Table 1.
Optimization of the reaction conditions for the Friedel–Crafts Michael-type reaction.[a]
| |||||
|---|---|---|---|---|---|
| Entry | Solvent [mL] | t [h] | Yield [%][b] | ee [%][c] | |
| 1 | CHCl3 (1.5) | 16 | 56 | 91 | |
| 2 | CH2Cl2 (1.5) | 15 | 59 | 87 | |
| 3 | toluene (1.5) | 21 | 60 | 88 | |
| 4 | PhCl (1.5) | 21 | 61 | 87 | |
| 5 | toluene (3.0) | 21 | 70 | 91 | |
| 6 | CH2Cl2 (3.0) | 24 | 68 | 91 | |
| 7[d] | CHCl3 (3.0) | 65 | 89 | 93 | |
| 8[d] | toluene (3.0) | 65 | 95 | 93 | |
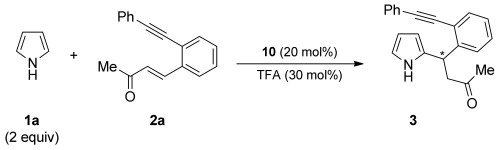
[a] General reaction conditions: 2 a (0.5 mmol), pyrrole 1 a (1.0 mmol), 10 (20 mol %), TFA (30 mol %), rt. [b] Yield of isolated 3. [c] Determined by HPLC analysis on a chiral stationary phase. [d] The reaction was performed at 0 °C.