chemical potential of water, is from the brain tissue to the hollow fibers containing aCSF (green arrows).
Further, when an osmotic agent is used within the lumen solution, the OTD is capable of treating the tissue by direct osmotherapy. With this treatment method, the flux of water, still with the chemical potential gradient of water in the direction of the hollow fibers (pink circles), is osmotically driven (Fig. 3E). The osmotically driven flux, J, can be approximated using the Kedem-Katchalsky equation (Equation 1)25:
 |
where ΔP is the transmembrane pressure, Δπ is the osmotic pressure, σ is the reflection coefficient that provides a measure of the membrane permselectivity to the osmotic agent, μ is the solution viscosity, Rm is the membrane resistance to the flow of water, and Rh is the hydratable material resistance to the flow of water.
Optimal OTD design includes minimization of the transmembrane pressure and resistances of the hollow fibers and hydratable material, maximization of the osmotic pressure within the lumen solution, and selection of the hollow fiber membrane MWCO such that the osmotic agent is completely rejected (preventing transport into the tissue) (σ=1).
With a sufficiently high osmotic pressure, such that the osmotic pressure is much larger than the transmembrane pressure (Δπ>ΔP), the osmotically driven flux is greater than the diffusive flux (shown by the increased magnitude of the green arrows). Osmotically driven water flux has the capability of controllability, which can be altered to provide a therapeutic effect for any duration and/or magnitude of edema.
Device design and efficacy
Developing an OTD to treat severe cerebral edema presents several technical challenges. First, the lumen solution needs to be carefully selected such that it provides a chemical potential gradient of water capable of removing water from the injured tissue. Herein, BSA was carried in aCSF to maintain ion similarities between the lumen solution and the tissue. In this study, the beneficial effect of an impermeable osmotic agent (BSA) was examined and, as observed by analyzing the right cerebral hemisphere brain water content, the osmotic agent is required for the reduction in brain water content after TBI.
Herein, we also showed that the selection of a hydratable material is paramount. The chosen hydratable material must offer structural support for the hollow fibers, provide minimal resistance to water transport, and prevent water flux from the hydratable material into the tissue (i.e., water flows only away from the injured tissue). Here, an agar hydrogel was utilized as the hydratable material. Though an agar hydrogel is capable of structural support and allowing easy passage of water, it fails to have negligible water transport in the direction of the tissue (specifically in the absence of an OTD). When an agar hydrogel is used with a craniectomy, the brain water content has a mean higher than that of untreated TBI animals. This observation is explained by the larger water content of the hydrogel (approximately 99%), compared to that of the brain tissue (approximately 78%). The use of a 0.3% agar hydrogel can be replaced with alternative hydratable materials, such as other U.S. Food and Drug Administration–approved materials (i.e., dura substitutes, which are hydratable collagen matrices), to achieve optimal direct osmotherapy by an OTD. An ideal hydratable material would have water content similar to, or lower than, that of uninjured brain tissue.
Implications for treatment
One limitation of our study is that our results are confined to an acute treatment of cerebral edema after a TBI. Here, edema was allowed to form for 3 h followed by a 2 h treatment; however, edema is known to increase over 24–48 h.21 Therefore, future studies need to address the treatment of maximum edema formation to determine the effectiveness direct osmotherapy by an OTD and length of treatment required to completely and irreversibly reduce edema after sTBI.
Another limitation of our study is that our results only show a reduction in brain water content; however, whether this translates to improved neurological outcome is unknown. Future studies also need to address the effects of direct osmotherapy by an OTD on the histological outcome, neurological function of animals receiving a sTBI, and validation studies in larger animal models of cerebral edema.
One advantage of our OTD device is that it appears to successfully normalize brain water content without dehydrating adjacent healthy tissue. Left (contralateral to injury site) cerebral hemisphere brain water content (Table 1) and cerebellum brain water content (Table 1) were not different from that of control animals. Thus, the OTD could be used in focal injuries without overdehydration of normal brain tissue.
The current design of the OTD is capable of significantly reducing brain water content in mice, which have brains more than 3000 times smaller than human brains. Though the effective therapeutic diffusional distance of the OTD has not been the focus of current or previous studies, it will be addressed in future experiments. However, careful scale-up of the ratio between the treatment surface area and the brain volume will allow for clinical translation for treatment of TBI patients.
Finally, future studies will also incorporate online measurement of edema/water content for real-time alterations of treatment duration and/or OTD parameters (such as BSA concentration and water flux) as part of a feedback control system for edema treatment.
Conclusion
In summary, we have validated the use of an OTD to directly remove water and reduce cerebral edema in a sTBI model. Future studies will need to validate the efficacy of an OTD to treat edema in a longer-term study, understand the device's effectiveness for improving histological and neurological outcome, optimize OTD device parameters, and incorporate online feedback control. We envision that, given the appropriate device parameters, direct osmotherapy with an OTD can be used to treat and reduce moderate to severe cerebral edema regardless of etiology.
Acknowledgments
This work was supported by the National Institutes of Health (NIH K08 grant NS-059674; to D.K.B.). D.W.M. was supported by an NSF IGERT Video Bioinformatics Fellowship (grant no.: DGE 0903667). All ongoing studies are being carried out on protocols approved by the institutional animal care and use committee of the University of California Riverside. Portions of this work were presented in abstract form and poster/oral form at UC Neurotrauma, September 9, 2012; Neurotrauma 2012, July 25, 2012; and The 13th Annual UC Systemwide Bioengineering Symposium at University of California Berkeley, June 22, 2012.
Author Disclosure Statement
This device and its applications are described in a patent application titled Compositions and Methods for Reducing Edema (# 20,130,115,267) submitted by the authors and the University of California Riverside on November 1, 2012. There is no commercial support at this time. The authors have no conflicts of interest to report.
References
- 1.Marmarou A. (2003). Pathophysiology of traumatic brain edema: current concepts. Acta Neurochir. (Suppl.) 86, 7–10 [DOI] [PubMed] [Google Scholar]
- 2.Park E., Bell J.D., and Baker A.J. (2008). Traumatic brain injury: can the consequences be stopped? Can. Med. Assoc. J. 178, 1163–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kettenmann H., and Ransom B.R. (2005). Neuroglia, 2nd ed. Oxford University Press: London [Google Scholar]
- 4.Bullock M.R., Povlishock J.T., Carney N.A., Ghajar J., Bratton S.L., Chestnut R.M., McConnell Hammond F.F., Harris O.A., Hartl R., Manley G.T., Nemecek A., Newell D.W., Rosenthal G., Schouten J., Shutter L., Timmons S.D., Ullman J.S., Videtta W., Wilberger J.E., and Wright D.W. (2007). Guidelines for the management of severe traumatic brain injury. J. Neurotrauma 24, S1–S106 [DOI] [PubMed] [Google Scholar]
- 5.Eisenberg H.M., Frankowski R.F., Contant C.F., Marshall L.F., and Walker M.D. (1988). High-dose barbiturate control of elevated intracranial pressure in patients with severe head injury. J. Neurosurgery 69, 15–23 [DOI] [PubMed] [Google Scholar]
- 6.Rabinstein A.A. (2006). Treatment of cerebral edema. Neurologist 12, 59–73 [DOI] [PubMed] [Google Scholar]
- 7.Timofeev I., Dahyot-Fizelier C., Keong N., Norje J., Al-Rawi P.G., Czosnyka M., Menon D.K., Kirkpatrick P.J., Gupta A.K., and Hutchinson P.J. (2009). Ventriculostomy for control of raised ICP in acute traumatic brain injury. Acta Neurochir. (Suppl.) 102, 99–104 [DOI] [PubMed] [Google Scholar]
- 8.Cooper D.J., Rosenfeld J.V., Murray L., Arabi Y.M., Davies A.R., D'Urso P., Kossman T., Ponsford J., Seppelt I., Reilly P., andWolfe R.; DECRA Trial Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. (2011). Decompressive craniectomy in diffuse traumatic brain injury. N. Engl. J. Med. 364, 1493–1502 [DOI] [PubMed] [Google Scholar]
- 9.Keyrouz S.G., Dhar R., and Diringer M.N. (2008). Variation in osmotic response to sustained mannitol administration. Neurocrit. Care 9, 204–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castillo L.B., Bugedo G.A., and Paranhos J.L. (2009). Mannitol or hypertonic saline for intracranial hypertension? A point of view. Crit. Care Resusc. 11, 151–154 [PubMed] [Google Scholar]
- 11.Hariri R.J. (1994). Cerebral edema. Neurosurg. Clin. North Am. 5, 687–706 [PubMed] [Google Scholar]
- 12.Bingaman W.E., and Frank J.I. (1995). Malignant cerebral edema and intracranial hypertension. Neurol. Crit. Care 13, 479–509 [PubMed] [Google Scholar]
- 13.Suarez J.I., Qureshi A.I., Bhardwaj A., Williams M.A., Schnitzer M.S., Mirski M., Hanley D.F., and Ulatowski J.A. (1998). Treatment of refractory intracranial hypertension with 23.4% saline. Crit. Care Med. 26, 1118–1122 [DOI] [PubMed] [Google Scholar]
- 14.Schwartz M.L., Tator C.H., Rowed D.W., Reid S.R., Meguro K., and Andrews D.F. (1984). The University of Toronto Head Injury Treatment Study: A prospective, randomized comparison of pentobarbital and mannitol. Can. J. Neurol. Sci. 11, 434–440 [DOI] [PubMed] [Google Scholar]
- 15.Ward J.D., Becker D.P., Miller J.D., Choi S.C., Marmarou A., Wood C., Newlon P.G., and Keenan R. (1985). Failure of prophylactic barbiturate coma in the treatment of severe head injury. J. Neurosurg. 62, 383–388 [DOI] [PubMed] [Google Scholar]
- 16.McBride D.W., Hsu M.S., Rodgers V.G.J., and Binder D.K. (2012). Improved survival following cerebral edema using a novel hollow fiber-hydrogel device. J. Neurosurg. 116, 1389–1394 [DOI] [PubMed] [Google Scholar]
- 17.Vilker V.L., Colton C.K., and Smith K.A. (1981). The osmotic pressure of concentrated protein solutions: effect of concentration and pH in saline solutions of bovine serum albumin. J. Coll. Interf. Sci. 79, 548–566 [Google Scholar]
- 18.Yousef M.A., Datta R., and Rodgers V.G.J. (1998). Understanding nonidealities of the osmotic pressure of concentrated bovine serum albumin. J. Coll. Interf. Sci. 207, 273–282 [DOI] [PubMed] [Google Scholar]
- 19.Buccola J.R. (2010). Significance of the choice of a concentration variable on viscosity model predictions for BSA in aqueous media. Bioengineering, University of California Riverside: Riverside, CA, 2010 [Google Scholar]
- 20.Csenkér É., Dioszeghy P., Fekete I., and Mechler F. (1982). Ion concentrations in serum and cerebrospinal fluid of patients with neuromuscular diseases. Arch. Psychiatry Nervenkr. 231, 251–258 [DOI] [PubMed] [Google Scholar]
- 21.Zweckberger K., Eros C., Zimmerman R., Kim S.W., Engel D., and Plesnila N. (2006). Effect of early and delayed decompressire craniectomy on secondary brain damage after controlled cortical impact in mice. J. Neurotrauma 23, 1083–1093 [DOI] [PubMed] [Google Scholar]
- 22.Gill A.S., Rajneesh K.F., Owen C.M., Yeh J., Hsu M.S., and Binder D.K. (2011). Early optical detection of cerebral edema in vivo. J. Neurosurg. 114, 470–477 [DOI] [PubMed] [Google Scholar]
- 23.McBride D.W. (2013). Further understanding the physical phenomena of crowded protein osmotic pressure and its application to medical devices. Ph.D. dissertation, University of California Riverside, Riverside, CA [Google Scholar]
- 24.Truskey G.A., Yuan F., and Katz D.F. (2004). Transport Phenomena in Biological Systems. Prentice Hall: Upper Saddle River, NJ [Google Scholar]
- 25.Kedem O., and Katchalsky A. (1958). Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochem. Biophys. Acta 27, 229–246 [DOI] [PubMed] [Google Scholar]