Abstract
Although they are less severe than a full blown concussive episodes, subconcussive impacts happen much more frequently and current research has suggested this form of head trauma may have an accumulative effect and lead to neurological impairment later in life. To investigate the acute effects that subconcussive head trauma may have on the default mode network of the brain resting-state, functional magnetic resonance was performed. Twenty-four current collegiate rugby players were recruited and all subjects underwent initial scanning 24 h prior to a scheduled full contact game to provide a baseline. Follow-up scanning of the rugby players occurred within 24 h following that game to assess acute effects from subconcussive head trauma. Differences between pre-game and post-game scans showed both increased connectivity from the left supramarginal gyrus to bilateral orbitofrontal cortex and decreased connectivity from the retrosplenial cortex and dorsal posterior cingulate cortex. To assess whether or not a history of previous concussion may lead to a differential response following subconcussive impacts, subjects were further divided into two subgroups based upon history of previous concussion. Individuals with a prior history of concussion exhibited only decreased functional connectivity following exposure to subconcussive head trauma, while those with no history showed increased connectivity. Even acute exposure to subconcussive head trauma demonstrates the ability to alter functional connectivity and there is possible evidence of a differential response in the brain for those with and without a history of concussion.
Key words: : concussion, default mode network, resting-state, subconcussive head trauma
Introduction
There is growing concern in the neuroscience community regarding the immediate and long-lasting effects from sports-related traumatic brain injury,1 especially the effects these may have on the risks for developing neurodegenerative diseases.2 Much of the research focused on sports-related traumatic brain injuries has been centered on concussions, while little attention has been placed on subconcussive impacts. However, subconcussive blows, which are below the threshold to cause or elicit any signs of a concussion,3 should not be overlooked as insignificant. Both animal and human research have shown that subconcussive blows can cause damage to the central nervous system and pathophysiological changes in the brain despite not evoking any apparent acute behavioral changes.4–6 These recent studies have identified that injury does not only come from full-blown concussive episodes but also from the repetitive nature of subconcussive blows.7 Similar to concussions, subconcussive impacts have the potential to transfer a high degree of linear and rotational acceleration forces to the brain8 and can cause pathophysiological changes in the brain.6 Yet unlike concussions, this type of repetitive head trauma in contact sports goes undiagnosed or unmanaged9,10 leading to a large number of these insults accumulated over the course of a season, let alone a career.9,10 Post-mortem studies have identified that repeated subconcussive impacts may have an accumulative effect,3 and it is thought they accelerate the cognitive aging process, leading to altered neuronal biology later in life.11
The effects of subconcussive head trauma in terms of neurocognitive, behavioral, and underlying neural substrates have not been sufficiently studied and currently are not well understood. Current research on the effects of subconcussive head trauma is divided, as there are reports that they have minimal impact on cognition12 while more recent studies have demonstrated adverse effects on cognitive and cerebral.13,14 Studies using neuropsychological testing metrics have reported mixed results, with some studies showing no effects12 while others observed chronic and acute effects from subconcussive trauma.14 Further, research shows that the accumulation of repetitive subconcussive impacts and concussions are linked to neurological impairment15 and disorders like early-onset Alzheimer's disease, dementia, depression, and even chronic traumatic encephalopathy (CTE).1,16,17
Studies using advanced neuroimaging on subconcussive head trauma are scarce and none to date have specifically investigated their acute effects. In this study, we used resting-state functional magnetic resonance imaging (rs-fMRI) to investigate the acute effects that subconcussive head trauma may have on the default mode network (DMN) of the brain. Recent fMRI reports have demonstrated alterations of resting-state functional connectivity in neurological disorders—including concussion—and have brought new insight into better understanding the pathophysiology of these disorders. Functional abnormalities of the brain are found to be associated with pathological changes in the connectivity of the structures that make up these resting-state networks.18 The examination of spontaneous oscillations of the blood-oxygen-level-dependent (BOLD) signal reflects the enduring and intrinsic properties of the brain19 and allows a characterization of possible brain dysfunction. The DMN is deactivated during attention or goal oriented tasks18 and is identified in the BOLD signal as low-frequency coherent oscillations.20
With advances in neuroimaging and data analysis techniques, the DMN has become the most studied resting-state network and a major focus in the neuroscience community.21 Accordingly, in this study we investigated the resting-state DMN after an acute exposure to subconcussive head trauma. Our central hypothesis is that repetitive subconcussive impacts received during participation in full contact sports will lead to altered functional connectivity in the DMN. Specifically, following subconcussive blows, there will be a decrease in the functional connectivity within the areas that make up the DMN. Secondary to this, we believe that a history of previous concussion will modulate this response.
Methods
Participants
Twenty-four current active collegiate athletes were recruited for this study (eight males, 16 females; average age, 20.2 years old; see Table 1 for detailed information on individual and group demographics). Specifically, all subjects under study were current collegiate rugby players and were further divided into two subgroups based upon history of previous diagnosed concussions (Hx mTBI and No Hx mTBI). All subjects signed an informed consent form and the Institutional Review Board of the Pennsylvania State University approved this protocol.
Table 1.
Demographic Information
| n | Group | Sex | Age | #mTBI | Group | Sex | Age | #mTBI | Last mTBI |
|---|---|---|---|---|---|---|---|---|---|
| 1 | No Hx mTBI | M | 22 | 0 | Hx mTBI | F | 21 | 1 | 5 |
| 2 | No Hx mTBI | F | 18 | 0 | Hx mTBI | M | 19 | 2 | 36 |
| 3 | No Hx mTBI | F | 19 | 0 | Hx mTBI | F | 20 | 1 | 13 |
| 4 | No Hx mTBI | M | 19 | 0 | Hx mTBI | M | 19 | 2 | 48 |
| 5 | No Hx mTBI | F | 19 | 0 | Hx mTBI | F | 19 | 1 | 8 |
| 6 | No Hx mTBI | M | 21 | 0 | Hx mTBI | F | 21 | 1 | 12 |
| 7 | No Hx mTBI | F | 20 | 0 | Hx mTBI | M | 20 | 2 | 24 |
| 8 | No Hx mTBI | F | 21 | 0 | Hx mTBI | M | 22 | 2 | 1 |
| 9 | No Hx mTBI | F | 19 | 0 | Hx mTBI | M | 23 | 2 | 12 |
| 10 | No Hx mTBI | F | 19 | 0 | Hx mTBI | F | 19 | 2 | 2 |
| 11 | No Hx mTBI | F | 20 | 0 | Hx mTBI | F | 22 | 1 | 48 |
| 12 | No Hx mTBI | F | 21 | 0 | Hx mTBI | F | 21 | 1 | 17 |
| Avg | 19.8 | 0 | 20.5 | 1.5 | 18.8 |
Demographic information for subjects under study, subgroups based upon history of concussion (Hx mTBI), or no previous history of concussion (No Hx mTBI). Last mTBI refers to the number of months since previous concussion.
Study design
We targeted rugby because the nature of the sport exposes players to numerous subconcussive impacts.22 Initially, quantification of subconcussive impacts was planned to be performed, yet a number of limitations made it not plausible. Rugby matches were recorded but due to limited resources and limited access to high speed cameras positioned in different angles, the standard one position video camera failed to accurately quantify the number of subconcussive impacts. Additionally, because rugby players do not wear protective equipment, including helmets,23 incorporation of commercially-available tools like the Head Impact Telemetry (HIT) system could not be employed.
Compounding the difficulty to quantify the number of subconcussive impacts in rugby are certain prominent elements of the game including the tackle and scrum.23 These events often results in a massive pileup of players and jeopardizes the accuracy of quantifying the number of subconcussive hits as players under the pile can experience anything from kicks, elbows, or any other type of blow to the head they may not be visible. To compensate for this shortcoming, an observational study of the matches was completed in order to see if the number of impacts were similar to those reported in the literature. Results from the observational study put the number of impacts at between 30 and 45 and were in agreement with those reported by in a study of 77 professional league rugby matches over the course of three seasons.24
The study was broken into two testing sessions, an initial baseline pre-game session and then a follow-up post-game session that included the acquisition of rs-fMRI. By eliminating the task, rs-fMRI excludes bias based upon performance.25 Pre-game scanning was performed 24 h prior to a scheduled full contact game, while the post-game session was performed within 24 h of the end of that game following a night's sleep. No sports-related subconcussive impacts were sustained between pre-game scanning and the actual game, as no contact practices were scheduled in the interim. Pre-game and post-game scanning was performed at the same time of the day to reduce any influence that may have been caused by circadian rhythms. No participants reported concussive symptoms at either session and additionally no players under study were diagnosed with a concussion on the field under supervision of certified athletic trainers as a part of the routine protocol of the Sport Concussion Program at the Pennsylvania State University.
MRI Set-up and Acquisition
Functional and anatomical images were acquired on a 3.0 Tesla Siemens Trio whole-body scanner (Siemens, Erlangen, Germany) using a 12-channel head coil. Subjects were asked to lay quietly with their eyes open and not fall asleep while in the scanner. Two-dimensional BOLD echo planar rs-fMRI sequence were acquired in the axial plane parallel to the anterior and posterior commissure axis covering the entire brain (3.0×3.0×3.0 mm resolution, TR=2490 ms, TE=24 ms, iPAT=none, EPI factor=74, echo spacing=0.48 ms, NSA=1, acquisition time=5:04). Three-dimensional isotropic T1 weighted magnetization prepared rapid gradient echo anatomical images were acquired in the sagittal plane parallel with the longitudinal fissure covering the entire brain (1 mm×1 mm×1 mm resolution, TE=3.46 ms, TR=2300 ms, TI=900 ms, flip angle=9°, 160 slices, iPAT=none, NSA=1).
Data analysis
We employed the commonly accepted and widely reported seed-based correlation analysis technique based upon our a priori hypothesis that subconcussive impacts will alter the areas of the brain that make up the DMN.18,19,20,32,34 Statistical Parametric Mapping (SPM) version 8 (www.fil.ion.ucl.ac.uk/spm/software/spm8), in conjunction with Functional Connectivity (CONN) toolbox (web.mit.edu/swg/software.htm) software were used for data analysis. Images were first pre-processed, which included realignment, co-registration, segmentation, normalization, and smoothing. During pre-processing, images were motion-corrected, registered with structural images, and normalized to the standard brain template from the Montreal Neurological Institute. After pre-processing, images were then band-pass filtered to 0.01 Hz-0.09 Hz and motion regressed to reduce the influence of noise.
White matter, cerebrospinal fluid, and physiological noise source reduction were taken as confounds, following the implemented CompCor strategy.26 Head motion was taken into account and rotational and translational motion parameters and their first-order temporal derivatives were regressed out. Further, whole-brain BOLD signal was excluded as a regressor to eliminate erroneous anti-correlations.27 The CONN toolbox performs seed-based correlation analysis based on the temporal low-frequency fluctuations of BOLD signals. Specifically, region of interest (ROI) analysis was performed by grouping voxels into ROIs based upon Brodmann areas. All Brodmann areas were imported as possible connections for our selected seed ROIs. The BOLD signal time series was averaged from all voxels compromising each ROI. Bi-variate correlations were calculated between each pair of ROIs as reflections of connections. Fisher transformed Z-scores were introduced to validate multiple comparisons and SPM functions were called by the CONN toolbox for spatial statistical tests. ROI-based analyses were performed for all subjects' data with a general linear model (GLM) test to determine significant resting-state connections at the individual level (1st level). Based upon first level results correlation coefficients were converted into standard scores, and an unpaired t-test was used with a threshold set at p<0.01 uncorrected to determine significant connections.
Functional connectivity is broadly defined as the temporal correlation of the BOLD signal between two distinct anatomical regions.28 Here, increases in connectivity indicate positive correlations between ROIs that are associated with increases in BOLD signal time series synchronization and reflected by higher Z-scores. Whereas decreased connectivity indicates negative correlations and decreased synchronicity between ROIs and reduction in Z-scores. ROIs were selected based upon the commonly reported and studied ROIs of the DMN and included dorsal frontal cortex, anterior prefrontal cortex, orbitofrontal cortex, medial prefrontal cortex, ventral posterior cingulate cortex, posterior cingulate cortex, precuneus, angular gyrus, retrosplenial cortex, and supramarginal gyrus.18,20,29
Results
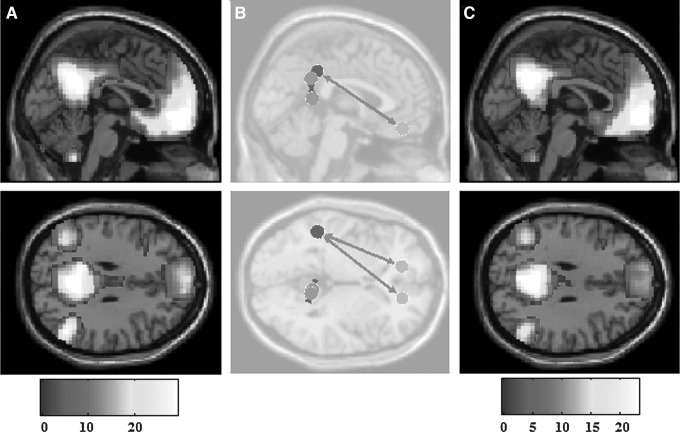
Analysis of all subjects as a whole revealed alterations to the DMN seen from pre-game to post-game in the form of both increased and decreased functional connectivity (Fig. 1). Specifically, increased connectivity between left (T=3.11; p=0.005; p-false discovery rate [FDR]=0.061) and right (T=3.30; p=0.003; p-FDR=0.07) orbitofrontal cortices and the left supramarginal gyrus. However, decreased connectivity was also observed between the right retrosplenial cingulate cortex and the right dorsal posterior cingulate cortex (T=−2.84; p=0.009; p-FDR=0.23).
FIG. 1.
Pre-game and post-game connectivity. Significant (p<0.01) differences in pre-game and post-game connectivity for all subjects. (A) Pre-game and (C) post-game default mode network activation. (B) Overall connectivity differences, with circles representing seed regions of interest, width of arrow representative of T-scores as well as directionality, (B) long range connection show increased connectivity, while short range connection depicts a decreased connectivity.
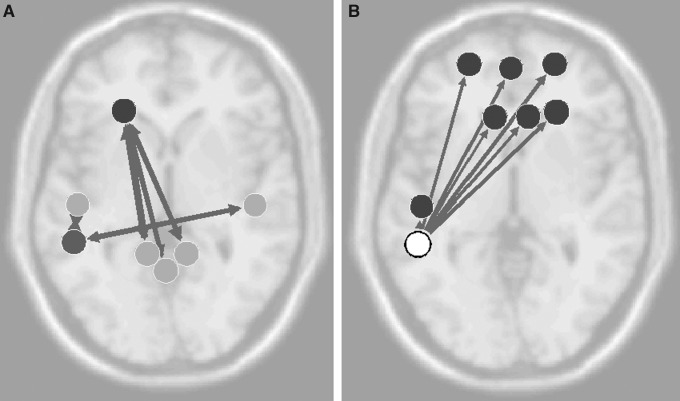
To further understand these mixed results and to infer if history of previous concussion may influence changes in functional connectivity, analysis was performed on the Hx mTBI and No Hx mTBI subgroups. The Hx mTBI cohort demonstrated only reductions in functional connectivity following exposure to subconcussive head trauma (Fig. 2). There was decreased connectivity (T=−3.52; p=0.005; p-FDR=0.112) from the left anterior prefrontal cortex and left right retrosplenial cingulate cortex, from the left inferior temporal gyrus to medial prefrontal cortex (T=−3.42; p=0.006; p-FDR=0.14), and lastly between right ventral posterior cingulate cortex and left fusiform gyrus (T=−3.31; p=0.007; p-FDR=0.17).
FIG. 2.
History of previously diagnosed concussions (Hx mTBI) connectivity differences. Significant (p<0.01) differences in pre-game and post-game connectivity in the Hx mTBI subgroup. (A) Overall connectivity differences with circles representing seed regions of interest, width of arrow representative of T-scores, as well as directionality. Examples of significant decreases in connectivity (color bar indicates the Z-score equivalent) in fMRI activation as reported for (B) left fusiform gyrus, (C) left anterior prefrontal cortex, and (D) left inferior temporal gyrus.
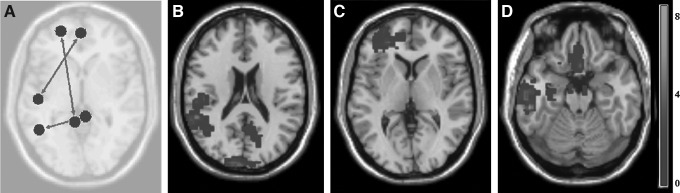
On the contrary, the No Hx mTBI group exhibited only increased connectivity (Fig. 3) changes from pre-game to post-game. Specifically, the left supramarginal gyrus increased functional connectivity to both the between left (T=3.87; p=0.003; p-FDR=0.064) and right (T=3.83, p=0.003, p-FDR=0.065) orbitofrontal cortices. Additional increases were seen between left retrosplenial cingulate cortex and right fusiform gyrus (T=3.4; p=0.006; p-FDR=0.147), as well as from the left fusiform gyrus (T=3.36; p=0.006; p-FDR=0.153) to medial prefrontal cortex.
FIG. 3.
No history of previously diagnosed concussions (Hx mTBI) connectivity differences. Significant (p<0.01) differences in pre-game and post-game connectivity in No Hx mTBI subgroup. (A) Overall connectivity differences, with circles representing seed regions of interest, width of arrow representative of T-scores as well as directionality. Examples of significant increases in connectivity (color bar indicates the Z-score equivalent) in fMRI activation as reported for (B) orbitofrontal cortex and (C) left retrosplenial cingulate cortex.
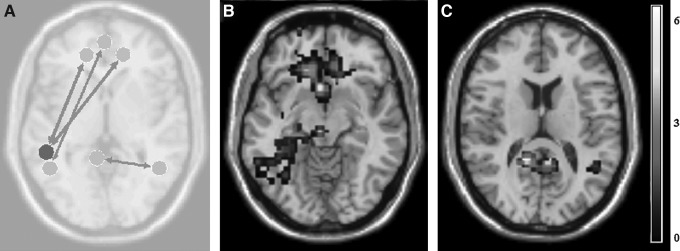
When evaluating the differences between the Hx mTBI and No Hx mTBI subgroups at pre-game scans and post-game scans, the Hx mTBI cohort displayed significant decreases in functional connectivity, compared with the No Hx mTBI subgroup (Fig. 4). Pre-game scanning showed reduced functional connectivity between the left dorsal frontal cortex with three other areas of the brain: left dorsal posterior cingulate cortex (T=−4.29; p=0.0003; p-FDR=0.007), right dorsal posterior cingulate cortex (T=−3.94; p=0.0007; p-FDR=0.016), and precuneus (T=−3.25; p=0.004; p-FDR=0.03). Additional pre-game decreases were seen from the left supramarginal gyrus to the left (T=−3.73; p=0.001; p-FDR=0.029) and right (T=−3.30; p=0.003; p-FDR=0.037) inferior temporal gyrus. Post-game analysis (Fig. 4) between subgroups revealed reduced connectivity from the left supramarginal gyrus to six other DMN ROIs: left (T=−2.79; p=0.008; p-FDR=0.045) and right (T=−3.38; p=0.003; p-FDR=0.037) anterior prefrontal cortices; left (T=−2.91; p=0.009; p-FDR=0.045) and right (T=−2.91; p=0.009; p-FDR=0.045) dorsal anterior cingulate cortices; right dorsal frontal cortex (T=−3.34; p=0.003; p-FDR=0.037); and left inferior temporal gyrus (T=−2.79; p=0.008; p-FDR=0.045).
FIG. 4.
Pre-game and post-game connectivity differences. Significant (p<0.05 false discovery rate) differences in (A) pre-game and (B) post-game connectivity between history of previous diagnosed concussions (Hx mTBI) and no Hx mTBI. Circles represent seed regions of interest, width of arrow representative of T-scores.
Discussion
In this study, we investigated the resting-state functional connectivity of the DMN in collegiate athletes that have been exposed to an acute bout of subconcussive head trauma incurred through participation of full contact sports. There are several findings of interest from this study. First, history of previous full-blown concussive episodes has the potential to infer long-term consequences as evident by significantly decreased functional connectivity within the DMN of the Hx mTBI group compared to the No Hx mTBI group assessed in the pre-game evaluation. Second, short-term exposure to subconcussive head trauma has the ability to alter functional connectivity patterns. And lastly, there is evidence to suggest that there is a differential response in the concussed and non-concussed brain to subconcussive head trauma, which may give insight to the susceptibility to recurrent concussions that has been previously reported in the literature.30,31 Moreover, it is important to address the problem of subject inhomogeneity when analyzing and interpreting results from concussion research as history of previous concussion may be an important confound.
Overall, our findings are consistent with the current advanced neuroimaging literature focused on concussion. Functional connectivity analysis of the DMN in full blown concussive episodes has revealed both hyper and hypoconnectivity. Mayer and colleagues32 reported decreases in connectivity within the DMN in the subacute phase of injury that were still present during the chronic phase. Similarly, Zhou and colleagues33 found reduced connectivity in the posterior hubs of the DMN in conjunction with increased connectivity within the ventromedial prefrontal cortex.
In our recent studies, we have found a reduced number of connections and strength of connections in the posterior cingulate cortex (PCC) and parietal cortices, in addition we saw an increased number of connections and strength of connections in the medical prefrontal cortex (MPFC).34 Regression analysis also indicated a further loss of connectivity as the number of concussive episodes increased. Here, we report significant decreases in the DMN of subjects with a history of previous concussion in both pre-game and post-game evaluations, compared with those with no prior full blown concussive events yet exposure to repetitive subconcussive head trauma. This is in line with previous research looking at more severe forms of traumatic brain injury, which have reported decreased DMN connectivity.35 Specifically, we observed reduced functional connectivity in the left supramarginal gyrus in both sessions, with greater decreases in this area noted in the post-game scan. The left supramarginal gyrus is an area that has been implicated in visuokinestics,36 motor attention in addition to verbal mediation,37 and memory retrieval.38 Further, this area also has been shown to exhibit cortical thinning in Alzheimer's diseases.39 The Hx mTBI group also showed decreased pre-game functional connectivity in the left prefrontal cortex, an area critical in decision making and emotion, and strongly associated with changes in behavior.40,41
There is limited research on the effects of subconcussive head trauma as assessed by fMRI or other advanced imaging techniques. Although using a task based approach Talavage and colleagues6 reported significant alterations in fMRI activation in a study of 11 high school football players attributed to subconcussive impacts sustained over the course of a single season, despite any clinically observable impairment. Specifically, there was decreased activation in the dorsolateral prefrontal cortex, middle and superior frontal gyri, and cerebellum that correlated to the number of subconcussive impacts. It should be noted that we looked at alterations in fMRI following an acute bout of subconcussive impacts, compared with the accumulation of them over the course of a season, which may explain why we did not reach a FDR-corrected statistical threshold for significance. We saw both increased connectivity in the prefrontal cortex and decreased connectivity in the posterior aspect of the DMN when all subjects were pooled together. This pattern of connectivity is consistent with the literature on the DMN that has been reported following concussion.32,33
However, when investigating the effect of history of prior concussion, we observed contradicting connectivity patterns between subgroups that may help explain the mixed patterns mentioned above. For the Hx mTBI cohort, we saw only decreased connectivity following exposure to subconcussive heads trauma, while the No Hx mTBI group exhibited increased connectivity. As noted earlier, increases and decreases in functional connectivity have been reported in the concussion literature; yet to date, no study has looked at the acute effects of subconcussive head trauma on the resting-state networks of the brain.
Acute changes in neurophysiology have been attributed to subconcussive head trauma and in a recent animal study Shultz and colleagues3 found that subconcussive injury caused acute neuroinflammation, despite lack of any significant axonal injury, cognitive, emotional, or sensorimotor alterations. There was a short-term increase in microglia, macrophages, and reactive astrogliosis, which returned to normal at a four week follow-up. Acute neuroinflammation has also been documented in other animal and human studies of TBI. This acute neuroinflammation may be one reason the No Hx mTBI group exhibited increased connectivity as inflammation is associated with changes in local vasculature, specifically increased blood flow and vascular permeability.42 It has also been thought that neuroinflammation may have neuroprotective qualities43 and that preconditioning in the form of gradual brain injury may allow for a so called “trauma resistance.”44 Moreover, Allen and colleagues45 reported in a rat model of repetitive brain injury that preconditioning served to preserve motor function following a severe TBI and also elicited activation of secondary sites in the brain that may aid in recovery.
Conversely, it also has been thought that repetitive head trauma, similar to neuroinflammation, may have cumulative effects leading to neurodegeneration3 and may be linked to behavioral impairments after concussion.46 This chronic neuroinflammation also could explain why we saw reduced connectivity in the pre-game scan as well as changes from pre- to post-game in the Hx mTBI cohort. Further, studies have shown that strength of functional connectivity has been strongly correlated to structural connectivity. Thereby, previous concussion that inflicts diffuse axonal injury (DAI) may alter structural connectivity which in turn decreases functional connectivity19 as seen in the Hx mTBI subgroup.
Several factors limit the conclusions that we can draw from this study. First, the number of subjects under study was small and the majority of them where female. We used a liberal statistical threshold for reporting differences between pre-game and post-game scans. However, it should be noted that corrected p values did approach significant values. Further, no subject reported any concussion-like symptoms at any time prior to scanning and therefore if significant alterations in functional connectivity were observed, we would have expected the presence of these symptoms. Additionally, quantification of subconcussive impacts was not performed due to the aforementioned limitations. However, further research is needed to investigate whether or not these changes in functional connectivity observed are acute transient alteration versus permanent neuronal reorganization.
In conclusion, impacts to the head in contact sports are unavoidable, and as serious as concussions are, subconcussive impacts happen much more often and are now being implicated as a source for the deterioration of cerebral structures and function later in life.8 It is important for future research to not only focus on concussive blows but varying degrees of head trauma that include subconcussive impacts, as well as history of previous sports-related head trauma.4 It is also important that clinicians take subconcussive head trauma seriously and change their thinking to take into account that despite not producing any identifiable symptoms, these events have the ability to alter neurophysiology, which may lead to long-term consequences.
Acknowledgments
This research was partly supported by The American Society of Radiological Technologists Education and Research Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.McKee A.C., Cantu R.C., Nowinski C.J., Hedley-Whyte E.T., Gavett B.E., Budson A.E., Santini V.E., Lee H.S., Kubilus C.A., and Stern R.A. (2009). Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exper. Neurol. 68, 709–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavett B., Stern R., Cantu R., Nowinski C., and McKee A. (2010). Mild traumatic brain injury: a risk factor for neurodegeneration. Alzheimers Res. Ther. 2, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shultz S.R., MacFabe D.F., Foley K.A., Taylor R., Cain D.P. (2012). Sub-concussive brain injury in the Long-Evans rat induces acute neuroinflammation in the absence of behavioral impairments. Behav. Brain Res. 229, 145–152 [DOI] [PubMed] [Google Scholar]
- 4.Dashnaw M.L., Petraglia A.L., and Bailes J.E. (2012). An overview of the basic science of concussion and subconcussion: where we are and where we are going. Neurosurg. Focus. 33, 1–9 [DOI] [PubMed] [Google Scholar]
- 5.Bauer J.A., Thomas T.S., Cauraugh J.H., Kaminski T.W., and Hass C.J. (2001). Impact forces and neck muscle activity in heading by collegiate female soccer players. J. Sports Sci. 19, 171–179 [DOI] [PubMed] [Google Scholar]
- 6.Talavage T.M., Nauman E.A., Breedlove E.L., Yoruk U., Dye A.E., Morigaki K.E., Feuer H., and Leverenz L.J. (2014). Functionally-detected cognitive impairment in high school football players without clinically-diagnosed concussion. J. Neurotrauma 31, 327–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiotta A.M., Shin J.H., Bartsch A.J., and Benzel E.C. (2011). Subconcussive impact in sports: a new era of awareness. World Neurosurg. 5, 175–178 [DOI] [PubMed] [Google Scholar]
- 8.Broglio S.P., Eckner J.T., Martini D., Sosnoff J.J., Kutcher J.S., and Randolph C. (2011). Cumulative head impact burden in high school football. J. Neurotrauma 28, 2069–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baugh C.M., Stamm J.M., Riley D.O., Gavett B.E., Shenton M.E., Lin A., Nowinski C.J., Cantu R.C., McKee A.C., Stern R.A. (2012). Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 6, 244–254 [DOI] [PubMed] [Google Scholar]
- 10.McKee A.C., Cantu R.C., Nowinski C.J., Hedley-Whyte E.T., Gavett B.E., Budson A.E., Santini V.E., Lee H.S., Kubilus C.A., and Stern R.A. (2009). Chronic traumatic encephalopathy in athletes. J. Neuropathol. Exper. Neurol. 68, 709–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broglio S.P., Eckner J.T., Paulson H.L., and Kutcher J.S. (2012). Cognitive decline and aging: the role of concussive and subconcussive impacts. Exerc. Sport Sci. Rev. 40, 138–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller J.R., Adamson G.J., Pink M.M., and Sweet J.C. (2007). Comparison of preseason, midseason, and postseason neurocognitive scores in uninjured collegiate football players. Am. J. Sports Med. 35, 1284–1288 [DOI] [PubMed] [Google Scholar]
- 13.Parker T.M., Osternig L.R., van Donkelaar P., and Chou L.S. (2008). Balance control during gait in athletes and non-athletes following concussion. Med. Eng. Phys. 30, 959–967 [DOI] [PubMed] [Google Scholar]
- 14.Gavett B.E., Stern R.A., and McKee A.C. (2011). Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med. 30, 179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witol A.D. and Webbe F.M. (2003). Soccer heading frequency predicts neuropsychological deficits. Arch. Clin. Neuropsychol. 18, 397–417 [PubMed] [Google Scholar]
- 16.Guskiewicz K.M., Marshall S.W., Bailes J., McCrea M., Cantu R.C., Randolph C., and Jordan B.D. (2005). Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 57, 719–724 [DOI] [PubMed] [Google Scholar]
- 17.McCrory P., Meeuwisse W., Johnston K., Dvorak J., Aubry M., Molloy M., and Cantu R. (2009). Consensus statement on concussion in sport—The 3rd International Conference on concussion in sport, held in Zurich, November 2008. J. Clin. Neurosci. 16, 755–763 [DOI] [PubMed] [Google Scholar]
- 18.Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., and Shulman G.L. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A. 98, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greicius M.D., Supekar K., Menon V., and Dougherty R.F. (2008). Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cort. 19, 72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greicius M.D., Krasnow B., Reiss A.L., Menon V. (2003). Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 100, 253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., and Beckmann C.F. (2009). Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 106, 13040–13045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shuttleworth-Rdwards A.B. and Radloff S.E. (2008). Compromised visuomotor processing speed in players of Rugby Union from school through to the national adult level. Arch. Clin. Neuropsychol. 23, 511–520 [DOI] [PubMed] [Google Scholar]
- 23.Marshall S.W. and Spencer R.J. (2001). Concussion in rugby: the hidden epidemic. J. Athl. Train. 36, 334–338 [PMC free article] [PubMed] [Google Scholar]
- 24.Gabbett T.J., Jenkins D.G., and Abernethy B. (2011). Physical collisions and injury in professional rugby league match-play. J. Sci. Med. Sport 14, 210–215 [DOI] [PubMed] [Google Scholar]
- 25.Wolf R.C., Sambataro F., Vasic N., Schmid M., Thomann P.A., Bienentreu S.D., and Wolf N.D. (2011). Aberrant connectivity of resting-state networks in borderline personality disorder. J. Psychiatry Neurosci. 36, 402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behzadi Y., Restom K., Liau J., and Liu T.T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy K., Birn R.M., Handwerker D.A., Jones T.B., and Bandettini P.A. (2009). The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? Neuroimage 44, 893–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horovitz S.G., Fukunaga M., de Zwart J.A., van Gelderen P., Fulton S.C., Balkin T.J., and Duyn J.H. (2008). Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum. Brain Mapp. 29, 671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raichle M.E. and Snyder A.Z. (2007). A default mode of brain function: a brief history of an evolving idea. Neuroimage 37, 1083–1090 [DOI] [PubMed] [Google Scholar]
- 30.Giza C.C. and Hovda D.A. (2001). The neurometabolic cascade of concussion. J. Athl. Train. 36, 228–235 [PMC free article] [PubMed] [Google Scholar]
- 31.Guskiewicz K.M. (2003). Cumulative effects associated with recurrent concussion in collegiate football players: The NCAA Concussion Study. JAMA 290, 2549–2555 [DOI] [PubMed] [Google Scholar]
- 32.Mayer A.R., Mannell M.V., Ling J., Gasparovic C., and Yeo R.A. (2011). Functional connectivity in mild traumatic brain injury. Hum. Brain Mapp. 32, 1825–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Y., Milham M.P., Lui Y.W., Miles L., Reaume J., Sodickson D.K., Grossman R.I., and Ge Y. (2012). Default-mode network disruption in mild traumatic brain injury. Radiology 265, 882–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson B., Zhang K., Gay M., Horovitz S., Hallett M., Sebastianelli W., and Slobounov S. (2012). Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. Neuroimage 59, 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanhaudenhuyse A., Noirhomme Q., Tshibanda L.J., Bruno M.A., Boveroux P., Schnakers C., Soddu A., Perlbarg V., Ledoux D., Brichant J.F., Moonen G., Maquet P., Greicius M.D., Laureys S., and Boly M. (2010). Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain 133, 161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seitz J.A. (2000). The bodily basis of thought. New Ideas Psychol. 8, 23–40 [Google Scholar]
- 37.Rushworth M.F.S., Krams M., and Passingham R.E. (2001). The attentional role of the left parietal cortex: the distinct lateralization and localization of motor attention in the human brain. J. Cog. Neurosci. 13, 698–710 [DOI] [PubMed] [Google Scholar]
- 38.Seghier M.L. (2013). The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist 19, 43–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dickerson B.C., Bakkour A., Salat D.H., Feczko E., Pacheco J., Greve D.N., Grodstein F., Wright C.I., Blacker D., Rosas H.D., Sperling R.A., Atri A., Growdon J.H., Hyman B.T., Morris J.C., Fischl B., and Buckner R.L. (2009). The cortical signature of Alzheimer's disease: regionallyspecific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb. Cort. 19, 497–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kringelbach M.L. and Rolls E.T. (2004). The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 72, 341–372 [DOI] [PubMed] [Google Scholar]
- 41.Bechara A., Damasio H., and Damasio A.R. (2000). Emotion, decision making and the orbitofrontal cortex. Cereb. Cort. 10, 295–307 [DOI] [PubMed] [Google Scholar]
- 42.Jacobs A.H. and Tavitian B.; INMiND consortium. (2012). Noninvasive molecular imaging of neuroinflammation. J. Cereb. Blood Flow Metab. 32, 1393–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt O.I., Heyde C.E., Ertel W., and Stahel P.F. (2005). Closed head injury—an inflammatory disease? Brain Res.Rev. 48, 88–399 [DOI] [PubMed] [Google Scholar]
- 44.Noble R.L. and Collip J.B. (1942). A quantitative method for the production of experimental traumatic shock without haemorrhage in unanaesthetized animals. Exp. Physiol. 31, 187–199 [Google Scholar]
- 45.Allen G.V., Gerami D., and Esser M.J. (2000). Conditioning effects of repetitive mild neurotrauma on motor function in an animal model of focal brain injury. Neuroscience 99, 93–105 [DOI] [PubMed] [Google Scholar]
- 46.Ramlackhansingh A.F., Brooks D.J., Greenwood R.J., Bose S.K., Turkheimer F.E., Kinnunen K.M., Gentleman S., Heckemann R.A., Gunanayagam K., Gelosa G., and Sharp D.J. (2011). Inflammation after trauma: microglial activation and traumatic brain injury. Ann. Neurol. 70, 374–383 [DOI] [PubMed] [Google Scholar]