Abstract
Buspirone, a 5-HT1A receptor agonist, and environmental enrichment (EE) enhance cognition and reduce histopathology after traumatic brain injury (TBI) in adult rats, but have not been fully evaluated after pediatric TBI, which is the leading cause of death in children. Hence, the aims of this study were to assess the efficacy of buspirone alone (Experiment 1) and in combination with EE (Experiment 2) in TBI postnatal day-17 male rats. The hypothesis was that both therapies would confer cognitive and histological benefits when provided singly, but their combination would be more efficacious. Anesthetized rats received a cortical impact or sham injury and then were randomly assigned to receive intraperitoneal injections of buspirone (0.08 mg/kg, 0.1 mg/kg, and 0.3 mg/kg) or saline vehicle (1.0 mL/kg) 24 h after surgery and once daily for 16 days (Experiment 1). Spatial learning and memory were assessed using the Morris water maze (MWM) on post-operative days 11–16, and cortical lesion volume was quantified on day 17. Sham controls for each condition were significantly better than all TBI groups. In the TBI groups, buspirone (0.1 mg/kg) enhanced MWM performance versus vehicle and buspirone (0.08 mg/kg and 0.3 mg/kg) (p<0.05) and reduced lesion volume relative to vehicle (p=0.038). In Experiment 2, buspirone (0.1 mg/kg) or vehicle was combined with EE after TBI, and the data were compared to the standard (STD)-housed groups from Experiment 1. EE lead to a significant enhancement of spatial learning and a reduction in lesion size versus STD. Moreover, the combined treatment group (buspirone+EE) performed markedly better than the buspirone+STD and vehicle+EE groups, which suggests an additive effect and supports the hypothesis. The data replicate previous studies assessing these therapies in adult rats. These novel findings may have important rehabilitation-relevant implications for clinical pediatric TBI.
Key words: : 5-HT1A receptor agonist, beam-walking; behavior; controlled cortical impact (CCI); functional recovery; hippocampus; learning and memory; Morris water maze; traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a significant health care issue worldwide with an estimated 10 million cases annually and 1.7 million of those occurring in the United States.1–5 Children comprise one of the high-risk groups for TBI.1 Specifically, 475,000 children under the age of 15 acquire a TBI each year, of which 37,000 are hospitalized, and more than 2600 do not survive, making it the leading cause of death and disability in the pediatric population.6–8 Of the surviving children, it is estimated that approximately half exhibit physical, cognitive, and/or psychosocial deficits.8–10 Because of the devastating consequences induced by TBI in a population that could be negatively affected for decades, studies that focus on functional recovery after pediatric TBI are critical.
Numerous pharmacotherapies, acting on various neurotransmitter systems, have been examined as treatments for functional outcome after experimental TBI.11–14 One class of drugs that has received attention is the serotonin1A (5-HT1A) receptor agonists. The 5-HT system, and in particular the 5-HT1A receptor subtype, is an integral component of cognitive processing.15–17 Several studies have shown that systemic administration of early-and-single (i.e., acute) as well as delayed-and-multiple (i.e., chronic) treatments with the 5-HT1A receptor agonists repinotan HCL and 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) confer neurobehavioral benefits after TBI.18–24 More recently, buspirone, also a 5-HT1A receptor agonist, has been reported to enhance cognitive performance as well as significantly reduce cortical lesion volume after TBI.25,26 This therapeutic paradigm, however, has not been evaluated as a treatment for experimental pediatric brain injury.
In addition to pharmacotherapies, noninvasive approaches have also been investigated after TBI. Environmental enrichment (EE), in particular, has been evaluated extensively in adult, and to a much lesser extent, in pediatric rodents as a pre-clinical model of neurorehabilitation. EE consists of expansive accommodations, which include cognitive and social stimulation that results in the reduction of histological damage and enhancement of functional recovery27 induced by either controlled cortical impact (CCI)20,23,26–34 or fluid percussion.35–40
Given that the 5-HT1A receptor agonist buspirone and EE confer recovery in adult rats after brain trauma, the goals of this study were to evaluate the potential efficacy of these therapies alone and in combination after pediatric TBI. It was hypothesized that buspirone and EE will attenuate histological damage and enhance cognitive recovery after CCI injury, but the combination will be more efficacious than either therapeutic approach individually. Two experiments were conducted to test the hypothesis; the first consisted of determining the best dose of buspirone in rats housed in standard (STD) living conditions, and the second combined the optimal dose of buspirone with EE to determine potential additive effects.
Methods
Experiment 1: Buspirone dose response
Subjects and surgery
Fifty-two male Sprague-Dawley pediatric rats (Harlan, Indianapolis, IN) were initially housed with their dams in a Plexiglas cage and maintained in a temperature (21±1°C) and light (on 0700 to 1900) controlled environment. The rats were all postnatal day-17 (PND-17) on the day of surgery, which consisted of a CCI injury that was produced as described previously,19,22,28,41,42 with the exception of some necessary modifications for the smaller rats. Specifically, surgical anesthesia was induced and maintained with inspired concentrations of 4% and 2% isoflurane (carried in 2:1 N2O/O2), respectively, via a nose cone. The rats were secured to a stereotaxic frame, and core temperature was monitored with a rectal thermometer and maintained at 37±0.5°C with a heating blanket.
Using aseptic procedures, a midline scalp incision was made, the skin and fascia were reflected to expose the skull, and a craniectomy was produced in the right hemisphere (between bregma/lambda and the sagittal suture/coronal ridge) with a high-speed dental drill. The impact tip (6 mm, flat) was lowered and centered through the craniectomy such that it produced a brain injury of moderate severity (2.2 mm tissue deformation at 4 m/sec). Immediately after the CCI, anesthesia was discontinued, and the incision was promptly sutured. Rats were assessed for acute neurological outcome and placed in a heated chamber (maintained at 37±0.5° C) until able to ambulate freely and then were returned to their dam in the colony. Sham rats underwent comparable surgical procedures, sans the CCI.
Post-surgery
After surgery, the rats were randomly assigned to four TBI groups, which are denoted as TBI+buspirone (0.08 mg/kg; n=9), TBI+buspirone (0.1 mg/kg; n=9), TBI+buspirone (0.3 mg/kg; n=9), TBI+vehicle (1.0 mL/kg; n=9), and four sham control groups (n=16) that received the same doses of buspirone and vehicle as their TBI counterparts. The pediatric pups were weaned on PND-28 and pair-housed in standard (STD) steel-wire mesh cages where they had ad libitum access to rat chow and water. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Every attempt was made to minimize discomfort, suffering, and the number of animals used.
Acute neurological evaluation
Hindlimb reflexive ability was assessed immediately after the discontinuation of anesthesia by gently squeezing the rat's paw every 5 sec and recording the latency to elicit a withdrawal response. Return of the righting reflex was determined by recording the time needed for the rat to return to the prone position after three consecutive supine placements.
Drug administration
Buspirone (Sigma-Aldrich; St. Louis, MO) was prepared daily by dissolving in sterile saline, which also served as the vehicle. Buspirone (0.08 mg/kg, 0.1 mg/kg, or 0.3 mg/kg) and vehicle (1.0 mL/kg) were administered intraperitoneally (i.p.) beginning 24 h after surgery and once daily for 16 days. On the days of behavioral testing, the injections were administered 1 h before testing to avoid behavioral symptoms associated with the serotonin syndrome (e.g., flat body posture) that could affect performance. The doses and route of administration were selected based on previous data from our laboratory showing a narrow therapeutic range.25
Cognitive function: spatial learning
Acquisition of spatial learning was assessed using a Morris water maze (MWM)43 task that has been shown to be a sensitive indicator of cognitive function after TBI.23,44–47 The maze consisted of a plastic pool (180 cm diameter; 60 cm high) filled with tap water (26±1°C) to a depth of 28 cm and situated in a room with prominent visual cues. The platform was a clear Plexiglas stand (10 cm diameter, 26 cm high) that was positioned 26 cm from the maze wall in the southwest quadrant and held constant for each rat.
Spatial learning consisted on providing four blocks of daily trials (four min intertrial interval) for 5 consecutive days (post-operative days 11–15) in which the rat was given a maximum of 120 sec to find the submerged platform (2 cm below the water surface (i.e., invisible to the rat). On post-operative day 16, the platform was raised 2 cm above the water surface (i.e., visible to the rat) as a control to determine the contributions of nonspatial factors (e.g., sensorimotor performance, motivation, and visual acuity) on cognitive performance. Each trial lasted until the rat climbed onto the platform or the maximum allotted time had elapsed, whichever occurred first. If the rats did not find the platform within the given time, they were manually guided to it. All rats remained on the platform for 30 cumulative sec and then were returned to a heated incubator between trials. The times of the four daily trials for each rat were averaged and used in the statistical analyses.
Cognitive function: memory retention
One day after the final acquisition training session in the MWM (i.e., day 16), all rats were given a single probe trial to measure memory retention. During this trial, the platform was removed from the pool, and the rats were placed in the maze from the location point most distal to the southwest quadrant (i.e., the “target quadrant” where the platform was previously located) and allowed to freely explore the pool for 30 sec and the percent time spent in the target quadrant was recorded. A spontaneous motor activity recording and tracking (SMART) system (San Diego Instruments, San Diego, CA) was used to record the data, which included time to locate the platform and percent time in the target quadrant.
Histology: quantification of cortical lesion volume
On post-operative day 17, the rats were anesthetized with pentobarbital (50 mg/kg, i.p.), and then perfused transcardially with 200 mL 0.1M phosphate-buffed saline (pH 7.4) and 300 mL 4% paraformaldehyde. The brains were extracted, post-fixed in the perfusate for 1 week, dehydrated with alcohols, and embedded in paraffin wax. Seven-micrometer-thick coronal sections were cut at 1-mm intervals throughout the lesion using a rotary microtome and mounted on gelatin-coated glass microscope slides. After drying at room temperature, the coronal sections were deparaffinized in xylenes, rehydrated, and stained with cresyl violet. Cortical lesion volumes (mm3) were determined by first calculating the area of the lesion (mm2) by outlining the inferred area of missing cortical tissue for each section taken at 1-mm intervals (MCID software; Imagining Research, Ontario, Canada), and then multiplying the distance between each section (1 mm) by the sum of the lesion area obtained from each section.
Statistical analysis
Data analyses were performed using StatView 5.0.1 software (Abacus Concepts, Inc., Berkeley, CA) by observers blinded to treatment conditions. The cognitive data were analyzed by repeated-measures analysis of variance (ANOVA), while the acute neurological evaluations, probe trial, swim speed, and histological data were analyzed by one-factor ANOVAs. When the ANOVA revealed a significant effect, the Fisher PLSD post-hoc test was used to determine specific group differences. The results are expressed as the mean±standard error of the mean (SEM) and are considered significant when p values are≤0.05.
Results
Experiment 1: Buspirone dose response
Sham controls did not differ from one another, regardless of treatments, and thus they were pooled into one group (denoted as “SHAM”).
Acute neurological function
No significant differences were detected among the TBI groups in hindlimb withdrawal response (right range=131.7±6.3 sec to 145.9±5.8 sec; left range=134.0±6.4 sec to 149.2±5.9 sec) or righting ability (range=217.3±4.5 sec to 266.6±38.9 sec) after the cessation of anesthesia (all p>0.05). The lack of significant differences with these acute neurological indices suggests that all groups experienced equivalent injury severity and level of anesthesia.
Cognitive function: acquisition of spatial learning
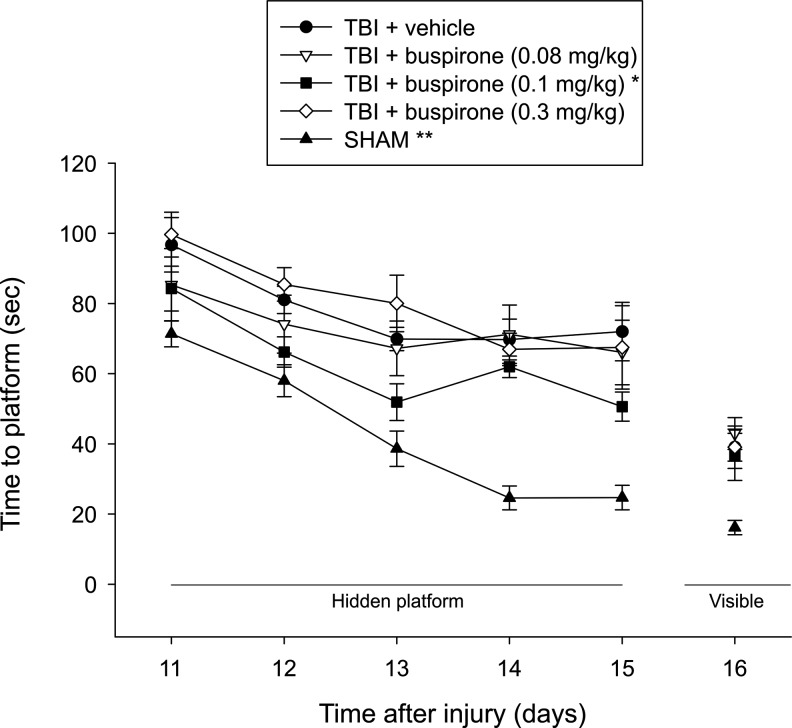
Analysis of the water maze data revealed significant Group (F4,47=24.346, p<0.0001) and Day (F4,188=24.464, p<0.0001) differences, but no Group×Day interaction (F16,188=1.418, p=0.14). The post-hoc analysis revealed that the SHAM group performed significantly better than all TBI groups, regardless of treatment (p<0.0001). Moreover, the TBI+buspirone (0.1 mg/kg) group learned the location of the escape platform significantly quicker over the 5 days of training relative to the TBI+buspirone (0.08 mg/kg), TBI+buspirone (0.3 mg/kg), and TBI+vehicle (1.0 mL/kg) groups (p<0.05; Fig. 1). No other group comparisons were significant (p>0.05). Regarding performance on the visible platform task, the ANOVA revealed a significant group difference (F4,47=7.207, p=0.0001), which was attributed to the SHAM control needing less time to reach the platform versus the TBI groups (p<0.001), which did not differ from one another (p>0.05).
FIG. 1.
Mean (±standard error of the mean) time (sec) to locate a hidden and visible platform in a water maze. For the hidden platform assessments, *p<0.05 versus TBI+buspirone (0.08 mg/kg), TBI+buspirone (0.3 mg/kg), and TBI+vehicle (1.0 mL/kg) and **p<0.0001 versus all TBI groups. For the visible platform assessment, the SHAM group was able to locate the platform significantly quicker than all TBI groups (p<0.001). No other group comparisons were significant.
Cognitive function: probe trial (memory retention)
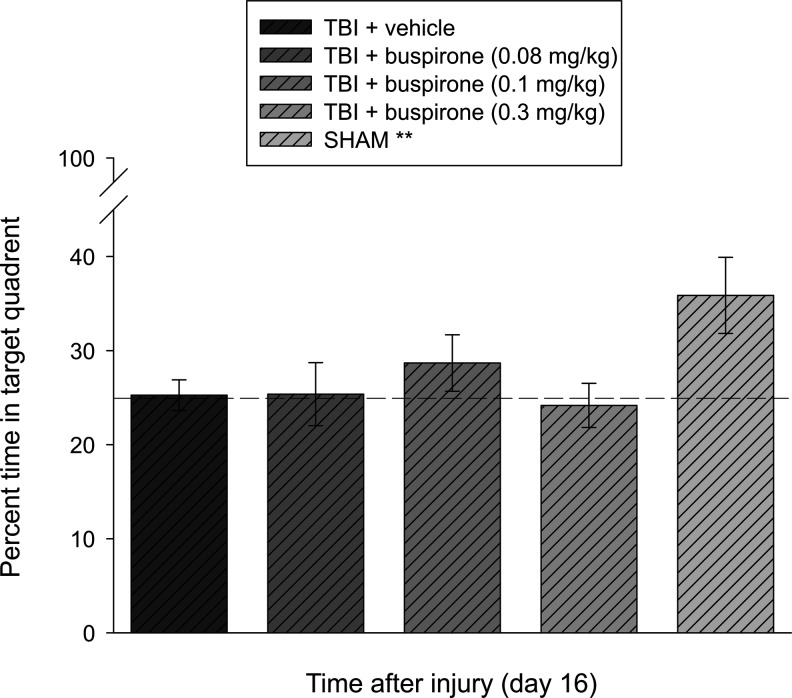
Analysis of the single probe trial data did not reveal a significant difference among groups (F4,47=2.376, p=0.052). Closer inspection of the data with the post-hoc test, however, showed that the SHAM group performed better than the TBI+buspirone (0.08 mg/kg), TBI+buspirone (0.3 mg/kg), and TBI+vehicle (1.0 mL/kg) groups with percent times in the target quadrant of 35.9±4.0 versus 25.4±3.2, 24.2±2.3, and 25.3±1.6, respectively (p<0.01). The SHAM group did not differ from the TBI+buspirone (0.1 mg/kg) group, which spent 28.7±1.6% of the allotted time in the target zone (p=0.13). No other group comparisons were statistically different (Fig. 2).
FIG. 2.
Mean (±SEM) percentage of time spent in the target quadrant (i.e., where platform was previously located) after a single probe trial 16 days after TBI or sham injury. No significant differences were observed among the TBI groups (p>0.05). **p<0.01 vs. TBI+buspirone (0.08 mg/kg), TBI+buspirone (0.3 mg/kg), and TBI+vehicle (1.0 mL/kg), but not TBI+buspirone (0.1 mg/kg) (p=0.13). The dotted line represents performance at the chance level (25%).
Histology: cortical lesion volume
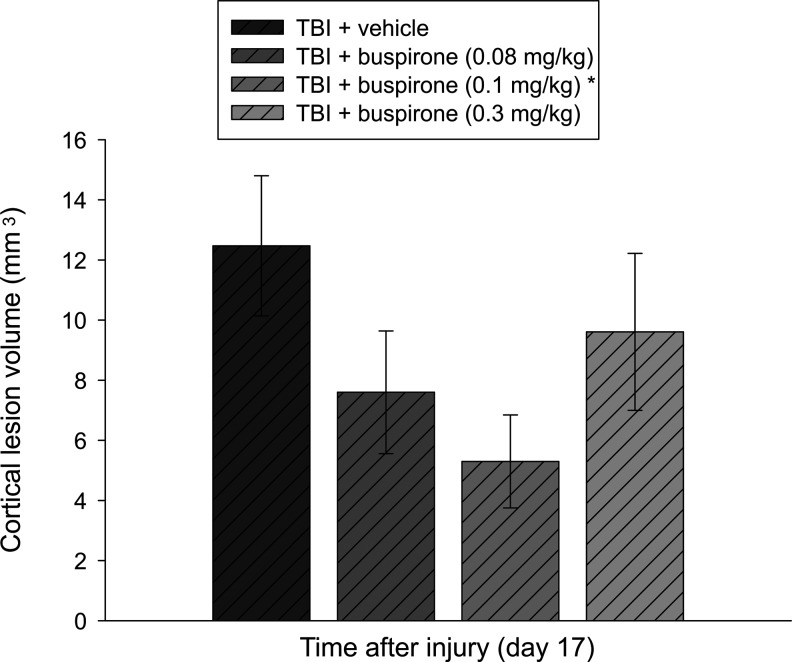
Analysis of the lesion data showed a significant difference between the TBI+vehicle (1.0 mL/kg) and TBI+buspirone (0.1 mg/kg) groups with mean cortical lesion volumes of 12.5±2.3 and 5.3±1.5 mm3, respectively (p=0.038). No other group comparisons were statistically different (Fig. 3).
FIG. 3.
Mean (±SEM) cortical lesion volume (mm3) at 17 days after cortical impact injury. *p<0.05 vs. TBI+vehicle (1.0 mL/kg). No other group comparisons were statistically different.
Methods
Experiment 2: Combined therapeutic paradigm: buspirone+EE
Subjects and surgery
An additional 26 pediatric male rats were added to this experiment, which consisted of two TBI (n=9 per group) and two sham control (n=4 per group) groups denoted as TBI+vehicle (1.0 mL/kg)+EE, TBI+buspirone (0.1 mg/kg)+EE, sham+vehicle (1.0 mL/kg)+EE, and sham+buspirone (0.1 mg/kg)+EE. Initial housing, surgery, and weaning were identical to that described for Experiment 1.
Drug administration
The dose of buspirone for comparison between the STD and EE groups was selected based on the finding from Experiment 1 showing that 0.1 mg/kg was most effective. Drug preparation and administration were identical to that described for Experiment 1.
Housing conditions: environmental manipulation
After weaning (PND-28), the rats were placed in their respective assigned housing conditions. The EE consisted of specifically designed 36×30×20-inch stainless steel wire cages consisting of three levels and ladders to ambulate from one level to another such that interaction with the various toys (e.g., balls, blocks, and tubes), nesting materials (e.g., paper towels), cage mates, and ad libitum food and water would be achievable (for depiction of EE cage, see 20,29). To maintain novelty, the objects were rearranged every day and changed each time the cage was cleaned, which was twice per week. Ten rats, which included TBI and sham controls, were housed together to minimize variability. Rats in the STD conditions were placed in standard steel wire mesh cages (two rats per cage) with only food and water available.
Cognitive function: spatial learning and retention
Assessment of the acquisition of spatial learning and memory retention was identical to that described for Experiment 1.
Histology: quantification of cortical lesion volume
Methods were identical to those described for Experiment 1.
Results
Experiment 2: Combined therapeutic paradigm: buspirone+EE
The sham controls provided buspirone or vehicle and housed in EE conditions did not differ from the shams in Experiment 1, and thus they were pooled into one group (denoted as “SHAM”).
Acute neurological function
No significant differences were detected among the TBI groups in hindlimb withdrawal response (right range=131.7±6.3 sec to 147.8±3.1 sec; left range=134.0±6.4 sec to 151.0±3.1 sec) or righting ability (range=226.9±13.5 sec to 266.6±38.9 sec) following the cessation of anesthesia (all p>0.05).
Cognitive function: acquisition of spatial learning
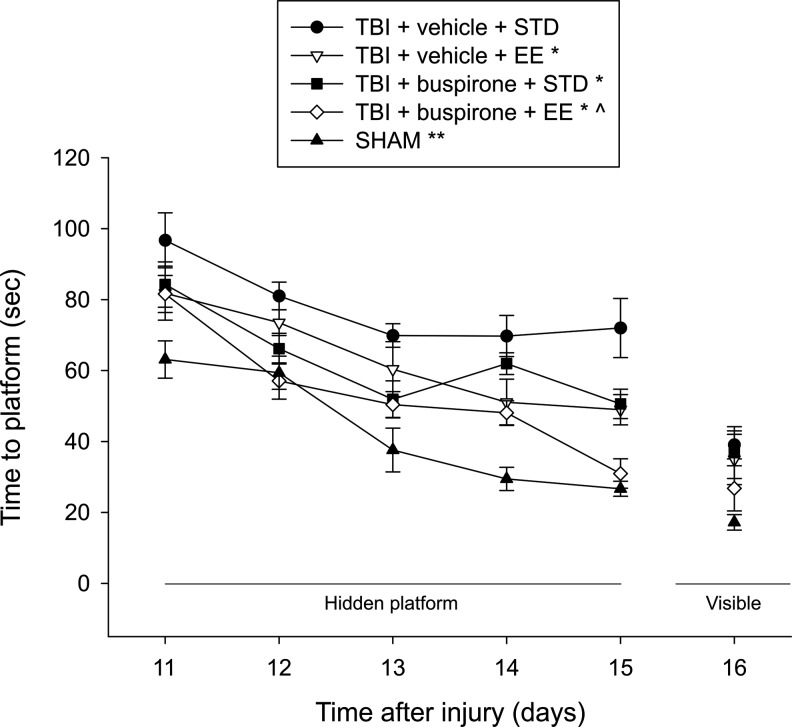
Analysis of the water maze data revealed significant Group (F4,47=22.793, p<0.0001) and Day (F4,188=39.877, p<0.0001) differences, but no Group×Day interaction (F16,188=1.238, p=0.21). The post-hoc analysis revealed several group differences. The SHAM group performed significantly better than all TBI groups, regardless of treatment (p<0.009). In addition, the TBI+vehicle (1.0 mL/kg)+EE, TBI+buspirone (0.1 mg/kg)+STD, and TBI+buspirone (0.1 mg/kg)+EE groups learned the location of the escape platform significantly quicker over the 5 days of training relative to the TBI+vehicle (1.0 mL/kg)+STD group (p<0.05; Fig. 4). Moreover, the TBI+buspirone (0.1 mg/kg)+EE group was significantly better than the TBI+vehicle (1.0 mL/kg)+EE and TBI+buspirone (0.1 mg/kg)+STD groups (p<0.05), which did not differ from one another (p> 0.05).
FIG. 4.
Mean (±SEM) time (sec) to locate a hidden and visible platform in a water maze. For the hidden platform assessments, *p<0.05 versus TBI+vehicle+STD, ^p<0.05 versus TBI+vehicle+EE and TBI+buspirone+STD groups, and **p<0.005 versus all TBI groups. For the visible platform assessment, the SHAM group was able to locate the platform significantly quicker than the TBI+vehicle+STD, TBI+vehicle+EE, and TBI+buspirone+STD groups (p<0.01), but not the TBI+buspirone+EE group (p=0.17). No other group comparisons were significant.
Regarding performance on the visible platform task, the ANOVA revealed a significant Group difference (F4,47=3.749, p=0.01), which was attributed to the SHAM controls needing less time to reach the platform versus the TBI+vehicle (1.0 mL/kg)+STD, TBI+vehicle (1.0 mL/kg)+EE, and TBI+buspirone (0.1 mg/kg)+STD groups (p<0.01), but not the TBI+buspirone (0.1 mg/kg)+EE group (p=0.17).
Cognitive function: probe trial (memory retention)
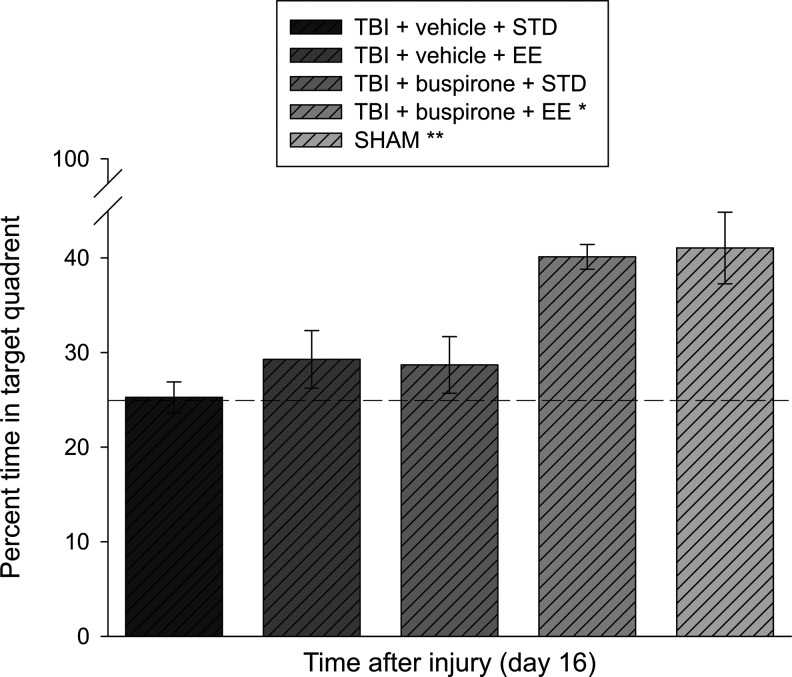
Analysis of the single probe trial data revealed a significant difference among groups (F4,46=4.041, p=0.007). The differences as delineated by the post-hoc test concluded that the TBI+buspirone (0.1 mg/kg)+EE group performed better than the TBI+vehicle (1.0 mL/kg)+STD, TBI+vehicle (1.0 mL/kg)+EE, and TBI+buspirone (0.1 mg/kg)+STD groups, with percent times in the target quadrant of 40.1±1.3 versus 25.3±1.6, 29.3±3.0, and 28.7±1.6%, respectively (p<0.05). The SHAM group, which spent 41.1±4.7% of the allotted time in the target zone, did not differ from the TBI+buspirone (0.1 mg/kg)+EE group (p>0.05), but was significantly better than all other TBI groups (p<0.05). No other group comparisons were significant (Fig. 5).
FIG. 5.
Mean (±SEM) percentage of time spent in the target quadrant (i.e., where platform was previously located) after a single probe trial 16 days after TBI or sham injury. *p<0.05 vs. all other TBI groups. **p<0.05 vs. all TBI groups, except TBI+buspirone+EE (p=0.85). The dotted line represents performance at the chance level (25%).
Histology: cortical lesion volume
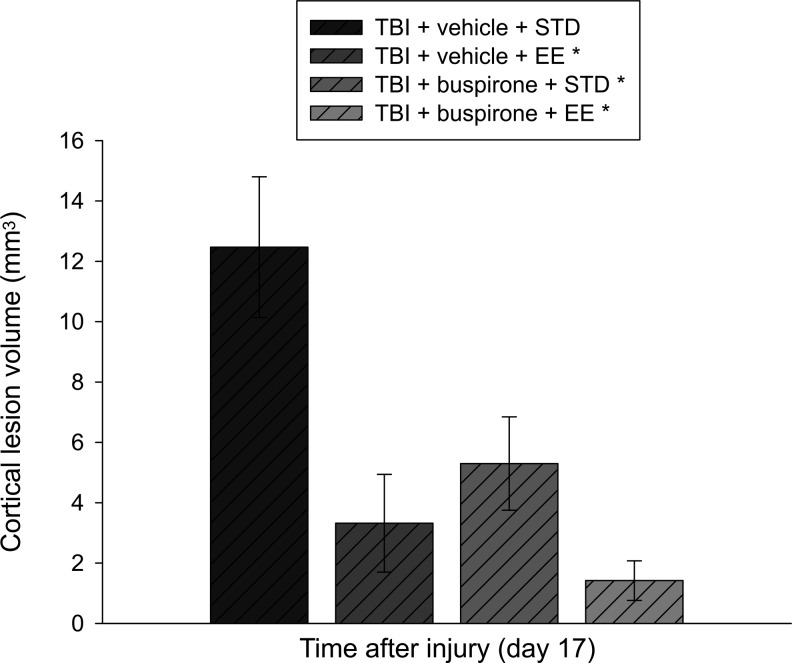
Analysis of the lesion data revealed that the TBI+vehicle (1.0 mL/kg)+EE, TBI+buspirone (0.1 mg/kg)+STD, and TBI+buspirone (0.1 mg/kg)+EE groups had smaller cortical lesion volumes relative to the TBI+vehicle (1.0 mL/kg)+STD group with mean lesion volumes of 3.3±1.6 mm3, 5.3±1.5 mm3, 1.4±0.6 mm3, and 12.5±2.3, respectively (p<0.05). No other group comparisons were statistically different (Fig. 6).
FIG. 6.
Mean (±SEM) cortical lesion volume (mm3) at 17 days after cortical impact injury. *p<0.005 vs. TBI+vehicle+STD. No other group comparisons were statistically different.
Discussion
Serotonin1A (5-HT1A) receptor agonists have been reported to improve cognitive performance and attenuate histological damage in adult male rats after CCI injury.18–26 Likewise, EE has also been shown to confer neurobehavioral and histological benefits in adult male rats after CCI20,23,26–34 and fluid percussion brain injury.35–40 Recently, the benefits of EE have also been extended to female rats after CCI.33 Despite the consistent benefits produced by these therapies in adult rodent models of TBI, they have received limited attention in pediatric studies. Determining potentially efficacious therapies for pediatric TBI, which often result from falls or sports-related activities, is of great concern because it is the leading cause of death and disability in this age population.6–8
Hence, the aim of this study was to determine whether buspirone, a 5-HT1A agonist, and EE would enhance cognition and attenuate histological damage in male pediatric rats. Two experiments were conducted to test the hypothesis that both treatments would confer benefits when provided alone and that their combination would be more efficacious.
The data from Experiment 1 revealed a significant enhancement in cognitive performance as determined by shorter times to find the escape platform in a MWM task after treatment with the middle dose of buspirone (0.1 mg/kg). Histological protection was also observed with the same dose of buspirone as demonstrated by a significant reduction in cortical lesion volume relative to the vehicle control group. These findings parallel studies in adult rats in which buspirone and other 5-HT1A receptor agonists (i.e., 8-OH-DPAT) benefitted neurobehavioral outcome and histology after experimental TBI.19–25,42 The middle dose of buspirone was also significantly better than the lowest and highest doses administered (0.08 mg/kg and 0.3 mg/kg, respectively) in the acquisition of spatial learning, but not in memory retention or histological outcome. The narrow dose response of buspirone in pediatrics is similar to that observed in adult rats treated with buspirone25 or 8-OH-DPAT19 and in non-TBI models of cognition.16
Experiment 2 was designed to test the potential additive effect of the best dose of buspirone from Experiment 1, which in this case was 0.1 mg/kg, and combining it with EE. The data revealed that the TBI group receiving EE plus vehicle (i.e., EE alone group) performed significantly better in the acquisition task in the MWM than the vehicle-treated STD housed group, which replicates numerous studies in adult models of TBI.18–33,36 EE alone, however, did not lead to an increase in memory retention, which is in contrast to that seen in adult models.23,26,29,33 As reported for Experiment 1, the buspirone (0.1 mg/kg) STD-housed group was also significantly better than the STD vehicle group in the MWM. The data also showed that the combination group (buspirone, 0.1 mg/kg+EE) performed markedly better than the single therapy groups (i.e., EE plus vehicle and STD buspirone) in both the acquisition of spatial learning and memory retention, which suggests an additive effect. This cumulative effect on cognition is novel after CCI injury because it has not been observed in adult rats using these therapies.26
Mechanisms for the synergy between EE and buspirone in the developing versus the adult rodent brain remain undetermined, but some comments are warranted. Regarding the benefits conferred by buspirone alone in adult rats, possible mechanisms may include those previously reported for other 5-HT1A receptor agonists, such as 8-OH-DPAT and repinotan HCL. It is well known that the 5-HT1A receptor subtype interacts with several neurotransmitter systems.15–17 Buspirone has been reported to increase dopamine (DA) levels in regions considered essential for cognitive processing such as the prefrontal cortex and hippocampus.15,48 In addition, numerous studies have shown that DA neurotransmission is important for spatial learning and memory (for excellent reviews, see 10–13).
Another potential mechanism may be neuroprotection or restoration of the cholinergic system as evidenced by data showing that 8-OH-DPAT reduced the TBI-induced loss of choline acetyltransferase-positive medial septal cells, which correlated with improved cognitive performance.23 Moreover, repinotan HCL has been shown to increase choline acetyltransferase activity,49 which is correlated with cholinergic neurotransmission. While the DA and 5-HT systems are significantly developed at the PND age of the immature rats used in the present study, the acetylcholine system, however, is not.50 Consequently, the DA system may have a greater role in pediatric rats compared with adults in response to buspirone treatment alone.
A number of structural and biochemical events have been associated with EE and plasticity-related reparative responses, such as increases in neurogenesis, synaptogenesis, dendritic growth/density, or nerve growth factor51–56 and may contribute, in part, to the benefits conferred by EE in pediatric rats. In addition, EE attenuates TBI-induced choline acetyltransferase-positive medial septal cell loss in adult rats,23 but this remains to be evaluated at the PND age of immature rats used in the present study. This mechanism is similar to that reported for 5-HT1A receptor agonists and could contribute to the additive cognitive benefit observed with buspirone plus EE in immature rats if medial septal sparing is ultimately found. A burst of synaptogenesis and the establishment of changes in synaptic efficiency associated with memory processing, however, have been documented at the PND ages used in the present study,57 which may make the developing brain more sensitive to EE treatment than the adult rat brain and contribute to the increased efficacy of the combination of EE and buspirone treatment in pediatric versus adult rat populations.
Overall, the data demonstrate that both buspirone and EE can provide cognitive and histological benefits after pediatric TBI. In addition, the combination of treatments leads to an additive effect as demonstrated by cognitive improvement beyond that observed with the individual therapies, which supports the hypothesis. These findings may have important rehabilitation-relevant implications for clinical pediatric TBI. Future studies should build on this initial, and beneficial, therapeutic approach for pediatric TBI by determining whether other 5-HT1A receptor agonists can also induce benefits as well as testing these pharmacotherapies alone or in conjunction with EE. In addition, to make the treatments even more clinically relevant, delayed and abbreviated EE studies should be incorporated into the experimental design as we have been doing in adult male and female rats after CCI injury.28,30–32
Acknowledgment
Supported, in part, by NIH grants HD046700, NS060005, and HD069620 (AEK).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Xu L., Wald M.M., and Coronado V.G. (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta [Google Scholar]
- 2.Summers C.R., Ivins B., and Schwab K.A. (2009). Traumatic brain injury in the United States: an epidemiologic overview. Mt. Sinai J. Med. 76, 105–110 [DOI] [PubMed] [Google Scholar]
- 3.Selassie A.W., Zaloshnja E., Langlois J.A., Miller T., Jones P., and Steiner C. (2008). Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 23, 123–131 [DOI] [PubMed] [Google Scholar]
- 4.Thurman D., and Guerrero J. (1999). Trends in hospitalizations associated with traumatic brain injury. JAMA 282, 954–957 [DOI] [PubMed] [Google Scholar]
- 5.Sosin D.M., Sniezek J.E., and Waxweiler R.J. (1995). Trends in death associated with traumatic brain injury, 1979 through 1992. JAMA 273, 1778–1780 [PubMed] [Google Scholar]
- 6.Langlois J.A., Rutland-Brown W., and Thomas K.E. (2004). Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta [Google Scholar]
- 7.Keenan H.T., Runyan D.K., Marshall S.W., Nocera M.A., and Merten D.F. (2004). A population-based comparison of clinical and outcome characteristics of young children with serious inflicted and noninflicted traumatic brain injury. Pediatrics 114, 633–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babikian T., and Asarnow R. (2009). Neurocognitive outcomes and recovery after pediatric TBI: meta-analytic review of the literature. Neuropyschology 23, 283–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson V., Catroppa C., Morse S., Haritou F., and Rosenfeld J. (2001). Outcome from mild head injury in young children: A prospective study. J. Clin. Exp. Neuropsychol. 23, 705–717 [DOI] [PubMed] [Google Scholar]
- 10.Hawley C.A., Ward A.B., Magnay A.R., and Long J. (2004). Outcomes following childhood head injury: a population study. J. Neurol. Neurosurg. Psychiatry 75, 737–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kokiko O.N., and Hamm R.J. (2007). A review of pharmacological treatments used in experimental models of traumatic brain injury. Brain Inj. 21, 259–274 [DOI] [PubMed] [Google Scholar]
- 12.Bales J.W., Wagner A.K., Kline A.E., and Dixon C.E. (2009). Persistent cognitive dysfunction after traumatic brain injury: A dopamine hypothesis. Neurosci. Biobehav. Rev. 33, 981–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheaton P., Mathias J.L., and Vink R. (2009). Impact of early pharmacological treatment on cognitive and behavioral outcome after traumatic brain injury in adults: a meta-analysis. J. Clin. Psychopharmacol. 29, 468–477 [DOI] [PubMed] [Google Scholar]
- 14.Garcia A.N., Shah M.A., Dixon C.E., Wagner A.K., and Kline A.E. (2011). Biologic and plastic effects of experimental traumatic brain injury treatment paradigms and their relevance to clinical rehabilitation. PM R 3, Suppl 1, S18–S27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes N.M., and Sharp T. (1999). A review of central 5-HT receptors and their function. Neuropharmacology 38, 1083–1152 [DOI] [PubMed] [Google Scholar]
- 16.Meneses A. (1999). 5-HT system and cognition. Neurosci. Biobehav. Rev. 23, 1111–1125 [DOI] [PubMed] [Google Scholar]
- 17.Meneses A., and Perez-Garcia G. (2007). 5-HT1A receptors and memory. Neurosci. Biobehav. Rev. 31, 705–727 [DOI] [PubMed] [Google Scholar]
- 18.Kline A.E., Yu J., Horváth E., Marion D.W., and Dixon C.E. (2001). The selective 5-HT1A receptor agonist repinotan HCl attenuates histopathology and spatial learning deficits following traumatic brain injury in rats. Neuroscience 106, 547–555 [DOI] [PubMed] [Google Scholar]
- 19.Kline A.E., Yu J., Massucci J.L., Zafonte R.D., and Dixon C.E. (2002). Protective effects of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin against traumatic brain injury-induced cognitive deficits and neuropathology in adult male rats. Neurosci. Lett. 333, 179–182 [DOI] [PubMed] [Google Scholar]
- 20.Kline A.E., Wagner A.K., Westergom B.P., Malena R.R., Zafonte R.D., Olsen A.S., Sozda C.N., Luthra P., Panda M., Cheng J.P., and Aslam H.A. (2007). Acute treatment with the 5-HT1A receptor agonist 8-OH-DPAT and chronic environmental enrichment confer neurobehavioral benefit after experimental brain trauma. Behav. Brain Res. 177, 186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng J.P., Aslam H.A., Hoffman A.N., Zafonte R.D., and Kline A.E. (2007). The neurobehavioral benefit conferred by a single systemic administration of 8-OH-DPAT after brain trauma is confined to a narrow therapeutic window. Neurosci. Lett. 416, 165–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng J.P., Hoffman A.N., Zafonte R.D., and Kline A.E. (2008). A delayed and chronic treatment regimen with the 5-HT1A receptor agonist 8-OH-DPAT after cortical impact injury facilitates motor recovery and acquisition of spatial learning. Behav. Brain Res. 194, 79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kline A.E., McAloon R.L., Henderson K.A., Bansal U.K., Ganti B.M., Ahmed R.H., Gibbs R.B., and Sozda C.N. (2010). Evaluation of a combined therapeutic regimen of 8-OH-DPAT and environmental enrichment after experimental traumatic brain injury. J. Neurotrauma 27, 2021–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yelleswarapu N.K., Tay J.K., Fryer W.M., Shah M.A., Garcia A.N., Cheng J.P., and Kline A.E. (2012). Elucidating the role of 5-HT1A and 5-HT7 receptors on 8-OH-DPAT-induced behavioral recovery after experimental traumatic brain injury. Neurosci. Lett. 515, 153–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsen A.S., Sozda C.N., Cheng J.P., Hoffman A.N., and Kline A.E. (2012). Traumatic brain injury-induced cognitive and histological deficits are attenuated by delayed and chronic treatment with the 5-HT1A-receptor agonist buspirone. J. Neurotrauma 29, 1898–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kline A.E., Olsen A.S., Sozda C.N., Hoffman A.N., and Cheng J.P. (2012). Evaluation of a combined treatment paradigm consisting of environmental enrichment and the 5-HT1A receptor agonist buspirone after experimental traumatic brain injury. J. Neurotrauma 29, 1960–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bondi C.O., Klitsch K.C., Leary J.B., and Kline A.E. (2014). Environmental enrichment as a viable neurorehabilitation strategy for experimental traumatic brain injury. J. Neurotrauma 31, 873–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman A.N., Malena R.R., Westergom B.P., Luthra P., Cheng J.P., Aslam H.A., Zafonte R.D., and Kline A.E. (2008). Environmental enrichment-mediated functional improvement after experimental traumatic brain injury is contingent on task-specific neurobehavioral experience. Neurosci. Lett. 431, 226–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sozda C.N., Hoffman A.N., Olsen A.S., Cheng J.P., Zafonte R.D., and Kline A.E. (2010). Empirical comparisons of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. J. Neurotrauma 27, 1047–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matter A.M., Folweiler K.A., Curatolo L.M., and Kline A.E. (2011). Temporal effects of environmental enrichment-mediated functional improvement after experimental traumatic brain injury in rats. Neurorehabil. Neural Repair 25, 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Witt B.W., Ehrenberg K.M., McAloon R.L., Panos A.H., Shaw K.E., Raghavan P.V., Skidmore E.R., and Kline A.E. (2011). Abbreviated environmental enrichment enhances neurobehavioral recovery comparably to continuous exposure after traumatic brain injury. Neurorehabil. Neural Repair 25, 343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng J.P., Shaw K.E., Monaco C.M., Hoffman A.N., Sozda C.N., Olsen A.S., and Kline A.E. (2012). A relatively brief exposure to environmental enrichment after experimental traumatic brain injury confers long-term cognitive benefits. J. Neurotrauma 29, 2684–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monaco C.M., Mattiola V.V., Folweiler K.A., Tay J.K., Yelleswarapu N.K., Curatolo L.M., Matter A.M., Cheng J.P., and Kline A.E. (2013). Environmental enrichment promotes robust functional and histological benefits in female rats after controlled cortical impact injury. Exp. Neurol. 247, 410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Briones T.L., Woods J., and Rogozinska M. (2013). Decreased neuroinflammation and increased brain energy homeostasis following environmental enrichment after mild traumatic brain injury is associated with improvement in cognitive function. Acta Neuropathol. Commun. 1, 57. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Hamm R.J., Temple M.D., O'Dell D.M., Pike B.R., and Lyeth B.G. (1996). Exposure to environmental complexity promotes recovery of cognitive function after traumatic brain injury. J. Neurotrauma 13, 41–47 [DOI] [PubMed] [Google Scholar]
- 36.Passineau M.J., Green E.J., and Dietrich W.D. (2001). Therapeutic effects of environmental enrichment on cognitive function and tissue integrity following severe traumatic brain injury in rats. Exp. Neurol. 168, 373–384 [DOI] [PubMed] [Google Scholar]
- 37.Hicks R.R., Zhang L., Atkinson A., Stevenon M., Veneracion M., and Seroogy K.B. (2002). Environmental enrichment attenuates cognitive deficits, but does not alter neurtrophin gene expression in the hippocampus following lateral fluid percussion brain injury. Neuroscience 112, 631–637 [DOI] [PubMed] [Google Scholar]
- 38.Giza C.C., Griesbach G.S., and Hovda D.A. (2005). Experience-dependent behavioral plasticity is disturbed following traumatic injury to the immature brain. Behav. Brain Res. 157, 11–22 [DOI] [PubMed] [Google Scholar]
- 39.Maegele M., Lippert-Gruener M., Ester-Bode T., Garbe J., Bouillon B., Neugebauer E., Klug N., Lefering R., Neiss W.F., and Angelov D.N. (2005). Multimodal early onset stimulation combined with enriched environment is associated with reduced CNS lesion volume and enhanced reversal of neuromotor dysfunction after traumatic brain injury in rats. Eur. J. Neurosci. 21, 2406–2418 [DOI] [PubMed] [Google Scholar]
- 40.Lippert-Gruener M., Maegele M., Garbe J., and Angelov D.N. (2007). Late effects of enriched environment (EE) plus multimodal early onset stimulation (MEOS) after traumatic brain injury in rats: Ongoing improvement of neuromotor function despite sustained volume of the CNS lesion. Exp. Neurol. 203, 82–94 [DOI] [PubMed] [Google Scholar]
- 41.Dixon C.E., Clifton G.L., Lighthall J.W., Yaghmai A.A., and Hayes R.L. (1991). A controlled cortical impact model of traumatic brain injury in the rat. J. Neurosci. Methods 39, 253–262 [DOI] [PubMed] [Google Scholar]
- 42.Kline A.E., Massucci J.L., Dixon C.E., Zafonte R.D., and Bolinger B.D. (2004). The therapeutic efficacy conferred by the 5HT1A receptor agonist 8-hydroxy-2- (di-n-propylamino)tetralin (8-OH-DPAT) after experimental traumatic brain injury is not mediated by concomitant hypothermia. J. Neurotrauma 21, 175–185 [DOI] [PubMed] [Google Scholar]
- 43.Morris R. (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11, 47–60 [DOI] [PubMed] [Google Scholar]
- 44.Hamm R.J., Dixon C.E., Gbadebo D.M., Singha A.K., Jenkins L.W., Lyeth B.G., and Hayes R.L. (1992). Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma 9, 11–20 [DOI] [PubMed] [Google Scholar]
- 45.Kline A.E., Bolinger B.D., Kochanek P.M., Carlos T.M., Yan H.Q., Jenkins L.W., Marion D.W., and Dixon C.E. (2002). Acute systemic administration of interleukin-10 suppresses the beneficial effects of moderate hypothermia following traumatic brain injury in rats. Brain Res. 937, 22–31 [DOI] [PubMed] [Google Scholar]
- 46.Kline A.E., Massucci J.L., Marion D.W., and Dixon C.E. (2002). Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J. Neurotrauma 19, 415–425 [DOI] [PubMed] [Google Scholar]
- 47.Ji J., Kline A.E., Amoscato A., Samhan-Arias A.K., Sparvero L.J., Tyurin V.A., Tyurina Y.Y., Fink B., Manole M.D., Puccio A.M., Okonkwo D.O., Cheng J.P., Alexander H., Clark R.S., Kochanek P.M., Wipf P., Kagan V.E., and Bayir H. (2012). Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat. Neurosci. 15, 1407–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakaue M., Somboonthum P., Nishihara B., Koyama Y., Hashimoto H., Baba A., and Matsuda T. (2000). Postsynaptic 5-hydroxytryptamine(1A) receptor activation increases in vivo dopamine release in rat prefrontal cortex. Br. J. Pharmacol. 129, 1028–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harkany T., Mulder J., Horvath K.M., Keijser J., van der Meeberg E.K., Nyakas C., and Luiten P.G. (2001). Oral post-lesion administration of 5-HT1A receptor agonist repinotan hydrochloride (BAY x 3702) attenuates NMDA-induced delayed neuronal death in rat magnocellular nucleus basalis. Neuroscience 108, 629–642 [DOI] [PubMed] [Google Scholar]
- 50.Herlenius E., and Lagercrantz H. (2004). Development of neurotransmitter systems during critical periods. Exp. Neurol. 190, Suppl 1, S8–S21 [DOI] [PubMed] [Google Scholar]
- 51.Torasdotter M., Metsis M., Henriksson B.G., Winblad B., and Mohammed A.H. (1998). Environmental enrichment results in higher levels of nerve growth factor mRNA in the rat visual cortex and hippocampus. Behav. Brain Res. 93, 83–90 [DOI] [PubMed] [Google Scholar]
- 52.van Praag K., Kempermann G., and Gage F.H. (2000). Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 1, 191–198 [DOI] [PubMed] [Google Scholar]
- 53.Ip E.Y., Giza C.C., Griesbach G.S., and Hovda D.A. (2002). Effects of enriched environment and fluid percussion injury on dendritic arborization within the cerebral cortex of the developing rat. (2002) J. Neurotrauma 19, 573–585 [DOI] [PubMed] [Google Scholar]
- 54.Frick K.M., and Fernandez S.M. (2003). Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol. Aging 24, 615–626 [DOI] [PubMed] [Google Scholar]
- 55.Leggio M.G., Mandolesi L., Federico F., Spirito F., Ricci B., Gelfo F., and Petrosini L. (2005). Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behav. Brain Res. 163, 78–90 [DOI] [PubMed] [Google Scholar]
- 56.Olson A.K., Eadie B.D., Ernst C., and Christie B.R. (2006). Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus 16, 250–260 [DOI] [PubMed] [Google Scholar]
- 57.Teyler T.J., Perkins A.T., and Harris K.M. (1989). The development of long-term potentiation in hippocampus and neocortex. Neuropsychologia 27, 31–39 [DOI] [PubMed] [Google Scholar]