Abstract
Studies on functional brain lateralization using functional magnetic resonance imaging (fMRI) have generally focused on lateralization of local brain regions. To explore the lateralization on the whole-brain level, lateralization of functional connectivity using resting-state fMRI (N=87, right handed) was analyzed and left- and right-lateralized networks were mapped. Four hundred two equally spaced regions of interest (ROI) covering the entire gray matter were divided into 358 task-positive and 44 task-negative ROIs. Lateralization of functional connectivity was analyzed separately for the task-positive and task-negative regions to prevent spuriously high lateralization indices caused by negative correlations between task-positive and task-negative regions. Lateralized functional connections were obtained using k-means clustering analysis. Within the task-positive network, the right-lateralized functional connections were between the occipital and inferior/middle frontal regions among other connections, whereas the left-lateralized functional connections were among fusiform gyrus and inferior frontal and inferior/superior parietal regions. Within the task-negative network, the left-lateralized connections were mainly between the precuneus and medial prefrontal regions. Specific brain regions exhibited different left- or right-lateralized connections with other regions, which suggest the importance of reporting lateralized connections over lateralized seed regions. The mean lateralization indices of the left- and right-lateralized connections were correlated, suggesting that the lateralization of connectivity may result from complementary processes between the lateralized networks. The potential functions of the lateralized networks were discussed.
Key words: : functional connectivity, hemispheric asymmetry, lateralization, resting state
Introduction
One of the most compelling enigmas in neuroscience is the functional lateralization of the brain. In the 19th century, pioneers, including Marc Dax, Paul Broca, and Carl Wernicke, observed that each cerebral hemisphere hosts specific cognitive function (Harris, 1999). Such observation has brought forth the concept of hemispheric dominance in functional specialization that has vastly shaped our understanding of the brain–behavior relation in cognitive and affective functions. Contemporary functional–anatomical evidence shows detailed asymmetric involvement of either hemisphere for specific task demands or mental states (Herve et al., 2013). In general, the left hemisphere is dominant for language processing (Vigneau et al., 2006), praxis planning (Haaland et al., 2000), and categorical spatial processing (Kosslyn et al., 1989), while the right hemisphere is specialized for spatial attention (Shulman et al., 2010), coordinate spatial processing (Jager and Postma, 2003), affective prosody (Ross and Monnot, 2008), and self-awareness (Keenan et al., 2001). This relative division of labor between the hemispheres may increase the brain's capacity to carry out simultaneous parallel processing in different domains (Fair et al., 2007).
Recent studies have examined intrinsic lateralization using functional connectivity to provide further insight of functional brain lateralization. Resting-state functional connectivity is the correlation of the time series between two spatially remote regions (Biswal et al., 1995). Despite the recent evidence demonstrating that the brain is intrinsically organized into two types of competing networks even when there is no demand for explicit tasks (Fox et al., 2005; Raichle, 2009; Raichle et al., 2001), the competing relationship between these networks has rarely been acknowledged in the laterality assessment of functional connectivity (Gee et al., 2011; Liu et al., 2009). One type of network is task negative, exhibiting decreased activity during task-dependent performance. Many suggest these task-negative brain regions support the default mode of the brain (Gusnard et al., 2001). The other type of network is task positive, demonstrating increased activity with a given task (Fox et al., 2005). A task-positive region and a task-negative region may be functionally segregated, typically supported by negative correlation of the time series.
In particular, Liu and colleagues (2009) computed hemispheric differences in heterotopic functional connectivity, including negative correlations between task-positive and task-negative regions, and reported 37 left-lateralized and 47 right-lateralized seed regions (Liu et al., 2009). Negative correlations might be problematic when computing laterality index. First, the difference between two negative values is opposite of the absolute difference between them, resulting in a directional change of laterality index (e.g., from left-lateralized to right-lateralized or vice versa). Second, computing laterality index between positive and negative functional connectivity results in a sum rather than the difference of the two values and thus increases the laterality index. Lastly, given that a functional connection is between a seed and a target region, it is unknown based on previous studies how these lateralized seed regions connect to other regions to compose the left- and right-lateralized networks.
The goal of the present study is to provide a comprehensive laterality assessment of functional connectivity. To do so, the right- and left-lateralized functional connections were mapped between the seed and the target regions. The influence of negative correlations was minimized when comparing functional connectivity between regions of each heterotopic pair by conducting separate analyses for task-positive and task-negative regions. Our main hypothesis was that the intrinsic lateralization of functional connectivity reflects the functional lateralization of the brain. Specifically, resting-state functional connections may exist among the inferior/middle prefrontal regions and parietal regions, which reflects the left-lateralized language and praxis networks. Conversely, the right-lateralized connections may be observed among the occipital, temporoparietal, and insular regions, reflecting the right-lateralized networks associated with the ventral attentional and the visual perception systems.
Methods
Subjects and data source
The functional magnetic resonance imaging (fMRI) data from the Oulu dataset were used, which are publicly available and part of the 1000 Functional Connectomes project (Biswal et al., 2010). The resting-state fMRI data were obtained using GE 1.5 T HDX scanner with an EPI GRE sequence (TR=1800 msec, TE=40 msec, FOV=25.6 cm×25.6 cm, matrix=64×64, slice thickness=4 mm, flip angle of 90°). A total of 245 images were obtained for each subject. The anatomical images were acquired with 3D FSPGR BRAVO sequence (TR 12.1 msec, TE 5.2 msec, FOV 24.0×24.0 cm, matrix=256×256, slice thickness=1.0 mm, flip angle 20°) (Jukuri et al., 2013; Littow et al., 2010). The dataset included 103 subjects, but 87 of them were included in the present analyses after excluding those who were left-handed or who showed large (>2 mm) head motion. Participants were between 20 and 22 years old, with 57 females and 30 males.
Data preprocessing
The functional and anatomical image preprocessing was performed using the SPM8 toolbox under MATLAB 7.7 software. First five functional images were discarded for each subject, and the remaining 240 images were motion-corrected. To account for differences in geometric configuration of the brain hemispheres, the original version and the left–right mirrored version of the standard six tissue probability maps were averaged to generate six symmetrical tissue probability maps. The anatomical images were segmented using the new segmentation routine in SPM8. The segmented white matter (WM) and cerebrospinal fluid (CSF) images were thresholded at p>0.99 to define the WM and CSF masks for the regression of nuisance signals. The functional images were coregistered to the subjects' own anatomical images. The deformation field maps obtained from the segmentation procedure were used to normalize all the functional images into the standard Montreal Neurological Institute (MNI) space.
Regions of interest
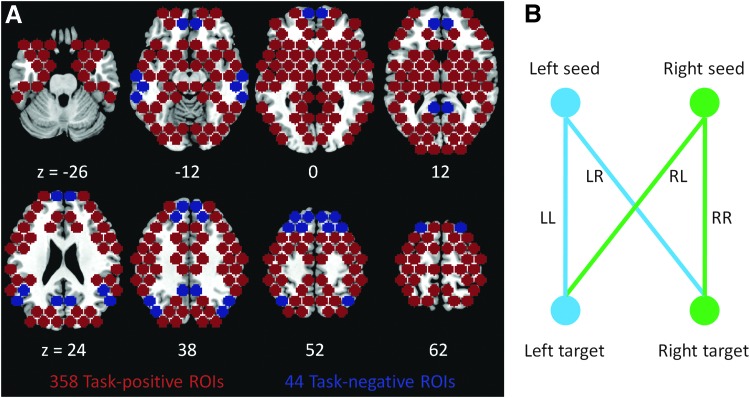
MarsBaR toolbox (Brett, 2002) was used to create regions of interest (ROIs) in the standard MNI template in SPM8 software. A closely spaced sphere packing algorithm was implemented to generate ROIs that tightly covered the entire brain hemispheres (Conway et al., 1999). All ROIs were spheres with a radius size of 7 mm. The distance between the center of a given ROI to the center of an adjacent ROI was 16 mm. The ROIs located in CSF and WM were removed, and also ROIs falling between the two cerebral hemispheres were excluded (Fig. 1A). Thus, 402 symmetrical ROIs covering the gray matter of the cerebral cortex were used in the current analysis. A full list of coordinates of these ROIs are available online (Di et al., 2014).
FIG. 1.
Regions of interest (ROIs) included in the present study (A) and illustration of functional connectivity lateralization index calculation (B). Task-positive ROIs and task-negative ROIs are color coded in red and blue, respectively. Z numbers represent z coordinates in Montreal Neurological Institute space.
The averaged time series of the voxels within these ROIs were obtained after regressing out the nuisance signals. The nuisance signals included six rigid-body head motion parameters, their first-order derivatives (Friston et al., 1996), and the first five principal components of WM and CSF signals (Chai et al., 2012). Lastly, a bandpass temporal filtering ranging between 0.01 and 0.1 Hz was applied.
Task-positive and task-negative regions
To differentiate the 402 ROIs into task-positive or task-negative regions, the k-means clustering algorithm was used. The k-means clustering algorithm uses an iterative partitioning to minimize the squared Euclidean distance of the within-cluster sums of point-to-cluster-centroid distances (Seber, 1984). The input to the k-means clustering algorithm was the mean correlation matrix across subject (402×402), and k was equal to 4. One of the clusters that resembled the task-negative regions was identified, and all other clusters were grouped as the task-positive regions. Since these clusters were not symmetrical, when a pair of symmetrical ROIs, also referred to as homotopic pairs, was not within the same network, the ROI pairs were manually placed into the task-positive network. A total of 44 symmetrical task-negative ROIs and 358 task-positive ROIs were identified (Fig. 1A).
Functional connectivity lateralization index
To calculate the functional connectivity laterality index (fcLI) of a given heterotopic group (i.e., two regions within a hemisphere and their mirrored regions from the other hemisphere), correlation analyses, yielding correlation coefficients, of the time series (1) between the left seed and the left target region (LL functional connectivity), (2) between the right seed and the right target region (RR functional connectivity), (3) between the right seed and the left target region (RL functional connectivity), and (4) between the left seed and the right target region (LR functional connectivity) were conducted (Fig. 1B). All possible estimates were computed for the task-positive ROIs (179×187 combinations) and the task-negative ROIs (22×21 combinations). Then, the fcLI was computed using Equation 1.
 |
A one-sample t-test was conducted across our 87 subjects to determine the fcLIs that were consistently lateralized across all the subjects using the Bonferonni-corrected p-values as the criteria (p<0.000001569 for task-positive ROIs; p<0.000108 for task-negative ROIs). We used BrainNet Viewer software (Xia and He, 2013) to visualize the strongest lateralized functional connectivity (fcLI>0.2 for left lateralization; fcLI<−0.2 for right lateralization) with their corresponding ROIs. Because a large number of lateralized functional connectivity lines are too dense to visualize specific networks, the k-means clustering analysis was conducted to divide the right and the left fcLIs into a number of clusters; k-means clustering analysis was conducted on the left fcLIs independently from the right fcLIs. Since there was no a priori assumption regarding the number of clusters (k) for the lateralized fcLIs, 100 iteration of k-means analysis for k between 2 and 10 was conducted. Each cluster analysis yielded a silhouette value, which provided a graphical representation of how well each data point lied within its cluster and was used to determine the number of clusters within a dataset (Kaufman and Rousseeuw, 1990). A silhouette value of 1 suggests that data within a given cluster are tightly grouped.
It was explored whether the lateralized networks exhibit complementary relationships. A subject-level correlation analysis was conducted to determine whether the degree of the mean left-lateralized fcLIs was correlated with the mean right-lateralized fcLIs within the task-positive and task-negative networks. The threshold for significance was p<0.05.
Lateralization of gray matter volume and the amplitude of low-frequency fluctuation
Because laterality index of functional connectivity between regions may be influenced by factors such as local brain anatomy and physiology, voxel-by-voxel gray matter volume (GMV) and amplitude of low-frequency fluctuation (ALFF) lateralization maps were also calculated to examine whether the spatial distributions of lateralization of GMV and ALFF resembled the lateralization of seed or target regions calculated in the functional connectivity lateralization analysis. Details of these analyses and results are reported in the Supplementary Materials (available online at www.liebertpub.com/brain).
Results
Distribution of functional connectivity lateralization index
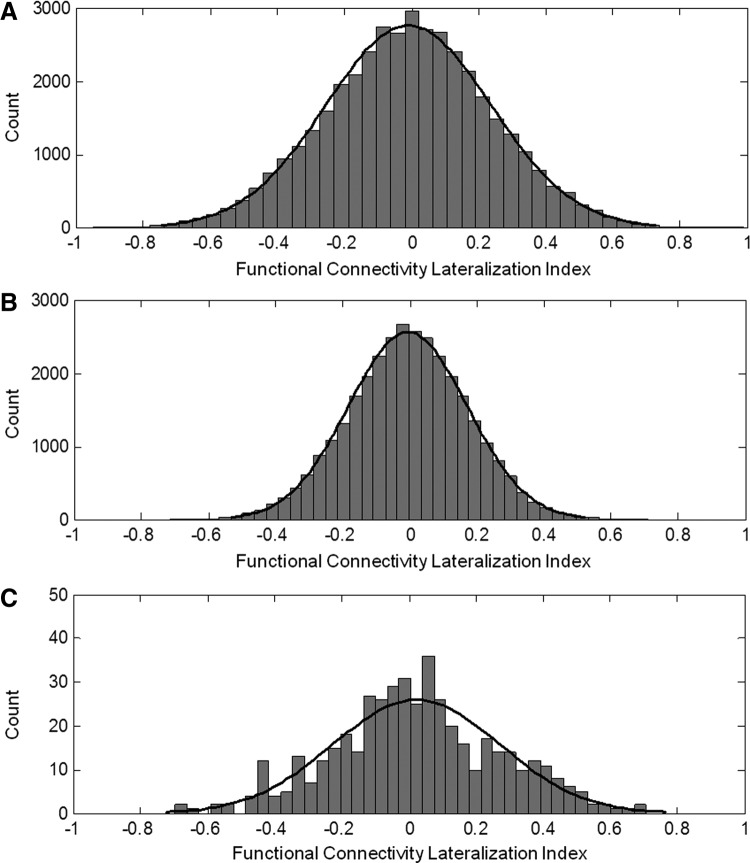
The mean distribution of functional connectivity laterality indices and their corresponding Gaussian fit curves are plotted for all ROIs, task-positive ROIs, and task-negative ROIs (Fig. 2). It was observed that the negative relationship between task-positive and task-negative ROIs in the laterality index analyses significantly increased the number of strongly lateralized functional connections (Fig. 2A,B; Kolmogorov–Smirnov test, p<0.001). The mean and standard deviation of fcLI was 0.0022±0.0228 and 0.0225±0.0362 for task-positive and task-negative ROIs, respectively.
FIG. 2.
Mean functional connectivity lateralization index distributions using all ROIs (A), task-positive ROIs (B), and task-negative ROIs (C).
Right- and left-lateralized task-positive regions
There were 22 left-lateralized and 33 right-lateralized seed ROIs and 31 left-lateralized and 44 right-lateralized target ROIs. To highlight that specific connections constitute lateralization of functional connectivity, seed and target ROIs that were involved in both the left-lateralized and right-lateralized networks were plotted (Fig. 3, green). There were 14 seed ROIs and 22 target ROIs that contributed to both the left- and right-lateralized networks. The overlapping seed ROIs were within the superior frontal gyrus, inferior/middle frontal gyrus, inferior parietal lobule, cuneus, and fusiform gyrus. The overlapping target ROIs were within the cuneus, middle occipital gyrus, inferior parietal lobule, inferior/temporal gyrus, paracentral lobule, and precentral gyrus.
FIG. 3.
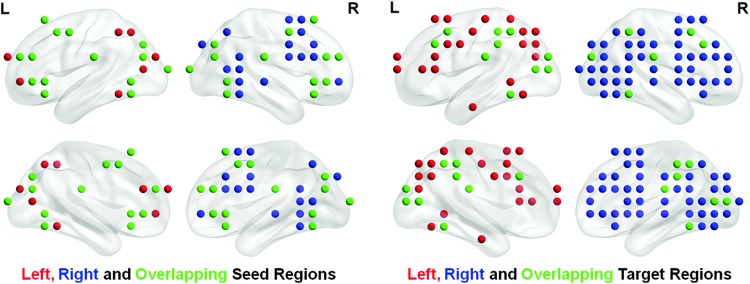
Strongly left-lateralized (red) and right-lateralized (blue) seed (left) and target (right) ROIs among task-positive networks. Overlapping ROIs between both hemispheres are shown in green.
Left- and right-lateralized functional connectivity among the task-positive regions
Ninety-six fcLIs were right-lateralized among the task-positive regions (Fig. 4, upper right panel). Fifty-five fcLIs were left-lateralized (Fig. 4, upper left panel). Although all major cortical areas were involved, the lateralized functional connectivity between the left and the right hemisphere differed as shown in Figure 4. Among other functional connections, right-lateralized functional connections were observed between occipital–frontal regions as well as between inferior/middle frontal–temporoparietal regions. The left-lateralized functional connections were among the inferior/middle frontal–parietal, frontal–temporal, and temporal–parietal regions.
FIG. 4.
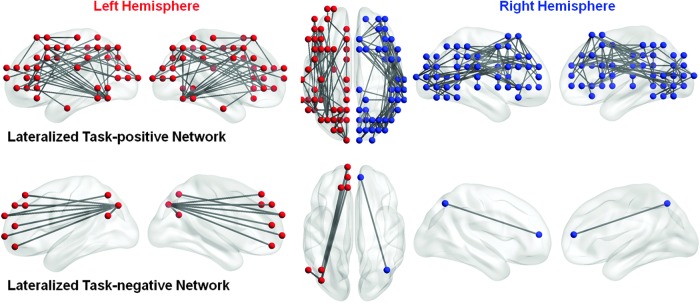
Lateralized functional connectivity among task-positive ROIs (upper) and task-negative ROIs (lower). Spheres in red denote regions with left-lateralized functional connectivity, and spheres in blue denote regions with right-lateralized functional connectivity.
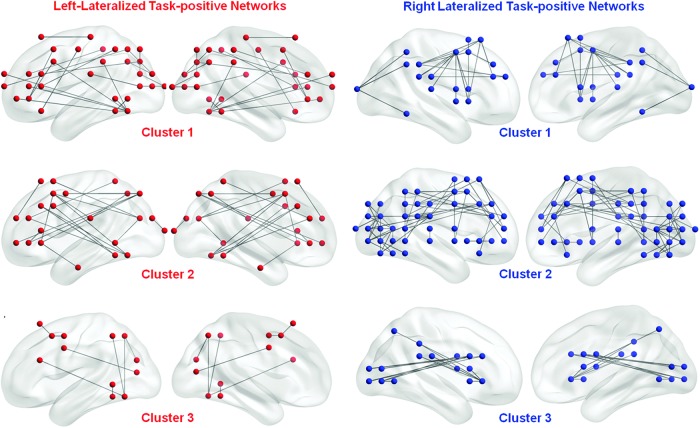
A cluster number of 3 for the left- and right-lateralized fcLIs generated high mean silhouette value that was closest to 1. The mean silhouette value for the left and the right fcLIs for k=3 was 0.77 (SD=0.01) and 0.75 (SD<0.001), respectively. The left- and right-lateralized clusters are shown in Figure 5.
FIG. 5.
The most left- and right-lateralized functional connectivity among task-positive network can be further decomposed into three networks by using cluster analysis, respectively. Three clusters of most left-lateralized task-positive networks are shown in red and in left columns, and right-lateralized task-positive networks are shown in blue and in right columns.
Left- and right-lateralized functional connectivity among the task-negative regions
The analysis resulted in eight left-lateralized fcLIs and one right-lateralized fcLI among the task-negative ROIs (Fig. 4). The left-lateralized connections were mainly between precuneus and medial prefrontal regions. One right-lateralized connection was found between the medial frontal gyrus and the superior parietal lobule.
Complementary left- and right-lateralized networks
Figure 6 demonstrates the mean left-lateralized fcLIs as a function of the mean right-lateralized fcLIs across all subjects. The degree of laterality in the left hemisphere was moderately correlated to the degree of laterality in the right hemisphere (r=−0.44, p<0.0001).
FIG. 6.
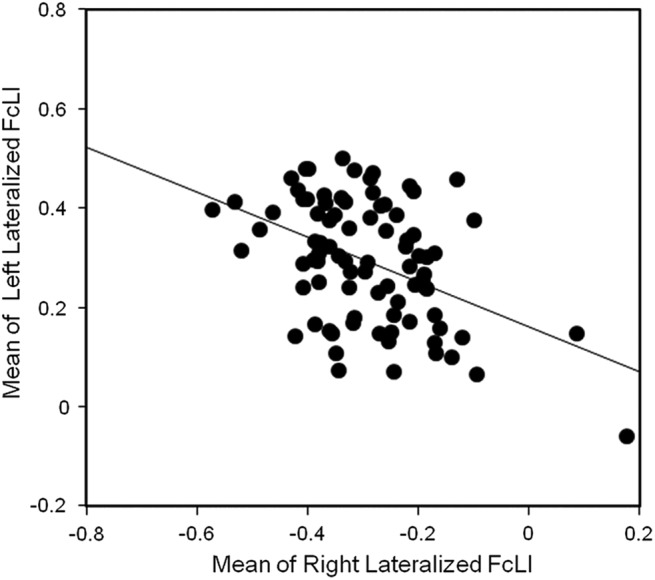
Linear regression between mean of left-lateralized fcLI and the mean of right-lateralized fcLI across subjects (p<0.0001).
Discussion
Studying lateralized functional connections between regions is likely to be informative in understanding functional brain lateralization and characterizing impairment altering brain laterality in clinical populations (e.g., schizophrenia, abnormal aging, and normal aging). Our finding demonstrates that the configuration of functional connections in the right-lateralized network is qualitatively different from that in the left-lateralized network. In addition, a given region may demonstrate different lateralized connections in the left- or right-lateralized networks; therefore, it is important to report lateralized connections in studies using laterality index of functional connectivity.
The right-and left-lateralized networks may reflect functional brain lateralization. For example, the left-lateralized connectivity among the inferior frontal and temporal regions (fusiform gyrus) may be associated with speech production and perceiving visual word forms (Deng et al., 2012; Pravata et al., 2011; Tomasi and Volkow, 2012; Wang et al., 2011; Zhang et al., 2010). Additional left-lateralized connectivity among premotor, superior/inferior parietal lobule, and prefrontal cortex may reflect the left-lateralized praxis network based on studies that have shown these regions' involvement during planning and executing actions (Haaland et al., 2000; Vingerhoets et al., 2012). The right-lateralized connectivity among the occipital regions (middle/superior occipital gyri) and various cortical regions, including the ventral frontal cortex, superior/inferior parietal lobule, and fusiform gyrus, insula, and angular gyrus, may reflect the right-lateralized visual perception and ventral attentional systems (Corbetta and Shulman, 2002; Shulman et al., 2010). Within the task-negative ROIs, the left-lateralized connections between the precuneus and medial prefrontal cortex suggest greater left-hemisphere involvement during self-related and internal-thought processing (Gusnard et al., 2001; Raichle et al., 2001; Spreng et al., 2009). However, our finding warrants validation from future studies exploring the relationship between lateralized task activations and lateralized resting-state functional networks.
The mean lateralization indices of the left- and right-lateralized connections were correlated, suggesting that the lateralization of connectivity may result from complementary processes between the lateralized networks. Several theories on the advantage of having two cerebral hemispheres suggest that lateralization divides the labor between the two hemispheres and increases the neural capacity (Cai et al., 2013; Hugdahl, 2000; Ringo et al., 1994). Specializing one hemisphere for a set of functions leaves the other hemisphere to perform another set of functions, prevents crowding effect, and avoids transmitting information across long distances between two hemispheres. In addition, our results show that the global mean of laterality index was zero. This global mean of laterality index of functional connectivity may be biased toward a given hemisphere in patients (e.g., stroke) and therefore be a clinically relevant parameter to assess hemispheric dominance.
The laterality index of functional connectivity measures is mainly related to the synchronous activity between two functionally related regions. Thus, anatomical differences in the gray matter volume (see Supplementary Fig. S1) (Good et al., 2001; Toga and Thompson, 2003) and WM tracts in the association fiber pathways (Thiebaut de Schotten et al., 2011) are most likely a major factor that influence laterality index of functional connectivity. In addition, physiological properties such as the vasculature and vascular activities detected by MRI may influence laterality index. Recently, our group has shown a positive correlation between the ALFF and functional connectivity (Di et al., 2013b). ALFF is highly correlated with the breath hold responses that reflect mostly the vascular activities of the local brain regions (Biswal et al., 2007; Di et al., 2013a). Furthermore, as shown in Supplementary Figure S2, hemispheric differences in ALFF were observed. These results suggest that functional lateralization may arise from the underlying anatomical–physiological hemispheric differences. Future studies are needed to investigate how anatomical–physiological factors influence laterality index of functional connectivity in order to reduce physiological influence in laterality assessment and yield functionally meaningful lateralized resting-state networks.
Using a stringent criterion to compute laterality index of functional connectivity may minimize the influence of methodological confounds that compromise the meaningfulness of the laterality index measure. Contrary to Liu and colleagues analyses, reducing negative correlations between task-positive and task-negative regions resulted in reduced number of lateralized seed regions (Liu et al., 2009). Therefore, the present method may provide an alternative approach to explore laterality of functional connectivity in healthy and clinical populations.
Conclusions
In the present study, potential negative correlations between task-positive and task-negative network regions were carefully controlled in calculation of fcLIs. This resulted in a narrower distribution of overall fcLIs. It is demonstrated that the same homotopic regions might show opposite lateralization of functional connectivity with different brain regions, which emphasizes the importance of reporting lateralized connections in addition to lateralized regions. Our analysis revealed three left-lateralized and three right-lateralized networks, which may be associated with different lateralized brain functions. In addition, the extent of lateralization of left-lateralized connections was correlated with the extent of lateralization of right-lateralized connections, suggesting complementary processes between left- and right-lateralized networks.
Supplementary Material
Acknowledgment
This study was supported by the National Institutes of Health grant 5R01AG032088.
Author Disclosure Statement
No competing financial interests exist.
References
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541 [DOI] [PubMed] [Google Scholar]
- Biswal BB, Kannurpatti SS, Rypma B. 2007. Hemodynamic scaling of fMRI-BOLD signal: validation of low-frequency spectral amplitude as a scalability factor. Magn Reson Imaging 25:1358–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, et al. . 2010. Toward discovery science of human brain function. Proc Natl Acad Sci U S A 107:4734–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B. Region of interest analysis using an SPM toolbox. Presented at the 8th International Conference on Functional Mapping of the Human Brain, June2–6, 2002, Sendai, Japan Available on CD-ROM in NeuroImage, Vol 16, No 2 [Google Scholar]
- Cai Q, Van der Haegen L, Brysbaert M. 2013. Complementary hemispheric specialization for language production and visuospatial attention. Proc Natl Acad Sci USA 110:E322–E330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Castañón AN, Ongür D, Whitfield-Gabrieli S. 2012. Anticorrelations in resting state networks without global signal regression. Neuroimage 59:1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway JH, Sloane NJA, Bannai E. 1999. Sphere Packings, Lattices, and Groups. New York: Springer; pp. 1–59 [Google Scholar]
- Corbetta M, Shulman GL. 2002. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:201–215 [DOI] [PubMed] [Google Scholar]
- Deng Y, Guo R, Ding G, Peng D. 2012. Top-down modulations from dorsal stream in lexical recognition: an effective connectivity FMRI study. PLoS One 7:e33337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Kannurpatti SS, Rypma B, Biswal BB. 2013a. Calibrating BOLD fMRI activations with neurovascular and anatomical constraints. Cereb Cortex 23:255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Kim EH, Chen P, Biswal BB. 2014. Symmetrical ROIs using spherical packing algorithm. http://dx.doi.org/10.6084/m9.figshare.951970 Last accessed Nov. 5, 2014
- Di X, Kim EH, Huang CC, Tsai SJ, Lin CP, Biswal BB. 2013b. The influence of the amplitude of low-frequency fluctuations on resting-state functional connectivity. Front Hum Neurosci 7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. 2007. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA 104:13507–13512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. 1996. Movement-related effects in fMRI time-series. Magn Reson Med 35:346–355 [DOI] [PubMed] [Google Scholar]
- Gee DG, Biswal BB, Kelly C, Stark DE, Margulies DS, Shehzad Z, Uddin LQ, Klein DF, Banich MT, Castellanos FX, Milham MP. 2011. Low frequency fluctuations reveal integrated and segregated processing among the cerebral hemispheres. Neuroimage 54:517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. 2001. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14:21–36 [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. 2001. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 98:4259–4264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, Knight RT. 2000. Neural representations of skilled movement. Brain 123:2306–2313 [DOI] [PubMed] [Google Scholar]
- Harris LJ. 1999. Early theory and research on hemispheric specialization. Schizophr Bull 25:11–39 [DOI] [PubMed] [Google Scholar]
- Herve PY, Zago L, Petit L, Mazoyer B, Tzourio-Mazoyer N. 2013. Revisiting human hemispheric specialization with neuroimaging. Trends Cogn Sci 17:69–80 [DOI] [PubMed] [Google Scholar]
- Hugdahl K. 2000. Lateralization of cognitive processes in the brain. Acta Psychol (Amst) 105:211–235 [DOI] [PubMed] [Google Scholar]
- Jager G, Postma A. 2003. On the hemispheric specialization for categorical and coordinate spatial relations: a review of the current evidence. Neuropsychologia 41:504–515 [DOI] [PubMed] [Google Scholar]
- Jukuri T, Kiviniemi V, Nikkinen J, Miettunen J, Maki P, Jaaskelainen E, Mukkala S, Koivukangas J, Nordstrom T, Taanila A, Moilanen I, Heinimaa M, Barnett JH, Jones PB, Murray GK, Veijola J. 2013. Default mode network in young people with familial risk for psychosis—the Oulu Brain and Mind study. Schizophr Res 143:239–245 [DOI] [PubMed] [Google Scholar]
- Kaufman L, Rousseeuw PJ. 1990. Finding Groups in Data: An Introduction to Cluster Analysis. Hoboken, NJ: Wiley & Sons, Inc [Google Scholar]
- Keenan JP, Nelson A, O'Connor M, Pascual-Leone A. 2001. Self-recognition and the right hemisphere. Nature 409:305. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Koenig O, Barrett A, Cave CB, Tang J, Gabrieli JD. 1989. Evidence for two types of spatial representations: hemispheric specialization for categorical and coordinate relations. J Exp Psychol Hum Percept Perform 15:723–735 [DOI] [PubMed] [Google Scholar]
- Littow H, Elseoud AA, Haapea M, Isohanni M, Moilanen I, Mankinen K, Nikkinen J, Rahko J, Rantala H, Remes J, Starck T, Tervonen O, Veijola J, Beckmann C, Kiviniemi VJ. 2010. Age-related differences in functional nodes of the brain cortex—a high model order group ICA study. Front Syst Neurosci 4pii:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Stufflebeam SM, Sepulcre J, Hedden T, Buckner RL. 2009. Evidence from intrinsic activity that asymmetry of the human brain is controlled by multiple factors. Proc Natl Acad Sci USA 106:20499–20503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravata E, Sestieri C, Mantini D, Briganti C, Colicchio G, Marra C, Colosimo C, Tartaro A, Romani GL, Caulo M. 2011. Functional connectivity MR imaging of the language network in patients with drug-resistant epilepsy. AJNR Am J Neuroradiol 32:532–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. 2009. A paradigm shift in functional brain imaging. J Neurosci 29:12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. 2001. A default mode of brain function. Proc Natl Acad Sci USA 98:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringo JL, Doty RW, Demeter S, Simard PY. 1994. Time is of the essence: a conjecture that hemispheric specialization arises from interhemispheric conduction delay. Cereb Cortex 4:331–343 [DOI] [PubMed] [Google Scholar]
- Ross ED, Monnot M. 2008. Neurology of affective prosody and its functional-anatomic organization in right hemisphere. Brain Lang 104:51–74 [DOI] [PubMed] [Google Scholar]
- Seber GAF. 1984. Multivariate Observations. Hoboken, NJ: John Wiley & Sons, Inc [Google Scholar]
- Shulman GL, Pope DL, Astafiev SV, McAvoy MP, Snyder AZ, Corbetta M. 2010. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci 30:3640–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. 2009. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci 21:489–510 [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Ffytche DH, Bizzi A, Dell'Acqua F, Allin M, Walshe M, Murray R, Williams SC, Murphy DG, Catani M. 2011. Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. Neuroimage 54:49–59 [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. 2003. Mapping brain asymmetry. Nat Rev Neurosci 4:37–48 [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. 2012. Resting functional connectivity of language networks: characterization and reproducibility. Mol Psychiatry 17:841–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N. 2006. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage 30:1414–1432 [DOI] [PubMed] [Google Scholar]
- Vingerhoets G, Acke F, Alderweireldt AS, Nys J, Vandemaele P, Achten E. 2012. Cerebral lateralization of praxis in right- and left-handedness: same pattern, different strength. Hum Brain Mapp 33:763–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yang J, Shu H, Zevin JD. 2011. Left fusiform BOLD responses are inversely related to word-likeness in a one-back task. Neuroimage 55:1346–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, Lu CM, Biswal BB, Zang YF, Peng DL, Zhu CZ. 2010. Detecting resting-state functional connectivity in the language system using functional near-infrared spectroscopy. J Biomed Opt 15:047003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.