Abstract
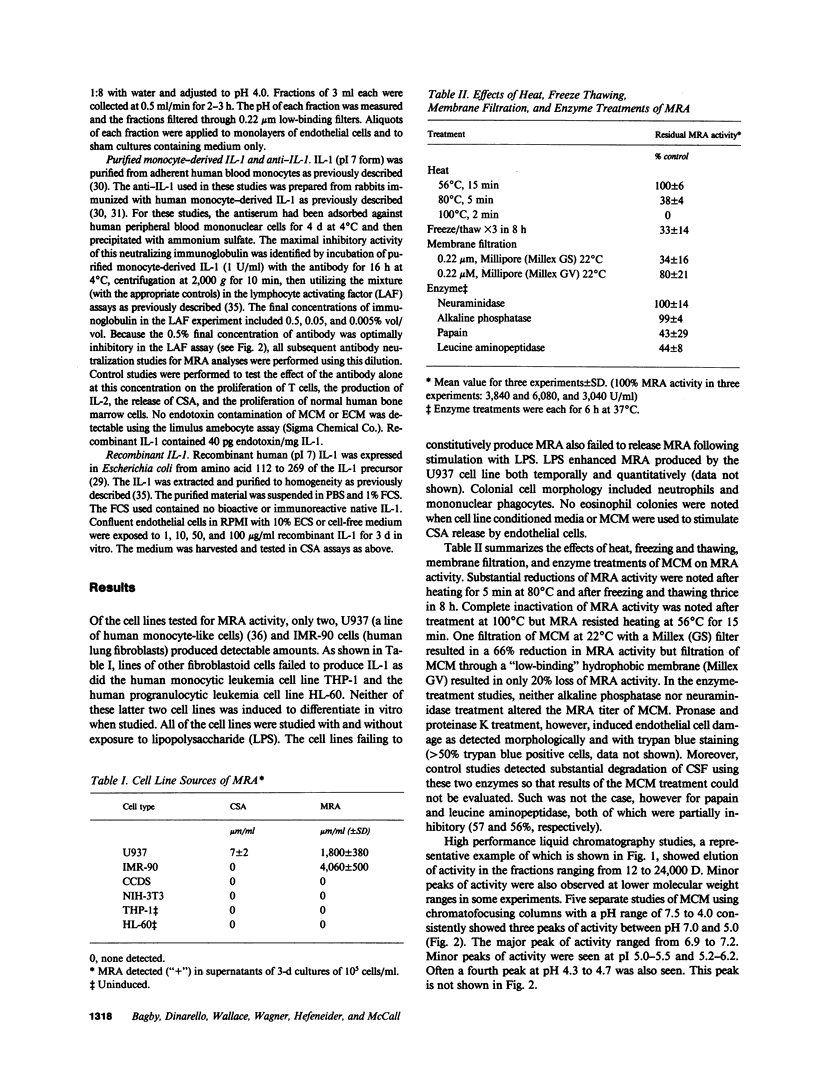
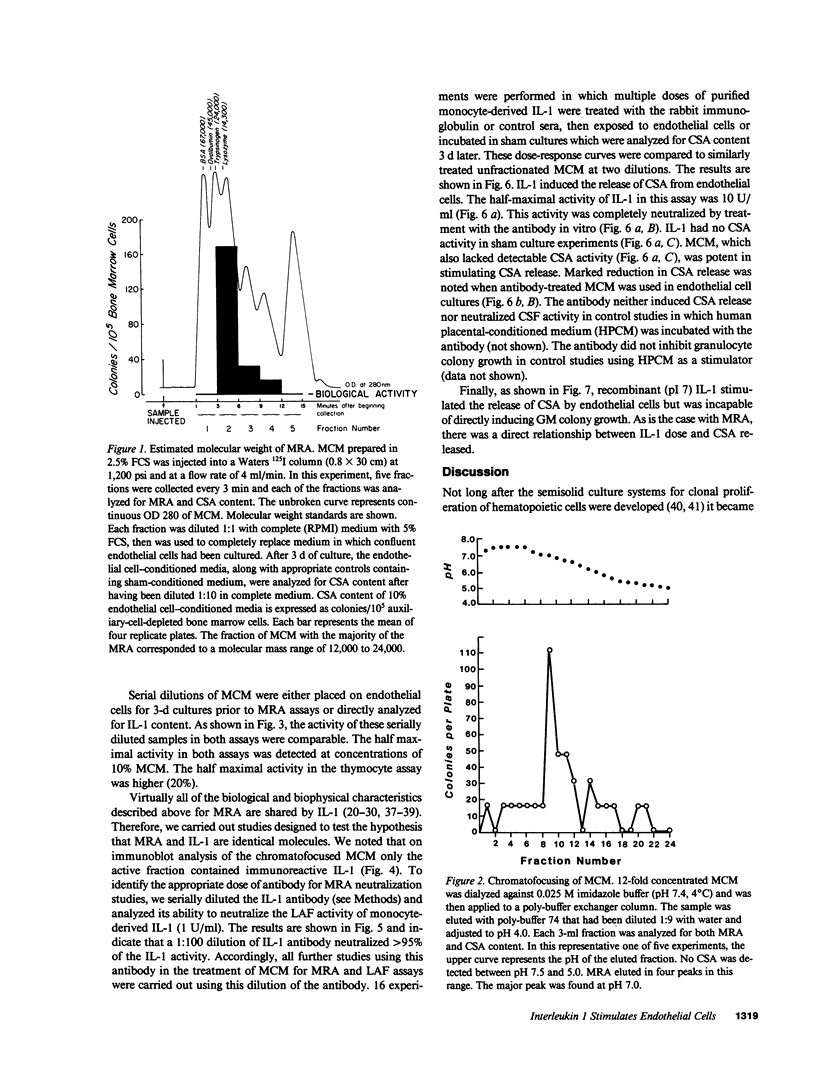
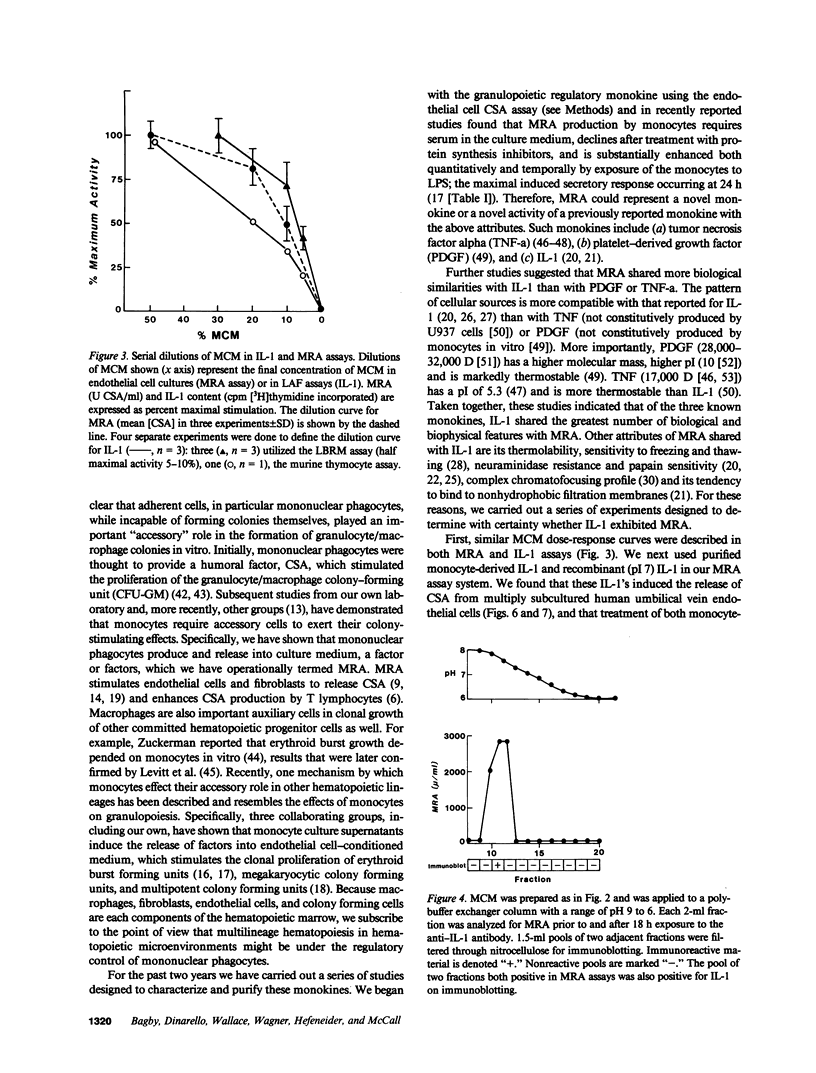
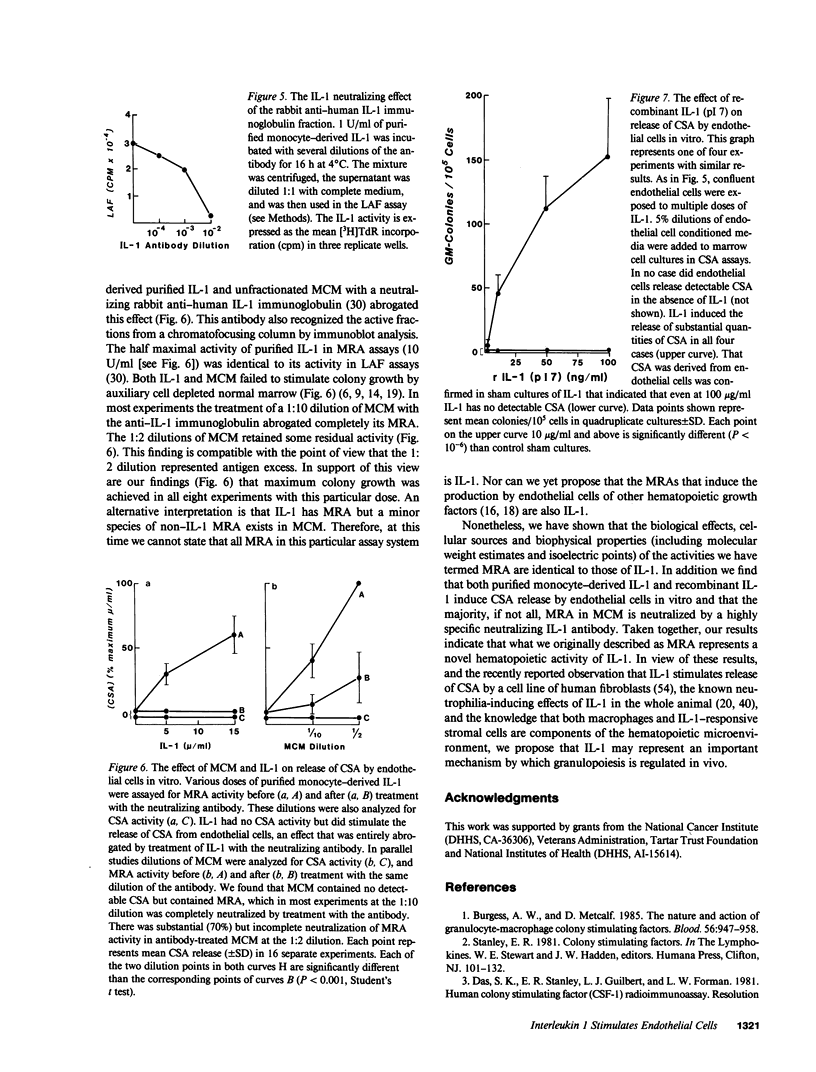
Studies designed to characterize monocyte-derived recruiting activity (MRA) a monokine that stimulates endothelial cells to produce granulocyte macrophage-colony-stimulating activity (CSA) by endothelial cells, show that it is a thermolabile protein of from 12,000 to 24,000 D which, on chromatofocusing, shows three separate peaks of eluted activity from pH 7.5 to 5.0. Because these and many other properties of MRA are identical to those of interleukin 1 (IL-1), we tested the hypothesis that MRA and IL-1 are identical. We cultured vascular endothelial cells with various concentrations of purified native and recombinant IL-1 (pI 7 form), then tested the endothelial cell supernatants for GM-CSA. Purified native IL-1 and recombinant IL-1 stimulated endothelial cells to release CSA. The MRA of native IL-1, recombinant IL-1, and unfractionated monocyte conditioned medium was neutralized by a highly specific rabbit anti-human IL-1 antiserum. Chromatofocusing fractions that contained MRA contained immunoreactive IL-1 on immunoblotting and the bioactivity was neutralized completely by treatment with the antiserum. We conclude that IL-1 induces the release of CSA by vascular endothelial cells, that IL-1 is constitutively produced by monocytes in vitro, and that MRA and IL-1 are biologically, biophysically and, immunologically identical.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Kohr W. J., Hass P. E., Moffat B., Spencer S. A., Henzel W. J., Bringman T. S., Nedwin G. E., Goeddel D. V., Harkins R. N. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985 Feb 25;260(4):2345–2354. [PubMed] [Google Scholar]
- Amento E. P., Kurnick J. T., Epstein A., Krane S. M. Modulation of synovial cell products by a factor from a human cell line: T lymphocyte induction of a mononuclear cell factor. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5307–5311. doi: 10.1073/pnas.79.17.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascensao J. L., Vercellotti G. M., Jacob H. S., Zanjani E. D. Role of endothelial cells in human hematopoiesis: modulation of mixed colony growth in vitro. Blood. 1984 Mar;63(3):553–558. [PubMed] [Google Scholar]
- Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., Dinarello C. A. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby G. C., Jr, McCall E., Bergstrom K. A., Burger D. A monokine regulates colony-stimulating activity production by vascular endothelial cells. Blood. 1983 Sep;62(3):663–668. [PubMed] [Google Scholar]
- Bagby G. C., Jr, McCall E., Layman D. L. Regulation of colony-stimulating activity production. Interactions of fibroblasts, mononuclear phagocytes, and lactoferrin. J Clin Invest. 1983 Feb;71(2):340–344. doi: 10.1172/JCI110774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby G. C., Jr, Rigas V. D., Bennett R. M., Vandenbark A. A., Garewal H. S. Interaction of lactoferrin, monocytes, and T lymphocyte subsets in the regulation of steady-state granulopoiesis in vitro. J Clin Invest. 1981 Jul;68(1):56–63. doi: 10.1172/JCI110254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Burgess A. W., Metcalf D. The nature and action of granulocyte-macrophage colony stimulating factors. Blood. 1980 Dec;56(6):947–958. [PubMed] [Google Scholar]
- Cannon J. G., Dinarello C. A. Increased plasma interleukin-1 activity in women after ovulation. Science. 1985 Mar 8;227(4691):1247–1249. doi: 10.1126/science.3871966. [DOI] [PubMed] [Google Scholar]
- Chervenick P. A., LoBuglio A. F. Human blood monocytes: stimulators of granulocyte and mononuclear colony formation in vitro. Science. 1972 Oct 13;178(4057):164–166. doi: 10.1126/science.178.4057.164. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Golde D. W. Production of colony-stimulating activity by human lymphocytes. Nature. 1974 Apr 19;248(5450):703–704. doi: 10.1038/248703a0. [DOI] [PubMed] [Google Scholar]
- Das S. K., Stanley E. R., Guilbert L. J., Forman L. W. Human colony-stimulating factor (CSF-1) radioimmunoassay: resolution of three subclasses of human colony-stimulating factors. Blood. 1981 Sep;58(3):630–641. [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S. Platelet-derived growth factor. Structure, function, and roles in normal and transformed cells. J Clin Invest. 1984 Sep;74(3):669–676. doi: 10.1172/JCI111482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Mier J. W., Bernheim H. A., LoPreste G., Lynn D. L., Love R. N., Webb A. C., Auron P. E., Reuben R. C. Multiple biological activities of human recombinant interleukin 1. J Clin Invest. 1986 Jun;77(6):1734–1739. doi: 10.1172/JCI112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Duff G. W., Atkins E. The inhibitory effect of polymyxin B on endotoxin-induced endogenous pyrogen production. J Immunol Methods. 1982 Aug 13;52(3):333–340. doi: 10.1016/0022-1759(82)90005-9. [DOI] [PubMed] [Google Scholar]
- Gerson S. L., Friedman H. M., Cines D. B. Viral infection of vascular endothelial cells alters production of colony-stimulating activity. J Clin Invest. 1985 Oct;76(4):1382–1390. doi: 10.1172/JCI112114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde D. W., Cline M. J. Identification of the colony-stimulating cell in human peripheral blood. J Clin Invest. 1972 Nov;51(11):2981–2983. doi: 10.1172/JCI107124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Russell R. G. Stimulation of the proliferation of human bone cells in vitro by human monocyte products with interleukin-1 activity. J Clin Invest. 1985 Apr;75(4):1223–1229. doi: 10.1172/JCI111819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J., Gillis S., Mizel S. B., Shevach E. M., Malek T. R., Dinarello C. A., Lachman L. B., Janeway C. A., Jr Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol. 1984 Sep;133(3):1339–1345. [PubMed] [Google Scholar]
- Keating A., Singer J. W., Killen P. D., Striker G. E., Salo A. C., Sanders J., Thomas E. D., Thorning D., Fialkow P. J. Donor origin of the in vitro haematopoietic microenvironment after marrow transplantation in man. Nature. 1982 Jul 15;298(5871):280–283. doi: 10.1038/298280a0. [DOI] [PubMed] [Google Scholar]
- Knudtzon S., Mortensen B. T. Growth stimulation of human bone marrow cells in agar culture by vascular cells. Blood. 1975 Dec;46(6):937–943. [PubMed] [Google Scholar]
- Lachman L. B. Human interleukin 1: purification and properties. Fed Proc. 1983 Jun;42(9):2639–2645. [PubMed] [Google Scholar]
- Levitt L., Kipps T. J., Engleman E. G., Greenberg P. L. Human bone marrow and peripheral blood T lymphocyte depletion: efficacy and effects of both T cells and monocytes on growth of hematopoietic progenitors. Blood. 1985 Mar;65(3):663–679. [PubMed] [Google Scholar]
- Luger T. A., Stadler B. M., Luger B. M., Mathieson B. J., Mage M., Schmidt J. A., Oppenheim J. J. Murine epidermal cell-derived thymocyte-activating factor resembles murine interleukin 1. J Immunol. 1982 May;128(5):2147–2152. [PubMed] [Google Scholar]
- Martinet Y., Bitterman P. B., Mornex J. F., Grotendorst G. R., Martin G. R., Crystal R. G. Activated human monocytes express the c-sis proto-oncogene and release a mediator showing PDGF-like activity. Nature. 1986 Jan 9;319(6049):158–160. doi: 10.1038/319158a0. [DOI] [PubMed] [Google Scholar]
- McCall E., Bagby G. C., Jr Monocyte-derived recruiting activity: kinetics of production and effects of endotoxin. Blood. 1985 Mar;65(3):689–695. [PubMed] [Google Scholar]
- Metcalf D., MacDonald H. R., Chester H. M., Metcalf D., MacDonald H. R., Chester H. M. Serum potentiation of granulocyte and macrophage colony formation in vitro. Exp Hematol. 1975 Aug;3(4):261–273. [PubMed] [Google Scholar]
- Mizel S. B. Physicochemical characterization of lymphocyte-activating factor (LAF). J Immunol. 1979 Jun;122(6):2167–2172. [PubMed] [Google Scholar]
- Männel D. N., Meltzer M. S., Mergenhagen S. E. Generation and characterization of a lipopolysaccharide-induced and serum-derived cytotoxic factor for tumor cells. Infect Immun. 1980 Apr;28(1):204–211. doi: 10.1128/iai.28.1.204-211.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Stadler B. M., Siraganian R. P., Mage M., Mathieson B. Lymphokines: their role in lymphocyte responses. Properties of interleukin 1. Fed Proc. 1982 Feb;41(2):257–262. [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The cloning of normal "mast" cells in tissue culture. J Cell Physiol. 1965 Dec;66(3):319–324. doi: 10.1002/jcp.1030660309. [DOI] [PubMed] [Google Scholar]
- Quesenberry P. J., Gimbrone M. A., Jr Vascular endothelium as a regulator of granulopoiesis: production of colony-stimulating activity by cultured human endothelial cells. Blood. 1980 Dec;56(6):1060–1067. [PubMed] [Google Scholar]
- Ruscetti F. W., Chervenick P. A. Release of colony-stimulating activity from thymus-derived lymphocytes. J Clin Invest. 1975 Mar;55(3):520–527. doi: 10.1172/JCI107958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlunk T., Schleyer M. The influence of culture conditions on the production of colony-stimulating activity by human placenta. Exp Hematol. 1980 Feb;8(2):179–184. [PubMed] [Google Scholar]
- Simon P. L., Willoughby W. F. The role of subcellular factors in pulmonary immune function: physicochemical characterization of two distinct species of lymphocyte-activating factor produced by rabbit alveolar macrophages. J Immunol. 1981 Apr;126(4):1534–1541. [PubMed] [Google Scholar]
- Souza L. M., Boone T. C., Gabrilove J., Lai P. H., Zsebo K. M., Murdock D. C., Chazin V. R., Bruszewski J., Lu H., Chen K. K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986 Apr 4;232(4746):61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Wagner C. R., Vetto R. M., Burger D. R. Expression of I-region-associated antigen (Ia) and interleukin 1 by subcultured human endothelial cells. Cell Immunol. 1985 Jun;93(1):91–104. doi: 10.1016/0008-8749(85)90391-0. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Creasey A. A., Ladner M. B., Lin L. S., Strickler J., Van Arsdell J. N., Yamamoto R., Mark D. F. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science. 1985 Apr 12;228(4696):149–154. doi: 10.1126/science.3856324. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- Wood D. D. Purification and properties of human B cell-activating factor. J Immunol. 1979 Nov;123(5):2395–2399. [PubMed] [Google Scholar]
- Zucali J. R., Dinarello C. A., Oblon D. J., Gross M. A., Anderson L., Weiner R. S. Interleukin 1 stimulates fibroblasts to produce granulocyte-macrophage colony-stimulating activity and prostaglandin E2. J Clin Invest. 1986 Jun;77(6):1857–1863. doi: 10.1172/JCI112512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman K. S., Bagby G. C., Jr, McCall E., Sparks B., Wells J., Patel V., Goodrum D. A monokine stimulates production of human erythroid burst-promoting activity by endothelial cells in vitro. J Clin Invest. 1985 Feb;75(2):722–725. doi: 10.1172/JCI111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman K. S. Human erythroid burst-forming units. Growth in vitro is dependent on monocytes, but not T lymphocytes. J Clin Invest. 1981 Mar;67(3):702–709. doi: 10.1172/JCI110086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zoelen E. J., van de Ven W. J., Franssen H. J., van Oostwaard T. M., van der Saag P. T., Heldin C. H., de Laat S. W. Neuroblastoma cells express c-sis and produce a transforming growth factor antigenically related to the platelet-derived growth factor. Mol Cell Biol. 1985 Sep;5(9):2289–2297. doi: 10.1128/mcb.5.9.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]


