Abstract
In the Czech Republic, the incidence of human tick-borne encephalitis (TBE) has been increasing over the last two decades. At the same time, populations of game have also shown an upward trend. In this country, the ungulate game is the main host group of hosts for Ixodes ricinus female ticks. This study examined the potential contribution of two most widespread game species (roe deer [Capreolus capreolus] and wild boar [Sus scrofa]) to the high incidence of TBE in the Czech Republic, using the annual numbers of culls as a proxy for the game population. This was an ecological study, with annual figures for geographical areas—municipalities with extended competence (MEC)—used as units of analysis. Between 2003 and 2011, a total of 6213 TBE cases were reported, and 1062,308 roe deer and 989,222 wild boars were culled; the culls of roe deer did not demonstrate a clear temporal trend, but wild boar culls almost doubled (from 77,269 to 143,378 per year). Statistical analyses revealed a positive association between TBE incidence rate and the relative number of culled wild boars. In multivariate analyses, a change in the numbers of culled wild boars between the 25th and 75th percentile was associated with TBE incidence rate ratio of 1.23 (95% confidence interval 1.07–1.41, p=0.003). By contrast, the association of TBE with culled roe deer was not statistically significant (p=0.481). The results suggest that the size of the wild boar population may have contributed to the current high levels and the rising trend in incidence of TBE, whereas the regulated population of roe deer does not seem to be implicated in recent geographical or temporal variations in TBE in the Czech Republic.
Key Words: : Tick-borne encephalitis, Incidence, Game, Wild boar, Roe deer
Introduction
Tick-borne encephalitis (TBE) is an acute neuroinfection caused by an RNA virus from the genus Flavivirus, family Flaviviridae. In the Czech Republic, the cause of TBE is a European subtype of this virus whose main vector is the tick Ixodes ricinus. TBE is a zoonosis—a vector-transmitted infection of wild animals, circulating, under suitable environmental conditions, independently from humans but accidentally communicable to humans. In most cases, this is as a result of the attachment of an infected tick to the skin or, less often, as a food-borne infection resulting from the consumption of heat-untreated milk from lactating pasture farm animals that were previously infected by tick-borne encephalitis virus (TBEV) and had been excreted it in milk.
I. ricinus is a three-host tick, with each of its three active developmental stages taking a single blood meal from a different host, at both the species and ecological group levels. The hosts of lower developmental stages are generally small ground mammals that are the major source of TBEV infection. The hosts of adult I. ricinus ticks are primarily large animals—wild bisulcate ungulates (game) or pasture farm animals. This parasite–host relationship is vital for the I. ricinus female, which attached to the host for 10–14 days to take a blood meal that is large enough to make it possible for it to lay viable eggs. This is crucial for a successful completion of the I. ricinus life cycle, which is the point of departure for a stable I. ricinus population involved in both the zoonotic circulation of TBEV and maintenance of natural foci of TBE.
In the Czech Republic, human cases of TBE have been increasing in the last two decades, with the incidence rates varying between administrative regions. At the same time, game populations have also shown an upward trend. In this context, the question arises as to whether and to what extent the two phenomena can be interrelated. In the Czech Republic, bisulcate game is the main host of I. ricinus tick females, because farm animals are pastured to a limited extent only. We focused on two game species most widespread in the Czech Republic—roe deer (Capreolus capreolus) and wild boar (Sus scrofa), whose contribution to the high incidence of TBE can be assumed.
Materials and Methods
The smallest territorial administrative unit of the Czech Republic, for which data since 2003 is reported to national databases on game populations as well as on relevant demographic and socioeconomic characteristics is the municipality with extended competence (MEC). The Czech Republic comprises 205 MECs with adjacent areas and the capital Prague as a separate entity (treated here as an additional MEC). A MEC has an average area of 416 km2. The mean altitude in MECs ranges from 170 meters to 800 meters above sea level. In the 2003–2011 study period, the mean MEC population ranged from 9200 to 370,000. The forest and agricultural area in MECs accounted for 52–94% of the total surface area. The source of land area and population data was the Czech Statistical Office.
TBE is a notifiable infectious disease in the Czech Republic. Laboratory-confirmed, clinically manifest TBE cases and/or deaths from TBE are subject to notification to the Epidat system. The records contain data on the date of disease onset, patient's age and gender, place of residence, previous immunization, and place of infection acquisition, whenever possible. Each TBE case was assigned to the respective MEC of residence. The mean annual incidence of TBE per 100,000 permanent residents was calculated.
Game populations were estimated from the numbers of culled roe deer and wild boars provided by the Forestry and Game Management Research Institute, Prague-Zbraslav. Annual data on culled game for each MEC were related to the size of the respective forest and agricultural area and population size. The data on culled animals have been shown repeatedly to be the only reliable data relevant to roe buck and wild boar population trends in the wild (Anděra and Geisler 2012). The temperature data were obtained from the Czech Hydrometeorological Institute, Prague.
Statistical analysis
Data were available for the period 2003–2011. The basic unit of the analysis was the MEC-year; the statistical analysis was based on annual MEC data (for each of 206 MECs, data for 9 consecutive years were available). Graphical and diagrammatic representations are derived from the mean values of indicators for the entire study period. The relationships between variables were characterized using Spearman correlation coefficients. The analysis of the association between clinically manifest TBE cases, size of the forest and agricultural area, and numbers of culled game in a MEC was based on population-averaged negative binomial regression model that allowed for lag-dependent correlation structure in the form of autoregression of order one. Robust standard errors estimates were used in all regression models.
A model of TBE incidence per 100,000 individuals, depending on the size of the forest and agricultural area and number of culled game, was derived from annual data. For the analysis, the predictors were rescaled and the results are presented as the incidence rate ratios (IRR) per interquartile range (IQR), i.e., IRR expresses the change in TBE incidence associated with a change in a predictor variable from the 25th to the 75th percentile. The calculations were adjusted to account for the differences among areas (at level NUTS2, the Czech Republic is divided into eight administrative areas called cohesion regions), years, and average yearly temperatures (allowing for quadratic dependency). Statistical tests were performed at a significance level of 0.05, and the analyses were done using the Stata software, version 9.2 (StataCorp LP, College Station, TX).
Results
In 2003–2011, a total of 6213 TBE cases were reported in the Czech Republic, with the lowest annual figure of 505 cases in 2004 and the annual peak of 1022 cases in 2006. In the same period, 1,062,308 roe deer individuals and 989,222 wild boars were culled. Whereas the numbers of culled roe deer slightly varied between years (from 98,811 to 131,612), the number of culled wild boars increased from 77,269 to 143,378, with considerable year-by-year fluctuations. Descriptive characteristics of variables used in the analyses for 206 MECs are shown in Table 1. For each MEC, the means and 25th and 75th percentiles of all variables over 9 years were computed.
Table 1.
Descriptive Characteristics of Annual Means of Analyzed Variables
| Variable | 25th percentile | Median | 75th percentile | Interquartile range |
|---|---|---|---|---|
| TBE mean annual incidence rate per 100,000 inhabitants | 2.11 | 4.21 | 9.90 | 7.79 |
| Mid-year population | 20,188 | 29,013 | 53,721 | 33,533 |
| Agriculture and forest area (in thousand ha) | 19,348 | 27,144 | 43,042 | 23,694 |
| Number of culled wild boars | 182.8 | 369.1 | 723.9 | 541.1 |
| Relative count of culled wild boars per 1000 ha of forest and agriculture area | 7.43 | 13.07 | 19.91 | 12.48 |
| Number of culled roe deer | 304.2 | 465.7 | 743.7 | 439.5 |
| Relative count of culled roe deer per 1000 ha of forest and agriculture area | 13.34 | 17.28 | 21.01 | 7.67 |
Based on yearly averages for 206 geographic areas (municipalities with extended competence) as individual units of analysis.
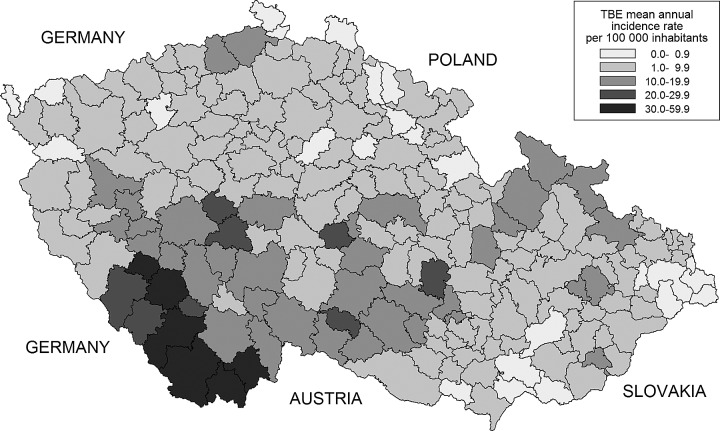
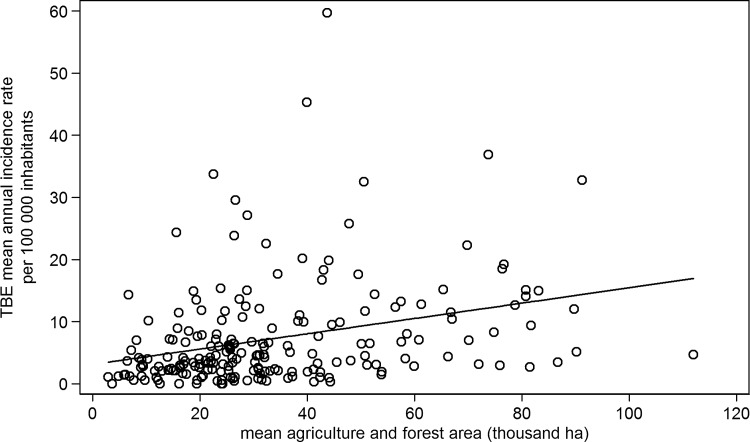
The geographical distribution of mean annual TBE incidence rates by MEC is shown in Figure 1. The overall mean annual TBE incidence rate was 6.7/100,000 population, and the mean rates in MECs ranged from 0 to 59.7/100,000, with a median of 4.2/100,000. The most affected areas were in the south of the Czech Republic, neighboring with Germany and Austria, and the northeast of the Czech Republic, neighboring with Poland. The association of the mean TBE incidence rates with the size of the forest and agricultural area in MECs over the study period is represented in Figure 2.
FIG. 1.
The mean annual tick-borne encephalitis (TBE) incidence rates per 100,000 inhabitants by municipalities with extended competence (MECs) in the Czech Republic 2003–2011.
FIG. 2.
The association of the mean tick-borne encephalitis (TBE) incidence rates per 100,000 inhabitants with the size of the forest and agricultural areas in municipalities with extended competence (MECs) in the Czech Republic in 2003–2011.
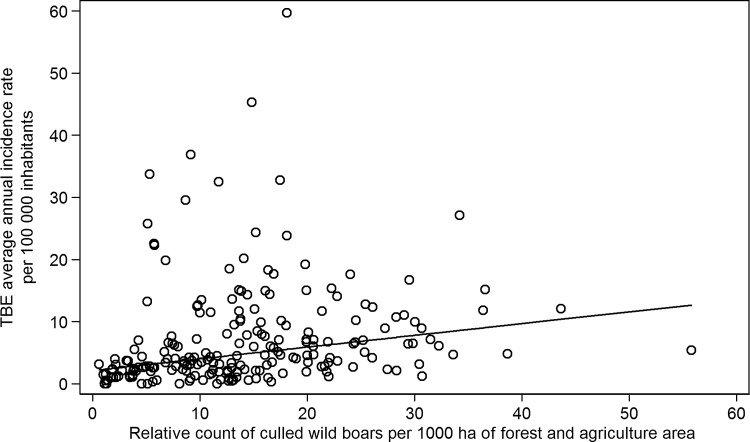
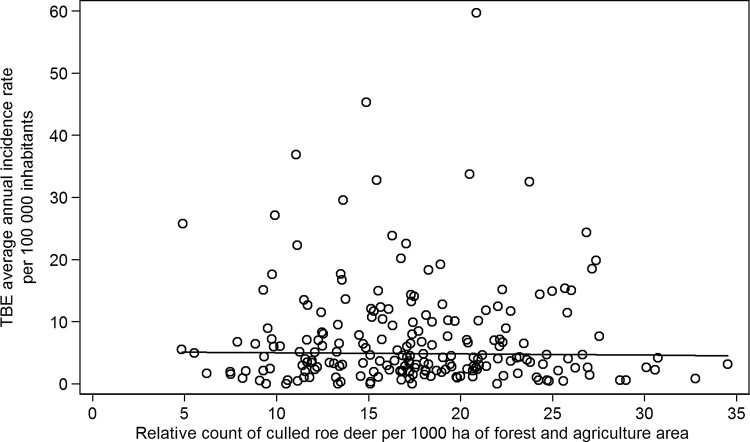
The association of the mean TBE incidence rates with numbers of culled animals is shown in Figure 3 (wild boars) and Figure 4 (roe deer). The figures show considerable variability in analyzed characteristics among MECs. Whereas numbers of culled wild boars show an association with TBE incidence, no relationship between roe deer and TBE was observed. This is also documented by correlation coefficients presented in Table 2. Moreover, the table shows that the correlations between agriculture and forest area and occurrence of game are relatively week. There is no correlation between numbers of culled wild boars and roe deer in MECs.
FIG. 3.
The association of the mean tick-borne encephalitis (TBE) incidence rates per 100,000 inhabitants with the relative count of culled wild boars per 1000 ha of the forest and agricultural areas in municipalities with extended competence (MECs) in the Czech Republic in 2003–2011.
FIG. 4.
The association of the mean tick-borne encephalitis (TBE) incidence rates per 100,000 inhabitants with the relative counts of culled roe deer per 1000 ha of the forest and agricultural area in municipalities with extended competence (MECs) in the Czech Republic in 2003–2011.
Table 2.
Spearman Correlation Coefficients Between Annual Averages of Analyzed Variables
| Variable | TBE mean annual incidence rate per 100,000 inhabitants | Agriculture and forest area | Relative count of culled wild boars per 1000 ha of forest and agriculture area |
|---|---|---|---|
| Agriculture and forest area | 0.365 p<0.001 |
||
| Relative count of culled wild boars per 1000 ha of forest and agriculture area | 0.393 p<0.001 |
0.283 p<0.001 |
|
| Relative count of culled roe deer per 1000 ha of forest and agriculture area | −0.020 p=0.774 |
−0.214 p=0.002 |
0.025 p=0.725 |
Based on yearly averages for 206 geographic areas (municipalities with extended competence) as individual units of analysis.
The left-hand part of Table 3 shows the associations between predictor variables and TBE incidence obtained from univariate regression models. The computations are based on the data for individuals MECs and individual years. The incidence rate ratios are expressed for change between 25th and 75th percentile of particular predictor variable. A significant positive association between the TBE incidence and size of the forest and agricultural area (p<0.001) and a borderline association between the TBE incidence and number of culled wild boars (p=0.080) were found. The TBE incidence was not associated with the number of culled roe deer (p=0.110).
Table 3.
Tick-Borne Encephalitis Incidence Rate Ratios by Change in Predictor Variables from 25th to 75th Percentile
| Univariate model | Multivariate model | |||||
|---|---|---|---|---|---|---|
| Predictor variable | IRR | 95% CI | p value | IRR | 95% CI | p value |
| Forest and agriculture area (in thousand ha) | 1.482 | 1.315–1.671 | <0.001 | 1.182 | 1.045–1.336 | 0.008 |
| Relative count of culled wild boars per 1000 ha of forest and agriculture area | 1.109 | 0.988–1.244 | 0.080 | 1.227 | 1.071–1.405 | 0.003 |
| Relative count of culled roe deer per 1000 ha of forest and agriculture area | 0.892 | 0.775–1.026 | 0.110 | 0.949 | 0.820–1.098 | 0.481 |
95% CI is 95% confidence interval for IRR.
The multivariate IRRs were adjusted to account for the territorial (NUTS2) impact, year, and average yearly temperature.
Based on the data for nine separately assessed years from 206 geographic areas (municipalities with extended competence) as units of analysis.
IRR, incidence rate ratio; CI, confidence interval.
The right-hand part of Table 3 presents the results of multiple regression model adjusted for covariates. The potential effect of all three predictors (size of the forest and agricultural area, number of culled wild boars, number of culled roe deer) was assessed simultaneously along with the adjustment for the effects of the area (NUTS2), year, and average yearly temperature. In this model, a significant positive association was seen between the TBE incidence rate and size of the forest and agricultural area (p=0.008) and between the TBE incidence rate and the relative number of culled wild boars per 1000 hectares of the forest and agricultural area (p=0.003). The effect of wild boars became stronger after adjustment (IRR=1.227), compared to univariate analysis (IRR=1.109). The association between the TBE incidence rate and the relative number of culled roe deer per 1000 hectares of the forest and agricultural area was not statistically significant after adjustment (p=0.481). The overall effects of both region and year were significant (p<0.001 for both factors). Compared to the national rates, significantly higher TBE incidence rates were observed in two areas (NUTS2), southwest and southeast (in the multiple regression model, IRR=2.295 and 1.511, respectively).
Discussion
In this ecological study, a significant positive association was detected between the geographical distribution of TBE incidence rates and the relative number of culled wild boars; on the other hand, the association between TBE incidence rate and the relative number of culled roe deer was not statistically significant. These results support the hypothesis that game populations in the Czech Republic contribute to TBE cases in humans.
A number of papers have provided evidence for the direct contact between roe deer and TBEV-infected ticks as documented, among others, by the detection of anti-TBEV antibodies in the serum of culled animals. In the Czech Republic, the rates of TBEV-positive roe deer ranged from 14.1% to 47.6% (Hubálek et al. 1993, Zeman and Januška 1999). In other European countries, the TBEV positivity rates were in the range of 8.7–50%, suggesting that roe deer is an important link in the spread of TBEV (Borcić et al. 1990, Skarphedinsson et al. 2005, Rizzoli et al. 2009, Kiffner et al. 2011, 2012).
Anti-TBEV antibodies were detected in the serum of 8.5% (Jurincová 1992) and 6.0% (Hubalek et al. 1993) of wild boars culled in the east of the Czech Republic. In Central Bohemia, wild boars had a TBEV seropositivity rate of 20% (Zeman and Januska 1999). In seven regions of The Netherlands, anti-TBEV antibodies were detected in 7% of wild boars (van Der Poel et al. 2005).
A parallel rise in I. ricinus tick and game populations has been observed, particularly over the last two decades. Moreover, this phenomenon is often associated with the spread to new areas (or to areas where these species were previously rare). Medlock et al. (2013) in his review concluded that increased host abundance leading to enhanced tick reproduction in endemic zones and dispersal of roe deer at higher altitude and latitude are key drivers for change in geographical distribution of I. ricinus ticks in Europe. High reproductive potential of roe deer and the capability for disseminating infected ticks to distant areas, far away from the original endemic area, were behind the spread of TBE to the North of Sweden (Jaenson et al. 2012).
Some authors went even further and hypothesized that the relationship between I. ricinus tick and game populations contributed to outbreaks caused by TBEV and other tick-borne pathogens in humans (e.g., Dorrestein et al. 1996, Zeman and Januska 1999, Carpi et al. 2008, Rizzoli et al. 2009, Kiffner et al. 2010). Such interpretation seems reasonable and plausible. However, factors involved in the contact of humans with ticks and subsequent interactions need to be considered when assessing the strength of these relationships. In addition to the tick populations and their role as a viral vector, anthropogenic factors need to be taken into account, namely the population density of the study area and behavior of this population. For this reason, demographic data were also included in our model.
Controversially, some authors concluded that more abundant populations of roe deer, acting as the host of I. ricinus ticks, are a “dead end” for the circulation of TBEV (e.g., Pugliese et al. 2008). This view, however, ignores the possibility of vertical transmission of TBEV during the development of I. ricinus ticks; even after a blood meal from an incompetent host, a female tick that acquired TBEV by transstadial transmission after infection at the nymph stage can pass it transovarially to some eggs and thus also to some larvae of the next generation. Furthermore, co-feeding should also be considered as a possible route of infection (Labuda et al. 1993a, b, c, Randolph et al. 1996). Game can disseminate ticks infected also to relatively distant areas. Engorged tick females drop off their hosts in new areas and lay eggs that are the starting point for new populations of infected ticks. These brief objections also apply to the assumption that greater populations of roe deer do not help the spread of TBEV, rather cause a dilution effect and thus reduce the risk of tick infection by TBEV (Perkins et al. 2006, Rosa and Pugliese 2007, Pugliese and Rosa 2008, Cagnacci et al. 2012, Bolzoni et al. 2012).
This study focused on two species of large game that are currently most widespread in the Czech Republic—roe deer and wild boar. The two species prefer the type of landscape that is also a suitable habitat for I. ricinus tick populations and the formation of natural foci of TBE. It is characterized by a patchwork pattern of forest growths alternated with agricultural areas. The analyses performed relate to these parts of the administrative territorial entities (MEC cadastres). Deer and mouflons are often kept in game preserves, particularly at higher altitudes, with restricted access to the public. Small animals such as hares have become rare over the last decades and hardly play a role in the transmission of TBE. Farm animals such as sheep, goats, and cows (Kriz at al. 2009), which can potentially be involved in the spread of TBE as a food-borne infection (resulting from the consumption of heat-untreated milk and dairy products), play a negligible role given the type of field agriculture in the Czech Republic.
In the Czech Republic, wild boars are more dispersed than roe deer across the forest and agricultural areas. Roe deer live in smaller forest habitats from which they expand to adjacent fields and return back to the woods. Wild boars live in groups and have a considerably greater radius of activity. However, under the conditions of the Czech Republic, it was found that the migration distance was under 5 km for 88% of wild boars and longer than 10 km for 2% of animals in two hunting grounds. An exception in this regard is the migration of males during the rutting season to distances of up to 40 km (Havránek 2005). Wild boars dig up the ground in search for roots to a depth of at least 10 cm, thus coming into contact with infested small rodents. The boars are not as tall as roe deer and while entering the thicket, their hairy bodies make it possible for the ticks to attach and thus travel to distant areas. Ticks can attach to various sites of their body, e.g., specific areas on the head, less hairy areas where legs join the trunk, and bisulcate feet. A significant association between the occurrence of wild boars and TBE cases in our data suggests an important role of these animals in the spread of TBEV infection.
Roe deer populations prefer mosaic landscapes where woods alternate with open areas with a high proportion of scattered greenery such as groves. During the summer, the deer stay in areas of several hectares to several tens of hectares and their long-term habitats range in size between 500 and 600 ha. Wild boars look preferably for deciduous or mixed woods, and they even move outside of the woods in the summer. During the vegetation period, they expand toward crop fields. Their home territory is 200–2000 ha in size, and exceptionally even larger.
Game management monitors several quantitative indicators for particular game species, e.g., the spring stock populations as derived from field observations and the annual numbers of culled individuals. In agreement with zoologists (Anděra and Gaisler 2012) who analyzed long-term changes in the game populations in the Czech Republic, we consider the numbers of culled individuals as a more reliable indicator of game population, and therefore we included this variable in our models.
The differences in the conditions of existence between the populations of the two study species, as well as the dynamics of trends in the Czech Republic, are clearly influenced by anthropogenic factors. The cull of wild boars is not regulated, mainly because they show rapid exponential growth, whereas the roe deer populations have been monitored on a long-term basis and their annual cull is regulated in terms of population numbers and their age and sex distribution.
Conclusions
The results of this study suggest a significant role of wild boars in the increasing incidence of TBE cases in the Czech Republic and support the involvement of this host of the I. ricinus tick as one of the contributors to the spread of TBE in the 1990s. This interpretation is consistent with the increasing unregulated populations of this species and its spread to areas outside the woods and, in the summer, to suburban areas with consequent adaptation to human presence, thus helping the spread of the vector tick I. ricinus. The currently regulated populations of roe deer are important hosts of ticks, contributing to the recent high level of TBE human cases. However, it is unlikely that roe deer are an important driver for further spread of TBE in the Czech Republic in recent years, although they may have played such a role in the second half of the past century when their populations were growing.
Acknowledgments
The authors wishes to thank Frantisek Havranek from the Forestry and Game Management Research Institute (FGMRI) for valuable help in gathering and evaluation of information regarding game animals. This study was partly supported by the Czech Ministry of Health Project (grant no.11425-5/2010).
Author Disclosure Statement
The authors declare that they have no competing interests.
References
- Anděra M, Gaisler J. Mammals of the Czech Republic. Prague: Academia Publishing House, 2012:285 pp. (In Czech) [Google Scholar]
- Borcić B, Raos B, Kranzelić D, Abu Eldan J, et al. [The role of large wildlife in the maintenance of natural foci of tick-borne meningoencephalitis in northern Croatia]. Acta Med Iugosl 1990; 44:399–406(in Croatian) [PubMed] [Google Scholar]
- Bolzoni L, Rosà R, Cagnacci F, Rizzoli A. Effect of deer density on tick infestation of rodents and the hazard of tick-borne encephalitis. II: Population and infection models. Int J Parasitol 2012; 42:373–381 [DOI] [PubMed] [Google Scholar]
- Cagnacci F, Bolzoni L, Rosà R, Carpi G, et al. . Effects of deer density on tick infestation of rodents and the hazard of tick-borne encephalitis empirical assessment. Int J Parasitolo 2012; 42:365–372 [DOI] [PubMed] [Google Scholar]
- Carpi G, Cagnacci E, Neteler M, Rizzoli A. Tick infestation on roe deer in geographic and remotely sensed climatic variables in a tick-borne encephalitis area. Epidemiol Infect 2008; 136:1416–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrestein GM, Jongejan F, Rijpkema S. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Vet Q 1996; 18(Suppl 3):S148. [PubMed] [Google Scholar]
- Havranek F, Bukovjan K., Urbanec R, Rehak L.Wild boar in the populated areas. Publication of the Czech Ministry of Agriculture, ISBN:80-7084-472-8 2005; 1–35 [Google Scholar]
- Hubálek Z, Juřincová Z, Svobodová S, Halouska J. A serological survey for some bacterial and viral zoonoses in game animals in the Czech Republic. J Wildlife Dis 1993; 29:604–607 [DOI] [PubMed] [Google Scholar]
- Jaenson TGT, Jaenson DGE, Eisen L, Petersson E, et al. . Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasites Vectors 2012; 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juřincová Z. Arbovirus antibodies in wild game caught in Moravia. Vet.Med (Praha) 1992; 11:633–666 [PubMed] [Google Scholar]
- Kiffner C, Lodige C, Alings M, Vor T, et al. . Attachment site selection of ticks on roe deer, Capreolus capreolus. Exp Appl Acarol 2011; 53:79–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiffner C, Vor T, Hagedorn P, Niedrig M, et al. . Determinants of tick-borne encephalitis virus antibody presence in roe deer (Capreolus capreolus) sera. Med Vet Entomol 2012; 26:18–25 [DOI] [PubMed] [Google Scholar]
- Kiffner Ch, Zucchini W, Schomaker P, Vor T, et al. . Determinants of tick-borne encephalitis in counties of southern Germany, 2001–2008. Int J Health Geographics 2010; 9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriz B, Beneš Č, Daniel M. Alimentary transmission of tick-borne encephalitis in the Czech Republic (1997–2008). Epidemiol Mikrobiol Imunol 2009; 58:98–103 [PubMed] [Google Scholar]
- Labuda M, Danielová V, Jones LD, Nuttall PA. Amplification of tick-borne encephalitis virus infection during co-feeding of ticks. Med Vet Entomol 1993a; 7:339–342 [DOI] [PubMed] [Google Scholar]
- Labuda M, Jones LD, Williams T, Danielová V, Nuttall PA. Efficient transmission of tick-borne encephalitis virus between cofeeding ticks. J Med Entomol 1993b; 30:295–299 [DOI] [PubMed] [Google Scholar]
- Labuda M, Nuttall PA, Kozuch O, Elecková E., et al. Non-viremic transmission of tick-borne encephalitis virus: A mechanism for arbovirus survival in nature. Experientia 1993c; 49:802–805 [DOI] [PubMed] [Google Scholar]
- Medlock JM, Hansford KM, Bormane A, Derdakova M, et al. . Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasit Vectors 2013; 6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins SE, Cattadori IM, Tagliapietra V, Rizzoli AP, et al. . Localized deer absence leads to tick amplification. Ecology 2006; 8:1981–1986 [DOI] [PubMed] [Google Scholar]
- Pugliese A, Rosa R. Effect of host populations on the intensity of ticks and the prevalence of tick-borne pathogens: How to interpret the results of deer exclosure experiments. Parasitology 2008; 135:1531–1544 [DOI] [PubMed] [Google Scholar]
- Randolph SE, Gern L, Nuttall PA. Co-feeding ticks, epidemiological significance for tick-borne pathogen transmission 1996. Parasitol Today 1996; 12:472–479 [DOI] [PubMed] [Google Scholar]
- Rizzoli A, Gern L, Nuttall PA, et al. . Forest structure and roe deer abundance predict tick-borne encephalitis risk in Italy. PLos One 2009; 4:4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa R.Pugliese A. Effects of tick population dynamics and host densities on the persistence of tick-borne infections. Math.Biosci 2007; 208:216–240 [DOI] [PubMed] [Google Scholar]
- Skarphedinsson S, Jensen PM, Kristiansen K. Survey of tickborne infections in Denmark. Emerg Infect Dis 2005;11:1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Poel WHM, Heide R, Bakker D, De Looff M, et al. . Attempt to detect evidence for tick-borne encephalitis virus in ticks and mammalian wildlife in The Netherlands. Vector Borne Zoonotic Dis 2005; 5:58–64 [DOI] [PubMed] [Google Scholar]
- Zeman P, Januška J. Epizootic background of dissimilar distribution of human cases of Lyme borreliosis and tick-borne encephalitis in joint endemic area. Comp Immunol Microbiol Infect Dis 1999; 22:247–260 [DOI] [PubMed] [Google Scholar]