Abstract
Amygdala hemodynamic responses to positive stimuli are attenuated in major depressive disorder (MDD) and normalize with remission. Real-time functional magnetic resonance imaging neurofeedback (rtfMRI-nf) training with the goal of upregulating amygdala activity during recall of happy autobiographical memories (AMs) has been suggested, and recently explored, as a novel therapeutic approach that resulted in improvement in self-reported mood in depressed subjects. In this study, we assessed the possibility of sustained brain changes as well as the neuromodulatory effects of rtfMRI-nf training of the amygdala during recall of positive AMs in MDD and matched healthy subjects. MDD and healthy subjects went through one visit of rtfMRI-nf training. Subjects were assigned to receive active neurofeedback from the left amygdale (LA) or from a control region putatively not modulated by AM recall or emotion regulation, that is, the left horizontal segment of the intraparietal sulcus. To assess lasting effects of neurofeedback in MDD, the resting-state functional connectivity before and after rtfMRI-nf in 27 depressed subjects, as well as in 27 matched healthy subjects before rtfMRI-nf was measured. Results show that abnormal hypo-connectivity with LA in MDD is reversed after rtfMRI-nf training by recalling positive AMs. Although such neuromodulatory changes are observed in both MDD groups receiving feedback from respective active and control brain regions, only in the active group are larger decreases of depression severity associated with larger increases of amygdala connectivity and a significant, positive correlation is found between the connectivity changes and the days after neurofeedback. In addition, active neurofeedback training of the amygdala enhances connectivity with temporal cortical regions, including the hippocampus. These results demonstrate lasting brain changes induced by amygdala rtfMRI-nf training and suggest the importance of reinforcement learning in rehabilitating emotion regulation in depression.
Key words: : amygdala, functional connectivity, major depressive disorder, neurofeedback, real-time fMRI, resting state
Introduction
Functional magnetic resonance imaging (fMRI) based on the blood-oxygen-level-dependent (BOLD) contrast is widely utilized to measure the patterns of brain activation that are associated with different cognitive processes (Friston, 2009). Real-time fMRI (rtfMRI) enables immediate access to brain activation patterns by analyzing data as quickly as they are acquired (Cox et al., 1995). Observing one's brain activation “live” via rtfMRI can be utilized as a form of biofeedback, that is, neurofeedback, which entails training patients to regulate their own brain activity through real-time fMRI neurofeedback (rtfMRI-nf) (deCharms, 2008). The continuously updated neurofeedback signal shows the activity level in the targeted brain region, thereby providing subjects with online information about their success in self-regulating their brain activity (Weiskopf et al., 2003). Studies have demonstrated that individuals can learn to self-regulate brain activity in structures relevant to emotional processing, including the insula (Caria et al., 2007; Johnston et al., 2010), amygdala (Johnston et al., 2010; Posse et al., 2003; Zotev et al., 2011), ventrolateral prefrontal cortex (Linden et al., 2012), and subgenual anterior cingulate cortex (Hamilton et al., 2011) through rtfMRI-nf. Emerging evidence also suggests that rtfMRI-nf has clinical utility in reducing the symptoms of chronic pain (deCharms et al., 2005), tinnitus (Haller et al., 2010), Parkinson's disease (Subramanian et al., 2011), and major depressive disorder (MDD) (Linden et al., 2012; Young et al., 2014).
MDD is a disabling and common medical condition (World Health Organization, 2004), for which approximately two-thirds of patients who seek pharmacological and/or psychological interventions will not respond fully to, and only one-half of treatment responders achieve sustained remission (Cain, 2007). However, treatment options beyond psychotherapy and/or pharmacotherapy such as electroconvulsive therapy (Merkl et al., 2009) and deep brain stimulation (Lozano et al., 2008) are very limited, invasive, and associated with significant adverse event risks.
A rtfMRI-nf training method for upregulating amygdala activity during positive autobiographic memory recall has been proposed (Zotev et al., 2011) and recently explored as a novel therapeutic approach for MDD (Young et al., 2014). The amygdala is a key element of the emotion processing circuit in the human brain (Drevets et al., 2008). Studies show that amygdala BOLD activity increases in response to both positive and negative emotional stimuli in healthy humans (Everitt et al., 2003; Sergerie et al., 2008; Victor et al., 2010). In MDD, however, amygdala responses are attenuated to positive stimuli and enhanced to negative stimuli (Suslow et al., 2010; Victor et al., 2010). Furthermore, more severe depression is found to be associated with more attenuated amygdala response to positive stimuli (Suslow et al., 2010), and this response increases after successful antidepressant pharmacotherapy (Victor et al., 2010) or Cognitive Control Therapy (Siegle et al., 2007). Therefore, rtfMRI-nf training of the amygdala with the goal of better controlling and upregulating amygdala activity to positive stimuli may exert therapeutic effects by normalizing this emotional processing bias (Harmer et al., 2009).
In order to achieve such upregulation, the strategy of positive autobiographical memory (AM) retrieval was selected based on findings of amygdala activity (with other medial temporal regions) during AM retrieval (Greenberg et al., 2005), and because it is commonly reported by participants post-hoc as an effective strategy in neurofeedback studies targeting emotional processing brain regions (Caria et al., 2007; Johnston et al., 2011; Linden et al., 2012). More importantly, depressed individuals are impaired at recalling specific and positive AMs (van Vreeswijk and De Wilde, 2004; Young et al., 2012, 2013); therefore, reinforcement in the associated brain circuit is hypothesized to occur with the neurofeedback training, which may also help normalize this deficit.
Our recent proof-of-concept study (Young et al., 2014) has tested this amygdala-targeted rtfMRI-nf system in a cohort of 21 unmedicated MDD subjects. MDD subjects were assigned to receive rtfMRI-nf from either left amygdala (LA) or a control region and instructed to contemplate happy AMs to raise the level of a bar representing the hemodynamic signal from the target region to a target level. Our results demonstrated that MDD subjects were able to increase their amygdala activity while recalling happy AMs, and this procedure resulted in improvements in self-reported mood. However, it was not clear whether such neurofeedback training had any plastic changes in the brain, especially in the circuit for emotion processing (e.g., amygdala) (Victor et al., 2010) and autobiographic memory recall (e.g., pregenual anterior cingulate cortex, or pgACC) (Young et al., 2013). Furthermore, it was not known whether such modulatory effects, if any, would be sustainable after an extended period of time.
Recent studies have shown that the human brain is intrinsically organized into spatiotemporally dissociable functional networks, which manifest endogenous connectivity within each network during a task-free resting state (Biswal et al., 1995; Fox and Raichle, 2007). The resting-state networks involving at least two key regions (the seeds)—the amygdala and the pgACC—are consistently found to be abnormal in depression (Davey et al., 2012; Ramasubbu et al., 2014). In order to answer the question of where or how lasting neuromodulatory effects of rtfMRI-nf exist in MDD, we used resting-state fMRI to measure the resting-state functional connectivity (RSFC) with regard to the active training target, that is, amygdala, before and several days after rtfMRI-nf. By first comparing MDD subjects with a cohort of matched healthy subjects, we identified regions of abnormal connectivity with regard to amygdala before treatment and then tested those regions for changes of connectivity after treatment in MDD. Our hypothesis was that rtfMRI-nf training of the amygdala acts to relieve depression, at least in part, by normalizing connectivity in the amygdala-associated circuits. Another part of this hypothesis is that rtfMRI-nf training with active neurofeedback should have reinforcement effects by strengthening the circuits implicated in AM recall relative to the control neurofeedback. Accordingly, we also tested whether the amygdala- and pgACC-associated network was differentially affected between the active neurofeedback group and control neurofeedback group.
Materials and Methods
Participants
Twenty-seven right-handed, unmedicated individuals aged 18–55 years with MDD in a current major depressive episode according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR; American Psychological Association, APA, 2000) participated in the study. Twenty-seven right-handed, medically and psychiatrically healthy subjects with gender and age (±3 years) matching the MDD individuals were also recruited. All participants, recruited from the community via advertisements, underwent medical and psychiatric screening evaluations at the Laureate Institute for Brain Research, which included the Structural Clinical Interview for DSM-IV Disorders (First et al., 2002) and the 21-item Hamilton Depression Rating Scale (HDRS; Hamilton, 1960). Exclusion criteria included general MRI exclusions, current pregnancy, psychosis, serious suicidal ideation, major medical or neurological disorders, exposure to any medication likely to influence cerebral function or blood flow within 3 weeks (8 weeks for fluoxetine), and meeting DSM-IV criteria for drug or alcohol abuse within the previous year or for alcohol or drug dependence (except nicotine) within the lifetime. Depressed participants with a 21-item HDRS score ≥17 (mean, 22.11±4.95) were included. Healthy participants had an HDRS score ≤7 (mean, 1.04±1.82). Twenty-three of the depressed participants had recurrent MDD, while four were experiencing a first episode. After receiving a complete explanation of the study procedures, all participants provided written informed consent as approved by the Western Institutional Review Board. Participants received financial compensation for their participation. All participants were naïve to rtfMRI-nf.
Experimental paradigm
All MDD participants underwent rtfMRI-nf in a protocol described in Young and colleagues (2014). Subjects were instructed to feel happy by evoking positive AMs while trying to raise the activation level of the targeted region of interest (ROI), that is, the LA for the active group and the horizontal segment of intraparietal sulcus (HIPS) for the control group, respectively. The LA was selected as the active ROI as a functional dissociation between left and right amygdala has been proposed such that the right is engaged in rapid/automatic detection of emotional stimuli, while the left is involved in detailed and elaborate stimulus evaluation (Baas et al., 2004; Glascher and Adolphs, 2003; Sergerie et al., 2008). In addition, the LA response to positive stimuli is specifically related to MDD symptoms and treatment response (Victor et al., 2010). The HIPS was chosen for control ROI, as it is implicated in number and not in emotional processing (Dehaene et al., 2003; Fias et al., 2007; Molko et al., 2003; Newman et al., 2011). MDD participants were informed that they would be assigned to receive neurofeedback from one of two brain regions: one region involved in emotional processing or another region that is independent of emotional processing and which may be difficult to regulate. Participants' group was assigned under double-blind conditions. Participants were not told their group assignment until the end of the study, and those in the control group were offered the opportunity to receive another session in which they received active neurofeedback training. Participants were instructed to retrieve positive AMs that potentially would help them control the level of activity in the target brain region.
Since depressed individuals are impaired at recalling specific and positive AMs (van Vreeswijk and De Wilde, 2004; Young et al., 2012), each participant was interviewed before scanning to facilitate their AM recall and ensure five highly arousing, vivid, specific, and happy AMs could be evoked during rtfMRI-nf. Participants were instructed to recall those or other happy AMs while attempting to increase the hemodynamic activity in the assigned ROI to that of the blue bar representing the target level of activation. They were informed to maintain this strategy even if they felt it was ineffective at raising their brain activity, though they could change the positive memories utilized or the aspects of the memories focused on.
One resting-state scan before the neurofeedback on the same visit and another resting state scan on a separate visit at least 2 days and less than a month after neurofeedback were performed on MDD subjects. Healthy subjects also participated in a single visit of neurofeedback training by recalling positive AMs in a similar protocol. One resting-state scan before neurofeedback was conducted in the healthy individuals and compared with the pre-neurofeedback resting state in MDD subjects. All subjects opened their eyes and fixated on a cross on the screen during resting scans. They were instructed to relax and not think about anything in particular for the resting scan.
On each visit before MRI, all participants completed clinician-administered rating scales, including HDRS, the Montgomery–Asberg Depression Rating Scale (Montgomery and Asberg, 1979), and the Hamilton Anxiety Rating Scale (HARS) (Hamilton, 1959).
Only data during neurofeedback from a subset of the MDD subjects included in this study (20) have been reported in Young and colleagues (2014). In addition, the working hypothesis, image data, and analysis in this study are completely different from Young and colleagues (2014). This study analyzed the resting-state image data in MDD subjects before and after neurofeedback and the resting-state data in healthy subjects before neurofeedback. The resting-state data before neurofeedback were contrasted between MDD and healthy subjects, while the post-versus-pre (post-nf minus pre-nf) resting-state data are contrasted between MDD participants who received active and control neurofeedback.
Data acquisition
MRI was conducted at the Laureate Institute for Brain Research using a General Electric Discovery MR750 whole-body 3 T MRI scanner (GE Healthcare) equipped with a custom rtfMRI system (Bodurka and Bandettini, 2008). A standard eight-channel receive-only head coil array was used. A single-shot gradient-recalled echo planar imaging (EPI) sequence with Sensitivity Encoding (SENSE) was employed for fMRI. The following EPI imaging parameters were used: field of view (FOV)/slice=240/2.9 mm, axial slices per volume=34, acquisition matrix=96×96, repetition/echo time (TR/TE)=2000/30 msec, SENSE acceleration factor R=2 in the phase encoding (anterior-posterior) direction, flip angle=90°, sampling bandwidth=250 kHz, and number of volumes=263. Each functional scan time lasted 8 min 46 sec. The EPI images were reconstructed into a 128×128 matrix, in which the resulting fMRI voxel volume was 1.875×1.875×2.9 mm3. In addition, simultaneous physiological pulse oximetry and respiration waveform recordings were conducted (with 50 Hz sampling) for each fMRI run. A photoplethysmograph with an infrared emitter placed under the pad of the subject's left index finger was used for pulse oximetry, and a pneumatic respiration belt was used for respiration measurements. A T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) sequence with SENSE was used to provide an anatomical reference for the fMRI analysis. It had the following parameters: FOV=240 mm, axial slices per slab=128, slice thickness=1.2 mm, image matrix=256×256, TR/TE=5/1.9 msec, acceleration factor R=2, flip angle=10°, delay time (TD)=1400 msec, inversion time (TI)=725 msec, and sampling bandwidth=31.2 kHz.
Real-time fMRI neurofeedback
The rtfMRI-nf was implemented using a custom rtfMRI system using Analysis of Functional NeuroImages (AFNI, http://afni.nimh.nih.gov/) (Cox, 1996) and a custom graphic user interface software. The neurofeedback was based on fMRI activation in the ROIs defined as spheres of 7 mm radius in the stereotaxic array of Talairach and Tournoux (1988) and placed in the LA (−21, −5, −16) or the left HIPS (−42, −48, 48) for the respective active or control group.
For each subject, a high-resolution MPRAGE image and a short (10-sec) EPI scan were acquired before the neurofeedback procedure. The MPRAGE image was transformed to the Talairach space. The target ROIs (as defined earlier) were defined in Talairach space. They were first transformed to the original MPRAGE space, and then to the EPI space defined by a single EPI volume from the short EPI scan (for steady state). During the rtfMRI-nf experiment (40-sec Happy, Count, and Rest conditions were repeatedly employed as described in detail in Zotev et al., 2011), all acquired EPI volumes were registered to the same single EPI volume. During Happy condition, subjects were instructed to feel happy by evoking positive AMs while trying to raise the level of a red bar on the screen that is proportional to a percent signal change of the targeted ROI relative to the preceding baseline Rest condition. This neurofeedback signal (percent signal change) was computed at every time point as a moving average of the current and two preceding fMRI percent signal change values, and was updated every 2 sec.
Resting-state fMRI data analysis
The fMRI data preprocessing was performed using AFNI. The first five volumes of each run were excluded from analysis to allow the BOLD signal to reach steady state. Major steps include respiration- and pulse-associated noise reduction using RETROICOR (Glover et al., 2000), slice timing and rigid body motion correction, spatial smoothing with a Gaussian kernel (full width at half maximum=6 mm), and temporal filtering with a bandpass filter (0.005–0.1 Hz). In addition, the low-frequency changes in respiration volume (Birn et al., 2006), six affine motion parameters, signal from a ventricular region of interest, and signal from a region centered in the white matter were regressed out from the dataset. Data points of excessive motion (root mean square larger than 0.3 mm) were excluded from regression and correlation analysis using the censoring option implemented in AFNI (afni_proc.py). The censoring step, in principle, is similar to the scrubbing approach proposed by Power and colleagues (2012, 2013). Specifically, the L2-norm of motion parameters estimated from motion registration was calculated per run and those time points of amplitude larger than 0.3 mm were censored/excluded in the regression or the later calculation of connectivity. The threshold 0.3 mm was slightly more moderate than what was suggested by Power and colleagues (2013, 0.2 mm), but it yielded balanced censoring ratios across subject groups in our data. Notably, the band-pass filtering was implemented in the linear regression fashion; therefore, censoring out points of extraordinary motion does not lead to an unusual edge effect as seen in filtering using a convolution form. The whole-brain global signal was not removed, as this may lead to spurious anti-correlation (Fox et al., 2005; Murphy et al., 2009; Saad et al., 2012). The fMRI data of each subject was first spatially coregistered to high-resolution anatomical images and then to the Talairach and Tournoux template brain (Talairach and Tournoux, 1988) with aid of the Advanced Normalization Tools (ANTS, http://picsl.upenn.edu/ANTS/) for the spatial normalization.
RSFC was computed as Pearson's correlation with regard to a seed region (Biswal et al., 1995). Correlation maps were produced by extracting the pre-processed BOLD time course from a seed region, averaging the signals within the seed region, then computing the correlation coefficient between the seed time course and the time course from all other brain voxels. For this study, we first examined the correlations associated with the active training target of rtfMRI-nf, that is, the LA. The seed region was a 7-mm-radius sphere centered at the LA (−21, −5, −16) in Talairach space. Another brain region critical to autobiographic memory recall and that is differentially active in healthy and MDD participants (Young et al., 2013), the pgACC, was also examined as seed region (7-mm-radius sphere centered at −3, 43, 6).
For statistical tests and group analysis, correlation coefficients were converted to a normal distribution by Fisher's z transform. These values were converted to z scores (i.e., zero mean, unit variance, and Gaussian distributions) by dividing by the square root of the variance [1/sqrt(n−3), where n is the degrees of freedom in the measurement]. The degrees of freedom were corrected for the temporal dependence across consecutive time points (Fox et al., 2005). Individuals' z-score maps were then submitted for a comparison and statistic test.
To examine whether there exists any therapeutic modulatory effect, we defined ROIs as regions of abnormal connectivity based on pre-nf data from the depressed and healthy subjects, then tested whether the abnormal pre-nf connectivity in the MDD was reversed in the post-nf data. We employed two approaches to define ROIs. One way was to identify ROIs using conjunction criteria of (1) connectivity that is different in the pooled MDD subjects as compared with the healthy (using an unpaired two-sided t test); therefore changes, if any, might be relevant to the pathology of depression; (2) connectivity that linearly co-varies with HDRS in the pooled MDD subjects; therefore, reversed changes, if any, would be related to the therapeutic effect of alleviating depression. Another definition of ROI is to only consider the regions of abnormal connectivity regardless of their co-variation with HDRS, which would enable examining modulatory effect in regions where connectivity is abnormal but does not necessarily follow a linear trend with the depression severity.
In each ROI, the connectivity before and after neurofeedback was compared using a paired one-sided t test, separately for the active and control groups. The post-versus-pre connectivity changes were also compared between the active and control MDD groups using an unpaired two-sided t test. To assess the relationship between the changes of ROI connectivity and changes in depression severity (HDRS), we utilized both a parametric method (linear correlation) and a non-parametric method (two-binned comparison). The linear correlation between HDRS and connectivity changes was calculated to evaluate whether these changes follow a linear pattern. Meanwhile, the nonparametric two-binned comparison assessed whether the larger decreases of HDRS were associated with larger increases of connectivity. Specifically, the individuals' HDRS changes are sorted in descending order; then, the connectivity changes associated with higher half of HDRS changes are compared with those with the lower half using an unpaired one-sided t test.
Furthermore, to evaluate the whole-brain modulatory effect associated with amygdala training, MDD individuals' pre-nf z-score maps were subtracted from the post-nf z-score maps and whole brain analysis was performed to test whether post-versus-pre connectivity changes differ between the active and control MDD groups using an unpaired two-sided t test.
Results
Demographic and clinical data
The demographic and clinical characteristics of all subjects are listed in Table 1. The healthy and the pooled MDD subjects exactly matched in gender and did not differ in age (t52=0.14, p=0.89). Before neurofeedback, HDRS and HARS ratings were higher in the MDD compared with the healthy subjects (both t52>3.48, p<0.001), while the active MDD group and the control MDD group did not differ in age (t25=1.24, p=0.23), HDRS (t25=1.65, p=0.11), or HARS (t25=0.94, p=0.35) scores. On average, the duration between the neurofeedback and returning visits for MDD subjects is 9.00±5.91 days (Mean±standard deviation) and did not differ between the active and control group (t25=0.1279, p=0.90). Post-nf HARS ratings decreased in both the active MDD group (t11=3.09, p=0.01) and control MDD group (t12=2.21, p=0.047), while in both groups the HDRS decreases were marginally significant (active MDD: t11=2.07, p=0.06; control MDD: t12=2.06, p=0.06). However, the active MDD group and control MDD group did not differ in the post-versus-pre difference of HDRS (t23=0.31, p=0.76) or HARS (t23=0.17, p=0.86) scores.
Table 1.
Participant Characteristics by Experimental Group
| Active MDD | Control MDD | Healthy subjects | |||
|---|---|---|---|---|---|
| Characteristic, mean (SD) | n=14 | n=13 | n=27 | ||
| Females | 11 | 11 | 22 | ||
| Age in years | 38 (10) | 35 (8) | 36 (9) | ||
| Number of previous major depressed episodes | |||||
| 1 Episode | 14.3% [n=2] | 23.1% [n=3] | N/A | ||
| 2 Episodes | 21.4% [n=3] | 9.1% [n=1] | N/A | ||
| >2 Episodes | 64.3% [n=9] | 69.2% [n=9] | N/A | ||
| HDRSa | Pre | Post | Pre | Post | Pre |
| 20.64 (4.63) | 17.42 (5.28) | 23.69 (4.96) | 20.92 (6.02) | 1.04 (1.82) | |
| HARSa | Pre | Post | Pre | Post | Pre |
| 19.93 (5.15) | 16.25 (5.34) | 22.15 (7.02) | 18 (5.28) | 1.31 (2.02) | |
| Post-nf days | 9.14 (6.54) | 8.85 (5.41) | N/A | ||
Post-neurofeedback ratings were not available in two MDD subjects of the active group.
SD, standard deviation; MDD, major depressive disorder; HDRS, Hamilton Depression Rating Scale; HARS, Hamilton Anxiety Rating Scale; N/A, not available.
ROI analysis
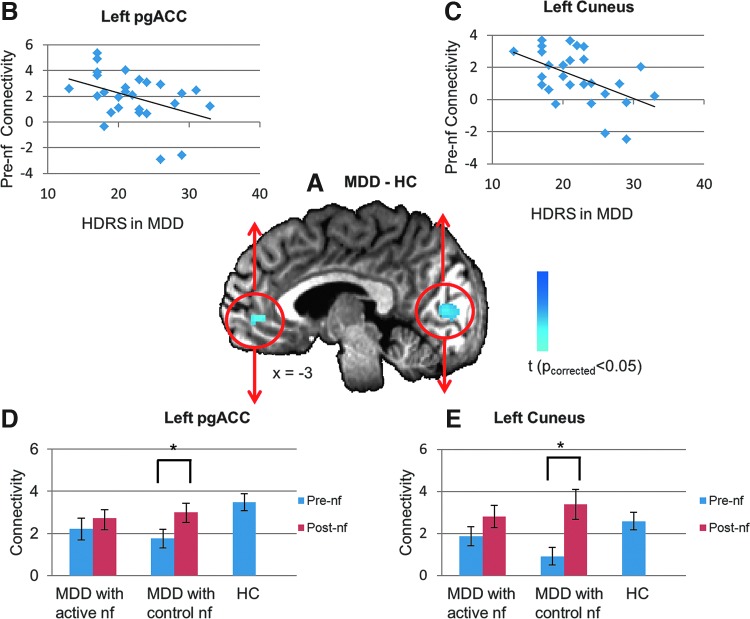
In order to examine the neuromodulatory effect of rtfMRI-nf on the amygdala connectivity, we first searched for regions that are likely to show the therapeutic effect, if any, based on the MDD and healthy subjects' pre-nf connectivity with seed at the active target, that is, LA. Thus, we performed a whole-brain conjunction analysis to identify the regions where the pre-nf amygdala RSFC maps in the MDD subjects (pooled active and control groups) differed from the pre-nf RSFC in healthy subjects and also covaried with their HDRS scores. Figure 1A shows the results of the conjunction analysis (p<0.05, cluster threshold p<0.05). Two regions were found: the pgACC (peak coordinates: −0.9, 44.1, 0.6, cluster size: 58 voxels) and the cuneus (peak coordinates: 10.3, −87.2, −3.1, cluster size: 498 voxels). Both the pgACC and the cuneus showed decreased connectivity with amygdala in the MDD subjects relative to healthy subjects, and the amygdala connectivity of both was negatively correlated with the HDRS (Fig. 1B, C).
FIG. 1.
(A) Regions where the resting-state functional connectivity with left amygdala (LA, seed) in subjects with major depressive disorder (MDD) differed from healthy control (HC) subjects and also was covaried with the Hamilton Depression Rating Scales (HDRS). The cold colors indicate the region where connectivity with amygdala was larger in healthy subjects than in MDD; (B) pre-neurofeedback (pre-nf) connectivity between left amygdala and pregenual anterior cingulate cortex (pgACC) in MDD subjects significantly linearly decreases with HDRS (cc=−0.41, p=0.04); (C) LA-cuneus pre-nf connectivity in MDD subjects significantly linearly decreases with HDRS (cc=−0.51, p=0.006); Group results of LA-pgACC (D), and LA-cuneus (E) pre-, and post-nf connectivity for MDD group with active, control neurofeedback, and the healthy group. *Indicates significance at p<0.05. nf stands for neurofeedback.
The pgACC and the cuneus plotted in Figure 1A were then identified as ROIs to examine the neuromodulatory effect of rtfMRI-nf on amygdala RSFC. At the pgACC, the pre-nf amygdala connectivity in the MDD ensemble were abnormally lower than in healthy subjects (pooled MDD: t52=2.83, p=0.007), and did not differ between the active and control MDD groups (t25=0.66, p=0.52). After neurofeedback, the amygdala connectivity with pgACC was no longer abnormal in either the active or control group (both p>0.1). The amygdala-pgACC connectivity was significantly increased in the control MDD group (t12=3.00, p=0.01) and also non-significantly increased in the active MDD group (t13=0.99, p=0.34). At the cuneus, the pre-nf amygdala connectivity in the MDD ensemble was significantly lower than in the healthy subjects (pooled MDD: t52=2.24, p=0.03), and did not differ between the active and control MDDs (t25=1.56, p=0.13). After neurofeedback, the amygdala connectivity with cuneus was no longer abnormal in either the active or control group (both p>0.1). Amygdala-cuneus connectivity was significantly increased in the control MDD group (t12=2.86, p=0.01) and also non-significantly increased in the active MDD group (t13=1.31, p=0.21).
The relationship between the clinical rating changes and connectivity changes were also investigated. Non-parametric comparison found that, in MDD individuals receiving active neurofeedback from LA, larger post-versus-pre increases of connectivity between LA and left cuneus are observed to be associated with larger decreases of HDRS at their returning visits (both p<0.01). In terms of linear relationship, the connectivity changes across MDD individuals tend to be inversely correlated with HDRS changes (LA vs. left cuneus: r=−0.45, p=0.09; LA vs. left pgACC: r =−0.39, p=0.16). However, the association between connectivity changes and HDRS changes is not significant in the MDD individuals with control neurofeedback from HIPS in either parametric or non-parametric analysis (p>0.1 for two-binned comparison for both ROIs; LA vs. left precuneus: r=−0.09, p=0.75; LA vs. left pgACC: r=−0.18, p=0.54). Interestingly, although MDD subjects' HARS scores were significantly reduced at the returning visits, there was no significant association found between HARS changes and the connectivity changes with either cuneus or pgACC (all p>0.1).
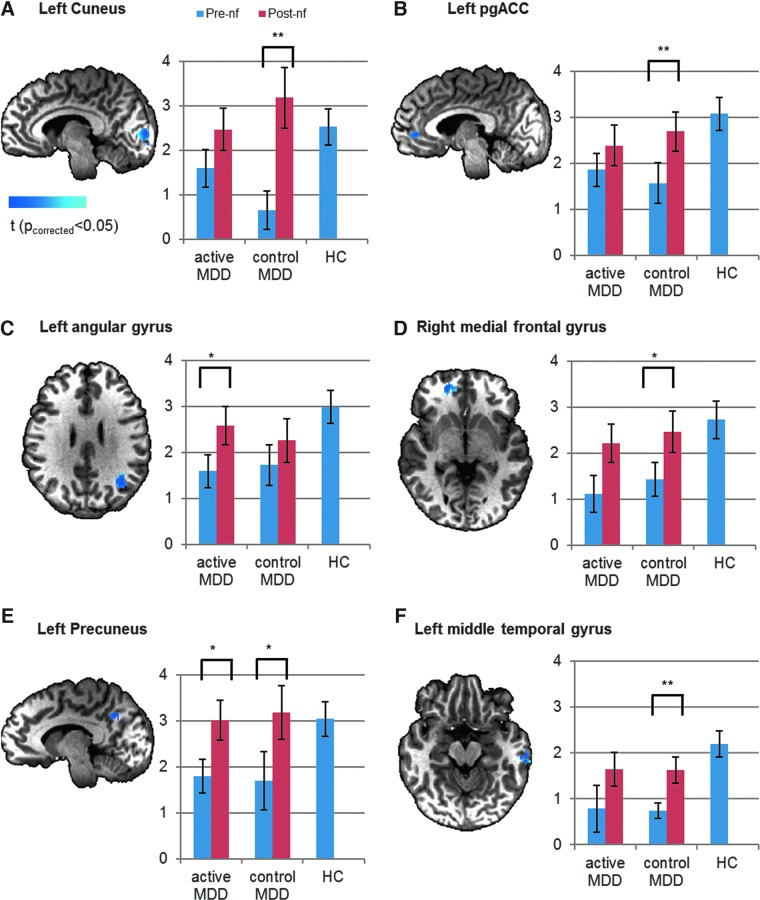
In order to further examine modulatory effect in regions that are of abnormal connectivity but are not necessarily subject to a linear trend with depression severity, another set of ROIs were defined by only considering the regions of abnormal connectivity (cluster size >30 voxels, thresholded at voxel p<0.005) regardless of their co-variation with HDRS. As shown in Figure 2, these ROIs include the two regions previously found to be of abnormal and HDRS-covaried connectivity, that is, the left cuneus and the left pgACC (Fig. 2A, B). Additional ROIs are left angular gyrus, right medial frontal gyrus, left precuneus, and left middle temporal gyrus (Fig. 2C–F). In all identified regions, the connectivity with LA in pooled MDD subjects was abnormally lower than in the healthy subjects (p<0.05 for all ROIs). After neurofeedback, the amygdala connectivity was no longer abnormal in either the active group (p>0.1 for all ROIs) or the control group (p>0.1 for all ROIs). The post-versus-pre increase of connectivity was significant for the active group in the left angular gyrus (p<0.05) and the left precuneus (p<0.05). Meanwhile, for the control group, the post-versus-pre increase of connectivity was significant in the left cuneus (p<0.01), the left pgACC (p<0.01), right medial frontal gyrus (p<0.05), left precuneus (p<0.05), and left middle temporal gyrus (p<0.01).
FIG. 2.
Resting-state functional connectivity in regions where the connectivity with LA (seed) in MDD differed from healthy subjects' regardless of co-variation with HDRS. (A) Left Cuneus; (B) Left pgACC; (C) Left angular gyrus; (D) Right medial frontal gyrus; (E) Left Precuneus; (F) Left middle temporal gyrus. Pre- and post-nf connectivity is shown for active and control MDD groups receiving neurofeedback from active and control brain regions (LA and horizontal segment of intraparietal sulcus, respectively). The cold colors indicate brain regions where connectivity with LA was larger in healthy subjects than in MDD; *indicates statistically significant post-nf versus pre-nf changes at p<0.05. **Indicates statistically significant post-nf versus pre-nf changes at p<0.01.
We also evaluated the sustainability of the neuromodulatory effect in terms of the relationship between post-versus-pre changes and days after neurofeedback. Interestingly, across MDD individuals who received active neurofeedback, a significant positive and linear correlation was observed between their post-nf increases of LA-cuneus connectivity and the time duration (days) after neurofeedback (r=0.76, p=0.0015, Fig. 5). However, no such positive linear correlation was observed in the MDD subjects with control neurofeedback (p>0.1 for all ROIs).
FIG. 5.
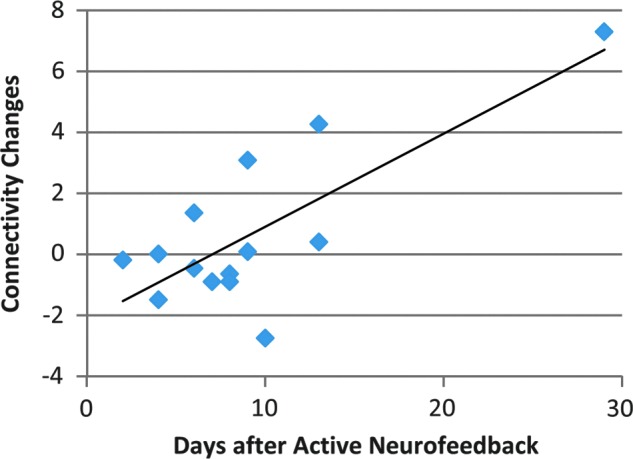
Relationship between post-nf versus pre-nf LA-cuneus connectivity changes (post-nf–pre-nf) and days after MDD subjects receiving active neurofeedback (r=0.76, p=0.0015).
Whole-brain analysis
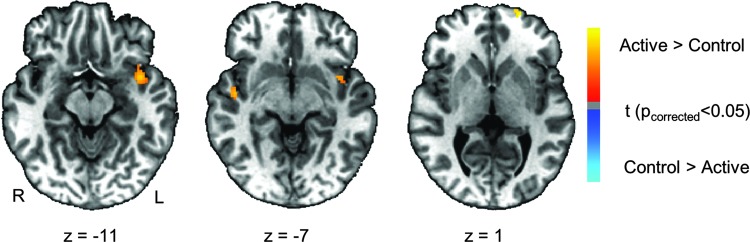
Results of the whole-brain analysis for the active neurofeedback associated effect are listed in Tables 2 and 3. The significance criterion for detecting activation was set at pcorrected<0.05 determined using the AFNI program 3dClustSim (cluster size >30 voxels, thresholded at voxel p<0.005). When we compared the post-versus-pre difference of amygdala connectivity between the active and control MDD group (Fig. 3), the active group had increased connectivity between LA at the right parahippocampal gyrus, right superior temporal gyrus, and bilateral middle frontal gyrus relative to the control group, whereas the control group had increased connectivity between the amygdala and right lingual gyrus. In addition, with regard to the pgACC seed, the active MDD group had increased connectivity with the left superior temporal cortex, the left superior frontal gyrus, and the right superior temporal gyrus (Fig. 4).
Table 2.
Group Difference of Post-Versus-Pre Connectivity Changes with Seed at Left Amygdala
| Peak coordinates | |||||
|---|---|---|---|---|---|
| Area | Cluster size (voxels) | x | y | z | t score |
| Active>Control | |||||
| Right middle frontal gyrus (BA10) | 224 | 38.4 | 57.2 | −3.1 | 3.63 |
| Right parahippocampal gyrus (BA45) | 31 | 21.6 | −25.3 | −12.5 | 3.24 |
| Control>Active | |||||
| Right lingual gyrus (BA18) | 82 | 17.8 | −81.6 | −5.0 | −3.24 |
BA, Brodmann area.
Table 3.
Group Difference of Post-Versus-Pre Connectivity Changes with Seed at Left Pregenual Anterior Cingulate Cortex
| Peak coordinates | |||||
|---|---|---|---|---|---|
| Area | Cluster size (voxels) | x | y | z | t score |
| Active>Control | |||||
| Left superior temporal gyrus (BA13/13) | 133 | −23.4 | −44.1 | 2.5 | 5.61 |
| Left superior frontal gyrus (BA10) | 115 | −19.7 | 60.9 | −1.2 | 3.72 |
| Right superior temporal gyrus, insula (BA22/21) | 37 | 45.9 | −10.3 | −6.9 | 4.00 |
FIG. 3.
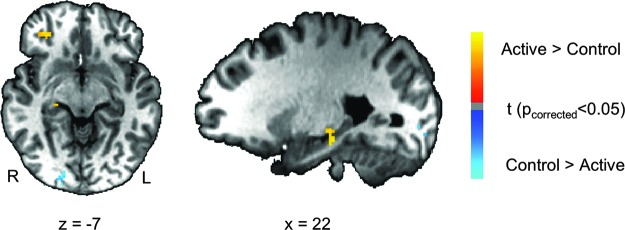
Maps of group difference of post-nf versus pre-nf connectivity changes (post-nf–pref-nf) with LA (seed). Warm colors indicate active>control. Cold colors indicate active<control. Maps are thresholded at pcorrected<0.05.
FIG. 4.
Maps of group difference of post-nf versus pre-nf connectivity changes (post-nf–pre-nf) with left pgACC (seed). Warm colors indicate active>control. Cold colors indicate active<control. Maps are thresholded at pcorrected<0.05.
Discussion
We investigated the neuromodulatory effect of training MDD patients to regulate the hemodynamic activity at their LA using rtfMRI-nf and recall of positive AMs. Our previous report (Young et al., 2014) demonstrated that, given appropriate direction, practice, and rtfMRI-nf information, MDD patients can enhance their amygdala BOLD activity by contemplating positive AMs within a single training session, and this procedure resulted in improvements in self-reported mood. Post-nf resting-state fMRI imaging in this study showed that abnormal hypo-connectivity was reversed and positive changes sustained in MDD subjects several days after using rtfMRI-nf. Although such neuromodulatory changes are observed in both MDD groups receiving feedback from respective active and control brain regions (LA and HIPS), only in the active group were larger decreases of depression severity associated with larger increases of amygdala connectivity. In addition, a significant, positive correlation was revealed between the connectivity changes and the days after active neurofeedback. Importantly, sustained neuromodulatory effects are observed after engaging in a single session of amygdala rtfMRI-nf using positive AMs.
In the pregenual ACC, BOLD functional connectivity with amygdala was lower in the MDD than in the healthy subjects at resting state. Moreover, more severe depression symptoms were associated with less amygdala-pgACC connectivity. This ACC subdivision has been implicated in emotional processing associated with both positively and negatively valenced stimuli (Bush et al., 2000; Grabenhorst et al., 2008; Pizzagalli, 2011; Rolls et al., 2008) and in AM deficits in patients with depression and individuals at high risk for depression (Young et al., 2012, 2013). Although both the pgACC and amygdala are hyperactive at rest in MDD (Drevets et al., 1992), pgACC is hyper-activated when recalling specific positive AMs (Young et al., 2013) whereas amygdala usually shows an attenuated response to positive stimuli (Victor et al., 2010), which may explain the abnormal lower functional connectivity between amygdala and pgACC found in our study at the resting state. Nonetheless, the difference of LA baseline connectivity between the MDD and healthy subjects was normalized after rtfMRI-nf training by recalling positive AMs. These findings suggest that modulation of LA-pgACC connectivity may underlie the observed therapeutic effects.
In the cuneus/BA17, the baseline amygdala connectivity was lower in MDD relative to healthy subjects and the effect of rtfMRI-nf training was to normalize this connectivity. Neuroimaging studies of AM recall reported occipital activation, including the cuneus (Addis et al., 2004; Cabeza et al., 2004; Conway et al., 1999), and this area has been implicated in visuospatial processing (Renier et al., 2010). Furthermore, a recent study also found activity in this region correlated with tolerance of distress in both healthy and remitted MDD samples, and increased activity in this area was associated with a lower risk for depression relapse (Farb et al., 2011). This may be particularly encouraging for the use of rtfMRI-nf, as post-nf connectivity in MDD seems to overshoot the baseline connectivity in healthy subjects (Fig. 1D).
In both the active and control groups, we found positive modulatory changes of abnormal resting-state LA functional connectivity in depressed subjects. These results suggest that the effect of enhancing amygdala connectivity may be more attributable to recalling happy autobiographic memories, which MDD subjects in both groups utilized as a mental strategy for neurofeedback. Since MDDs in the control group received neurofeedback from the HIPS region, where the activation during neurofeedback was not significantly different from zero (Young et al., 2014), it is likely that subjects in the control group exerted substantial effort during memory recall when the bar seemed not to move in the intended direction, which may have contributed to the observed increased connectivity between the LA and other brain regions. Consistent with our observation, several other neurofeedback studies (Hamilton et al., 2011; Scharnowski et al., 2012; Yoo et al., 2006) have also reported that both active and control neurofeedback have similar effects on behavior or brain activity in the initial stage, but such plastic effects by active neurofeedback tend to outgrow those of control neurofeedback with multiple training visits.
Although the active and control groups did not differ in amygdala connectivity enhancement, our results also found important differences in the connectivity changes between the active and control group. Post-nf connectivity between amygdala and hippocampus, and between pgACC and temporal cortical regions, was significantly strengthened by active relative to control neurofeedback. These strengthened connections are likely the result of reinforcement learning when active feedback is provided. Many of these regions share extensive anatomical and functional connections with the amygdala and are recruited during emotional learning (Kim et al., 2011) and in the modulation of emotional processes (Bush et al., 2000). We previously implicated the left superior temporal gyrus in the transfer run (i.e., AM recall without neurofeedback) immediately after the neurofeedback training (Young et al., 2014), showing that the group with active neurofeedback had increased activity relative to the control group when maintaining elevated amygdala activity after training but in the absence of neurofeedback. In addition to such increased activation in the superior temporal gyrus in the active group during transfer run, our present results further show elevated resting-state connectivity between the pgACC and the superior temporal gyrus in the active group several days after neurofeedback training. Superior temporal regions are involved in emotional processing and social cognition (Allison et al., 2000; Gallagher and Frith, 2003; Olson et al., 2007), and are less active in MDD versus healthy individuals (Canli et al., 2004; Drevets et al., 2008; Fitzgerald et al., 2008). Therefore, the increased activity and connectivity in these regions in MDD patients suggests that this neurofeedback procedure effectively recruits other regions that are important in emotional regulation which show abnormal BOLD responses in MDD, further suggesting the potential for rtfMRI-nf in MDD treatment.
Moreover, our results found that, only in the active group, larger decreases of depression severity are associated with larger increases of amygdala connectivity and a significant, positive correlation is found between the connectivity changes and the days after active neurofeedback. These findings suggest that the therapeutic effect of active neurofeedback may be intimately associated with the alleviation of depression and is more likely to sustain after neurofeedback. Although MDD participants in the active group did not significantly differ from the control group in their degree of clinical improvement at the single follow-up imaging visit after neurofeedback, we speculate and predict that the enhanced connectivity with hippocampus and temporal regions after active neurofeedback may lead to more prominent and long-lasting mood effects after repeated rtfMRI-nf sessions. However, to test this hypothesis, future studies involving repeated rtfMRI-nf training and multiple follow-up visits are needed.
The main limitation of this study is the lack of a control group that attempts self-regulation based only on the cognitive task of positive AM recall but without any neurofeedback. Without such a control group, we cannot completely exclude the possibility that mere practice of positive AM recalls led to the changes of amygdala functional connectivity. Nonetheless, autobiographic memory deficits have been well documented by many studies showing that individuals with MDD tend to generate fewer specific, more categorical, and fewer positive AMs compared with healthy control subjects (van Vreeswijk and De Wilde, 2004; Young et al., 2012, 2013). Therefore, simply practicing positive AM recall without any help of neurofeedback is intrinsically limited by depression-related deficits. Indeed, a proof-of-concept study by Linden and colleagues (2012) reported that the control group of depressed subjects that underwent a training procedure with only cognitive strategies of generating positive emotions but without neurofeedback did not improve clinically, whereas the group receiving active neurofeedback achieved significant improvement in clinical symptoms. Moreover, several other rtfMRI-nf studies that included control groups who received either control feedback or no feedback have firmly established that neurofeedback is necessary for learning to self-regulate brain activity [e.g., for the anterior cingulate cortex (Hamilton et al., 2011), for the inferior frontal gyrus (Rota et al., 2009), and for the visual cortex (Scharnowski et al., 2012; Shibata et al., 2011)]. Our findings that active neurofeedback is associated with a positive correlation between the plastic effect and the duration post-nf and that active neurofeedback more effectively engages the memory circuit further highlight the potential of neurofeedback training of the amygdala for inducing a lasting and sustainable therapeutic effect in patients with depression.
Conclusion
In conclusion, our results show lasting brain changes after the rtfMRI-nf training during happy autobiographic memory recall in subjects with major depression. Post-nf imaging results show that the abnormal hypo-connectivity with LA was reversed, suggesting a therapeutic effect of rtfMRI-nf using positive AM recall in MDD. Although such neuromodulatory changes are observed in both MDD groups receiving feedback from active and control brain regions, only in the active group are larger decreases of depression severity associated with larger increases of amygdala connectivity and a significant, positive correlation is found between the connectivity changes and the days after neurofeedback, suggesting long-term durability of amygdala-targeted rtfMRI-nf. Moreover, active neurofeedback from LA, rather than feedback from a control brain region irrelevant to emotion regulation or AM recall, further enhances the connectivity with temporal cortical regions, including the hippocampus. These findings have implications for our understanding of depression pathophysiology and a neurofeedback mechanism, as well as for efforts to enhance existing and to develop new, more effective depression treatments.
Acknowledgments
This research was supported by the Laureate Institute for Brain Research and the William K. Warren Foundation.
Author Disclosure Statement
The authors have declared that no competing interests exist. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of this article.
References
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP. 2004. Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach. Neuroimage 23:1460–1471 [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. 2000. Social perception from visual cues: role of the STS region. Trends Cogn Sci 4:267–278 [DOI] [PubMed] [Google Scholar]
- American Psychological Association (APA). 2000. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association [Google Scholar]
- Baas D, Aleman A, Kahn RS. 2004. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Res Rev 45:96–103 [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. 2006. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage 31:1536–1548 [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541 [DOI] [PubMed] [Google Scholar]
- Bodurka J, Bandettini P. 2008. Real-time software for monitoring MRI scanner operation. Neuroimage 41:S85 [Google Scholar]
- Bush G, Luu P, Posner MI. 2000. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:215–222 [DOI] [PubMed] [Google Scholar]
- Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, LaBar KS, Rubin DC. 2004. Brain activity during episodic retrieval of autobiographical and laboratory events: an fMRI study using a novel photo paradigm. J Cogn Neurosci 16:1583–1594 [DOI] [PubMed] [Google Scholar]
- Cain RA. 2007. Navigating the sequenced treatment alternatives to relieve depression (STAR*D) study: practical outcomes and implications for depression treatment in primary care. Prim Care 34:505–519, vi [DOI] [PubMed] [Google Scholar]
- Canli T, Sivers H, Thomason ME, Whitfield-Gabrieli S, Gabrieli JD, Gotlib IH. 2004. Brain activation to emotional words in depressed vs healthy subjects. Neuroreport 15:2585–2588 [DOI] [PubMed] [Google Scholar]
- Caria A, Veit R, Sitaram R, Lotze M, Weiskopf N, Grodd W, Birbaumer N. 2007. Regulation of anterior insular cortex activity using real-time fMRI. Neuroimage 35:1238–1246 [DOI] [PubMed] [Google Scholar]
- Conway MA, Turk DJ, Miller SL, Logan J, Nebes RD, Meltzer CC, Becker JT. 1999. A positron emission tomography (PET) study of autobiographical memory retrieval. Memory 7:679–702 [DOI] [PubMed] [Google Scholar]
- Cox RW. 1996. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173 [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A, Hyde JS. 1995. Real-time functional magnetic resonance imaging. Magn Reson Med 33:230–236 [DOI] [PubMed] [Google Scholar]
- Davey CG, Harrison BJ, Yücel M, Allen NB. 2012. Regionally specific alterations in functional connectivity of the anterior cingulate cortex in major depressive disorder. Psychol Med 42:2071–2081 [DOI] [PubMed] [Google Scholar]
- deCharms RC. 2008. Applications of real-time fMRI. Nat Rev Neurosci 9:720–729 [DOI] [PubMed] [Google Scholar]
- deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D, Gabrieli JD, Mackey SC. 2005. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci U S A 102:18626–18631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Piazza M, Pinel P, Cohen L. 2003. Three parietal circuits for number processing. Cogn Neuropsychol 20:487–506 [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. 2008. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 213:93–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. 1992. A functional anatomical study of unipolar depression. J Neurosci 12:3628–3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. 2003. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci 985:233–250 [PubMed] [Google Scholar]
- Farb NA, Anderson AK, Bloch RT, Segal ZV. 2011. Mood-linked responses in medial prefrontal cortex predict relapse in patients with recurrent unipolar depression. Biol Psychiatry 70:366–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fias W, Lammertyn J, Caessens B, Orban GA. 2007. Processing of abstract ordinal knowledge in the horizontal segment of the intraparietal sulcus. J Neurosci 27:8952–8956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York, NY: New York State Psychiatric Institute, Biometrics Research [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. 2008. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp 29:683–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. 2007. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711 [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. 2009. Modalities, modes, and models in functional neuroimaging. Science 326:399–403 [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. 2003. Functional imaging of ‘theory of mind’. Trends Cogn Sci 7:77–83 [DOI] [PubMed] [Google Scholar]
- Glascher J, Adolphs R. 2003. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J Neurosci 23:10274–10282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. 2000. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 44:162–167 [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET, Bilderbeck A. 2008. How cognition modulates affective responses to taste and flavor: top-down influences on the orbitofrontal and pregenual cingulate cortices. Cereb Cortex 18:1549–1559 [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, Labar KS. 2005. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia 43:659–674 [DOI] [PubMed] [Google Scholar]
- Haller S, Birbaumer N, Veit R. 2010. Real-time fMRI feedback training may improve chronic tinnitus. Eur Radiol 20:696–703 [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Glover GH, Hsu JJ, Johnson RF, Gotlib IH. 2011. Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Hum Brain Mapp 32:22–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. 1959. The assessment of anxiety states by rating. Br J Med Psychol 32:50–55 [DOI] [PubMed] [Google Scholar]
- Hamilton M. 1960. A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, O'Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, Goodwin GM, Cowen PJ. 2009. Effect of acute antidepressant administration on negative affective bias in depressed patients. Am J Psychiatry 166:1178–1184 [DOI] [PubMed] [Google Scholar]
- Johnston S, Linden DE, Healy D, Goebel R, Habes I, Boehm SG. 2011. Upregulation of emotion areas through neurofeedback with a focus on positive mood. Cogn Affect Behav Neurosci 11:44–51 [DOI] [PubMed] [Google Scholar]
- Johnston SJ, Boehm SG, Healy D, Goebel R, Linden DE. 2010. Neurofeedback: a promising tool for the self-regulation of emotion networks. Neuroimage 49:1066–1072 [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. 2011. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res 223:403–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DE, Habes I, Johnston SJ, Linden S, Tatineni R, Subramanian L, Sorger B, Healy D, Goebel R. 2012. Real-time self-regulation of emotion networks in patients with depression. PLoS One 7:e38115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. 2008. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol Psychiatry 64:461–467 [DOI] [PubMed] [Google Scholar]
- Merkl A, Heuser I, Bajbouj M. 2009. Antidepressant electroconvulsive therapy: mechanism of action, recent advances and limitations. Exp Neurol 219:20–26 [DOI] [PubMed] [Google Scholar]
- Molko N, Cachia A, Riviere D, Mangin JF, Bruandet M, Le Bihan D, Cohen L, Dehaene S. 2003. Functional and structural alterations of the intraparietal sulcus in a developmental dyscalculia of genetic origin. Neuron 40:847–858 [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. 1979. A new depression scale designed to be sensitive to change. Br J Psychiatry 134: 382–389 [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. 2009. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? 44:893–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SD, Willoughby G, Pruce B. 2011. The effect of problem structure on problem-solving: an fMRI study of word versus number problems. Brain Res 1410:77–88 [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. 2007. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain 130:1718–1731 [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. 2011. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology 36:183–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posse S, Fitzgerald D, Gao K, Habel U, Rosenberg D, Moore GJ, Schneider F. 2003. Real-time fMRI of temporolimbic regions detects amygdala activation during single-trial self-induced sadness. Neuroimage 18:760–768 [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2013. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage 76:439–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubbu R, Konduru N, Cortese F, Bray S, Gaxiola-Valdez I, Goodyear B. 2014. Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Front Psychiatry 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier LA, Anurova I, De Volder AG, Carlson S, VanMeter J, Rauschecker JP. 2010. Preserved functional specialization for spatial processing in the middle occipital gyrus of the early blind. Neuron 68:138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F, Parris BA. 2008. Warm pleasant feelings in the brain. Neuroimage 41:1504–1513 [DOI] [PubMed] [Google Scholar]
- Rota G, Sitaram R, Veit R, Erb M, Weiskopf N, Dogil G, Birbaumer N. 2009. Self-regulation of regional cortical activity using real-time fMRI: the right inferior frontal gyrus and linguistic processing. Hum Brain Mapp 30:1605–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW. 2012. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect 2:25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharnowski F, Hutton C, Josephs O, Weiskopf N, Rees G. 2012. Improving visual perception through neurofeedback. J Neurosci 32:17830–17841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony JL. 2008. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 32:811–830 [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Ghinassi F, Thase ME. 2007. Neurobehavioral therapies in the 21st century: summary of an emerging field and an extended example of cognitive control training for depression. Cognit Ther Res 31: 235–262 [Google Scholar]
- Shibata K, Watanabe T, Sasaki Y, Kawato M. 2011. Perceptual learning incepted by decoded fMRI neurofeedback without stimulus presentation. Science 334:1413–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian L, Hindle JV, Johnston S, Roberts MV, Husain M, Goebel R, Linden D. 2011. Real-time functional magnetic resonance imaging neurofeedback for treatment of parkinson's disease. J Neurosci 31:16309–16317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schoning S, Ohrmann P, Bauer J, Pyka M, Kersting A, et al. . 2010. Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry 67:155–160 [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. 1988. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System—An Approach to Cerebral Imaging. New York: Thieme Medical Publishers [Google Scholar]
- Van Vreeswijk MF. and De Wilde EJ. 2004. Autobiographical memory specificity, psychopathology, depressed mood and the use of the autobiographical memory test: a meta-analysis. Behav Res Ther 42:731–743 [DOI] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. 2010. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry 67:1128–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf N, Veit R, Erb M, Mathiak K, Grodd W, Goebel R, Birbaumer N. 2003. Physiological self-regulation of regional brain activity using real-time functional magnetic resonance imaging (fMRI): methodology and exemplary data. Neuroimage 19:577–586 [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2004. The World Health Report 2004—Changing History. Geneva: WHO [Google Scholar]
- Yoo SS, O'Leary HM, Fairneny T, Chen NK, Panych LP, Park H, Jolesz FA. 2006. Increasing cortical activity in auditory areas through neurofeedback functional magnetic resonance imaging. Neuroreport 17:1273–1278 [DOI] [PubMed] [Google Scholar]
- Young KD, Bellgowan PS, Bodurka J, Drevets WC. 2013. Behavioral and neurophysiological correlates of autobiographical memory deficits in patients with depression and individuals at high risk for depression. JAMA Psychiatry 70:698–708 [DOI] [PubMed] [Google Scholar]
- Young KD, Erickson K, Nugent AC, Fromm SJ, Mallinger AG, Furey ML, Drevets WC. 2012. Functional anatomy of autobiographical memory recall deficits in depression. Psychol Med 42:345–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KD, Zotev V, Phillips R, Misaki M, Yuan H, Drevets WC, Bodurka J. 2014. Real-time FMRI neurofeedback training of amygdala activity in patients with major depressive disorder. PLoS One 9:e88785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V, Krueger F, Phillips R, Alvarez RP, Simmons WK, Bellgowan P, Drevets WC, Bodurka J. 2011. Self-regulation of amygdala activation using real-time FMRI neurofeedback. PLoS One 6:e24522. [DOI] [PMC free article] [PubMed] [Google Scholar]