Abstract
The cardiac steroid ouabain binds to Na+, K+-ATPase and inhibits its activity. Administration of the compound to animals and humans causes an increase in the force of contraction of heart muscle and stabilizes heart rate. In addition, this steroid promotes the growth of cardiac, vascular, and neuronal cells both in vitro and in vivo. We studied the effects of ouabain on mouse recovery following closed head injury (CHI), a model for traumatic brain injury. We show that chronic (three times a week), but not acute, intraperitoneal administration of a low dose (1 μg/kg) of ouabain significantly improves mouse recovery and functional outcome. The improvement in mouse performance was accompanied by a decrease in lesion size, estimated 43 d following the trauma. In addition, mice that underwent CHI and were treated with ouabain showed an increase in the number of proliferating cells in the subventricular zone and in the area surrounding the site of injury. Determination of the identity of the proliferating cells in the area surrounding the trauma showed that whereas there was no change in the proliferation of endothelial cells or astrocytes, neuronal cell proliferation almost doubled in the ouabain-treated mice in comparison with that of the vehicle animals. These results point to a neuroprotective effects of low doses of ouabain and imply its involvement in brain recovery and neuronal regeneration. This suggests that ouabain and maybe other cardiac steroids may be used for the treatment of traumatic brain injury.
Key words: : cardiac steroids; cell viability; closed head injury; Na+, K+-ATPase
Introduction
Traumatic brain injury (TBI) is the leading cause of death and severe disability in people younger than age 45 in the Western industrialized countries.1 Although advances in emergency and intensive care medicine have substantially ameliorated the mortality rate associated with TBI, every year hundreds of thousands of people worldwide die of TBI. Patients who survive the injury are subjected to its catastrophic consequences, including an array of neurological deficits and motor dysfunctions.
TBI is manifested by functional deficits, owing to both primary and secondary mechanisms.2 Primary injury is a result of the immediate mechanical damage occurring at the time of injury. Secondary injury evolves over a period of hours to days, even months, after the primary insult, and is the result of biochemical and physiological events that ultimately lead to neuronal cell death. In the last few decades, several biochemical processes responsible for secondary injury have been demonstrated, including perturbation of cellular calcium homeostasis, increased free radical generation and lipid peroxidation, mitochondrial dysfunction, inflammation, and apoptosis.3 Repair mechanisms based on the proliferation of cells have been identified in the post-traumatic brain, although spontaneous repair is not sufficient to induce significant recovery.4
Ouabain is a steroid extracted from the seeds and dry leaves of herbs from the genus digitalis and other plants.5,6 The steroid belongs to inotropic agents collectively termed cardiac steroids. These compounds are used as drugs to increase the force of contraction of heart muscle in congestive heart failure patients and stabilize heart rhythm in patients with atrial arrhythmias.6 Because of the small therapeutic index of cardiac steroids (such as digoxin), their use is limited and that of ouabain was abandoned.7,8 The only established receptor for ouabain is the ubiquitous plasma membrane transporter Na+, K+-ATPase. The activity of this enzyme is essential for the regulation of cell volume and osmolarity, pH and calcium concentrations, maintenance of the plasma membrane electric potential and the co-transport of ions, glucose and other nutrients across the plasma membrane.9,10 The binding of ouabain to Na+, K+-ATPase inhibits its hydrolytic and ion transporting activities and induces the assembly of multiple protein complexes into functional micro-domains that activate diverse signaling pathways.11,12
The effects of ouabain on brain function are well recognized: the interaction of ouabain with Na+, K+-ATPase has profound effects on the neuronal electrical resting potential,13 the basal metabolic rate of neurons and glia, and the release and reuptake of several neurotransmitters.14 Further, ouabain was found to stimulate the viability and proliferation of several cell types present in the brain. The administration of ouabain was shown to increase retinal ganglion cell survival15 and to induce the proliferation of rat astrocytes.16 We recently demonstrated that ouabain at nM concentrations stimulates the viability and proliferation of NT2 cells, precursors for human neuronal cells.17 Administration of ouabain also proved to protect cortical neurons from apoptosis following excitotoxic stress18 and stimulated the proliferation of endothelial cells.19
Further, previous studies have shown that cardiac and related steroids provide neuroprotection against ischemia in several animal experimental models.20,21 The effect of ouabain on cell survival and proliferation and its beneficial influence against ischemia suggest that it may have an effect on functional neurologic recovery following TBI. We show in the present study that chronic intraperitoneal administration of low doses of ouabain significantly improves functional outcome in the mouse following closed head injury (CHI). This effect was accompanied by a decrease in lesion volume and an elevation in neuronal proliferation.
Methods
Animals
The study was conducted according to the Institutional Animal Care and Use Committee guidelines in compliance with National Institutes of Health guidelines (MD-11-12783-4). Adult Sabra male mice weighing 40 g were housed in a specific pathogen-free facility according to a 12:12-h light-dark cycle. Food and water were provided ad libitum.
Trauma model
TBI was induced according to the CHI model, using a weight drop device.22,23 In brief, after isoflurane anesthesia, a midline longitudinal incision was performed, exposing the skull. A Teflon-tipped cone (2 mm diameter) was placed 1 to 2 mm lateral to the midline in the midcoronal plane. The head was manually held in place and a 95 g weight was dropped on the cone from a prefixed height (18 cm), resulting in a focal injury to the left hemisphere. Following recovery from anesthesia, the mice were returned to their home cages with postoperative care and free access to food and water. Ouabain (1 μg/Kg) or saline as control were injected (intraperitoneally) into the mice (n=12) according to two protocols. In the first, ouabain or saline were given 1, 24, 48, and 72 h after injury. In the second, ouabain was administered 1 and 24 h following injury and then three times a week until the end of the experiment (43 d post-injury).
BrdU injections
During the last 10 d of the experiment (Days 33 to 43), all the animals received intraperitoneal injections of the tracer 5-bromo-2-deoxyuridine (BrdU; 50 mg/kg/day) to label dividing cells.
Neurobehavioral evaluation
The functional status of the mice was evaluated according to a Neurological Severity Score (NSS) by a blind observer. The score is a 10-point scale that assesses the functional neurologic status based on the presence of some reflexes and the ability to perform motor and behavioral tasks, such as beam walking, beam balance, and spontaneous locomotion.24 Animals are awarded one point for failure to perform a task, thus the NSS increases with the severity of dysfunction. The NSS obtained 1 h after CHI reflects the initial severity of injury.25 NSS values were measured at 1 and 24 h following injury. Additional measurements were made at 4, 7, 14, 21, 28, 35, and 40 d after injury.
Injury size
At 43 d after CHI, the animals were deeply anesthetized with pentobarbital and perfused with 4% paraformaldehyde. Their brains were removed and frozen-sectioned into 10 μm slices. Brain slices at 200 μm intervals between bregma +1.42 and bregma - 0.8 were stained with Giemsa stain-modified solution (1:1; Fluka, Sigma-Aldrich Corporation, St Louis, MO) and digitally photographed. The volume of injured tissue was measured using ImageJ software (National Institutes of Health, Bethesda, MD). Damaged tissue volume was calculated by dividing the volume of the injured hemisphere by that of the non-injured hemisphere.26
Immunohistochemistry
For each brain, three evenly-spaced frozen sections between bregma +1.42 and bregma - 0.8 were fixed in 4% paraformaldehyde for 15 min. Deoxyribonucleic acid was denatured by incubating the sections in 2 N HCl for 30 mins at 37°C followed by neutralization with immersion in 0.1 mol/L borate buffer. After washing with phosphate buffered saline (PBS), the sections were blocked in 5% normal goat serum containing 0.3% triton X-100, and incubated overnight at 4°C with the appropriate primary antibodies in a humidified chamber. Rat anti-BrdU was used as a marker for cell proliferation (1:200; Accurate, New York, NY), mouse anti-GFAP was used to detect astrocytes (1:200; Abcam Inc., Cambridge, MA), mouse anti-NeuN was used to identify mature neurons (1:100 Abcam Inc., Cambridge, MA) and mouse anti-CD31 was used as a marker for endothelial cells (1:100; Abcam Inc., Cambridge, MA). Following the incubation, the sections were rinsed and incubated for 2 h with Alexa 488 and Alexa 555 conjugated secondary antibodies (1:200; Molecular Probes, Leiden, the Netherlands). After an additional three washings in PBS, specimens were mounted in medium containing 4′,6-diamidino-2-phenylindole (DAPI; Sigma, Israel) to visualize the nuclei.
Image analysis and cell counting
For image analysis of each brain section, five high magnification (×400) images were obtained of specific area of interest, including the entire area surrounding the injured cortex and the subventricular zone (SVZ), using a confocal or epifluorescent Olympus (Tokyo, Japan) microscope equipped with a camera. Cells were counted using a cell counter plug-in for the ImageJ software. To measure cell proliferation, the percentage of BrdU-positive cells among the total DAPI stained cells was calculated. To determine the identity of the proliferating cells, the number of double-positive stained cells for BrdU and either CD31, GFAP or NeuN was determined and divided by the total number of DAPI stained cells.
Statistical analysis
The data are presented as the mean±standard error of the mean as indicated in the figure legends. Neurobehavioral evaluation values were compared at the selected time points using the non-parametric Mann-Whitney U test. Other tests were evaluated by Student's t-test. A p value of≤0.05 was considered significant for all comparisons.
Results
Ouabain improves functional recovery following CHI
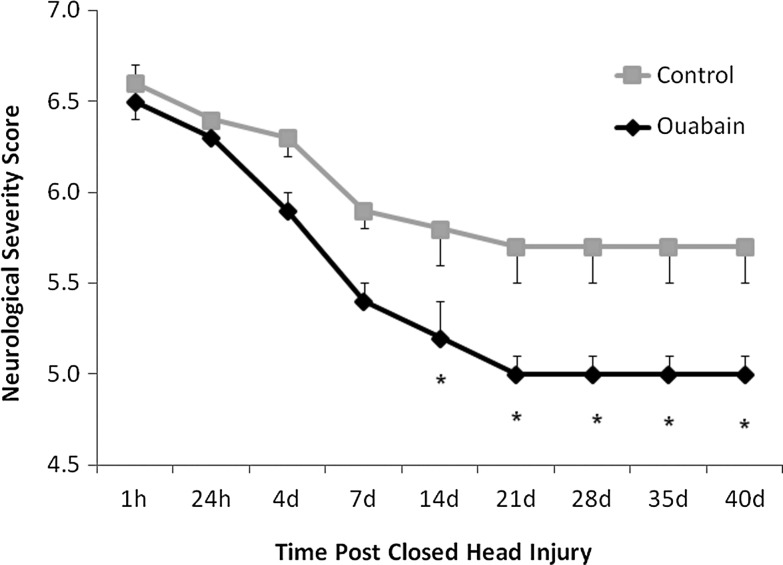
Ouabain (1 μg/Kg), was administered intraperitoneally to mice, 1 and 24 h following CHI and then three times a week for six weeks. Mice receiving saline served as control. As shown in Figure 1, ouabain-treated animals showed a significant improvement in the neurological function, as determined by the NSS. The inter-group differences in NSS were apparent as early as Day 14 after CHI, suggesting a neuroprotective effect of ouabain. A shorter treatment paradigm, in which ouabain at the same dosage was given 1, 24, 48, and 72 h following CHI, resulted in a slight and not significant improvement in NSS (data not shown).
FIG. 1.
Effect of long-term ouabain treatment on functional recovery of mice following closed head injury (CHI). Traumatic brain injury was induced in mice according to the CHI model, using a weight drop device. Ouabain (1 μg/kg) or saline as control was injected (intraperitoneally) into mice 1 and 24 h after injury and then three times a week until the end of the experiment (43 d post-injury). The functional status of the mice was evaluated by neurological examination (see Methods) by a blind observer. The values are expressed as the mean±standard error of the mean (error bars; n=12), *Significantly lower score than that of the control mice; p<0.05.
Ouabain decreases lesion volume following CHI
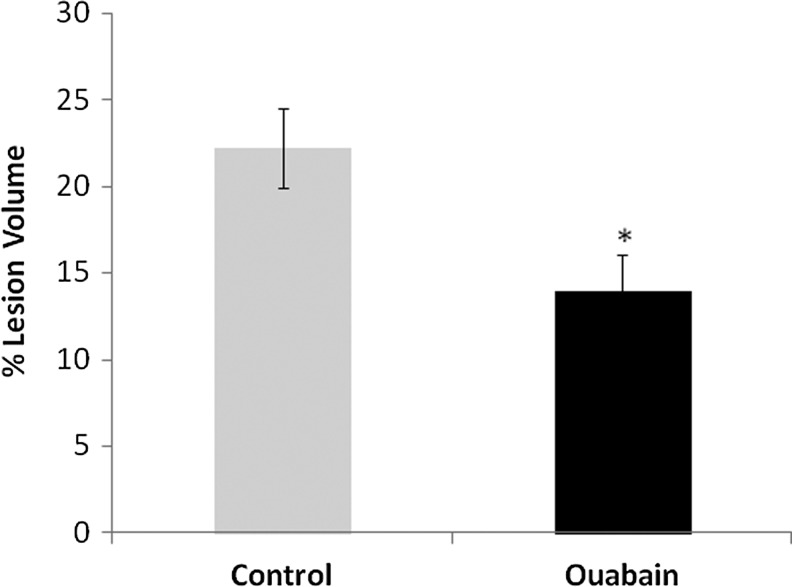
The beneficial effect of a chronic administration of ouabain also was manifested by reduced lesion volume. At 43 d after CHI, lesion sizes were measured and found to be smaller by about 40% in the ouabain-treated group versus those in the control group (Fig. 2).
FIG. 2.
Effect of long-term ouabain treatment on lesion size in mice following closed head injury (CHI). Brains from mice (43 days after CHI) subjected to ouabain or saline (control) treatment, as described in Figure 1, were sectioned and stained with Giemsa. The volume of injured tissue was measured using ImageJ software. Lesion volume was calculated by dividing the volume of the injured hemisphere by that of the non-lesioned hemisphere. The results are expressed as the percentage of hemispheric tissue. The values are expressed as the mean±standard error of the mean (error bars; n=36), *Significantly lower than control; p<0.01.
Ouabain increases cell proliferation following CHI
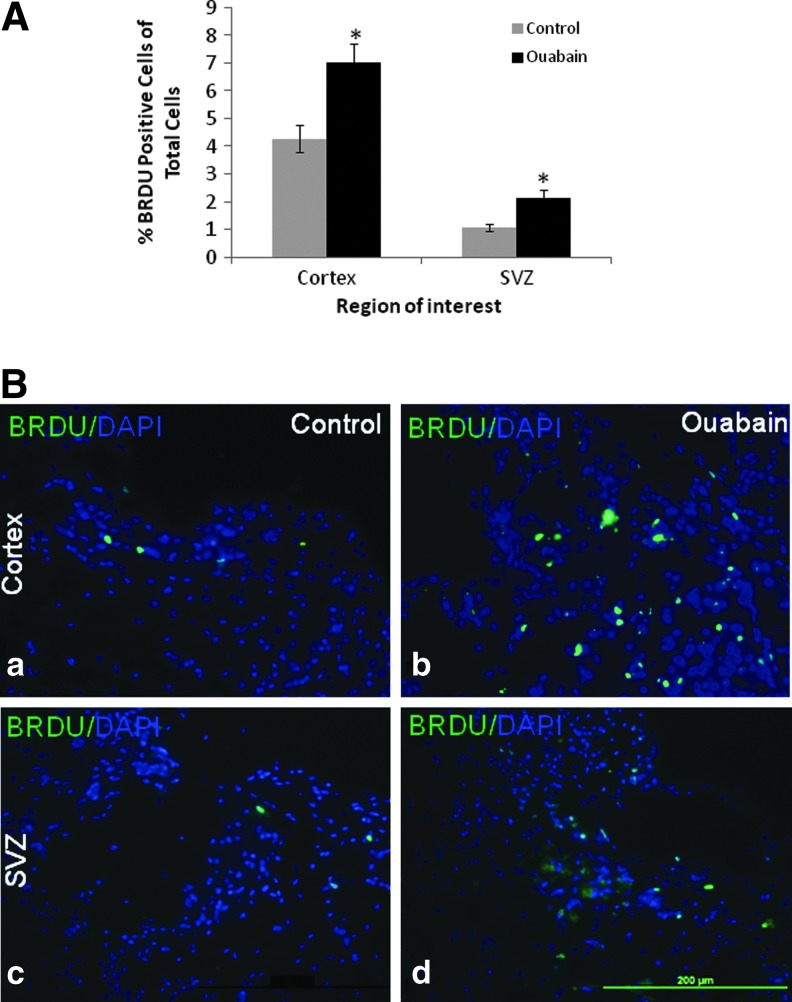
The SVZ represents a niche of neural progenitors. Following a cortical injury these cells proliferate, migrate to the injured area, and differentiate.27 To assess the effect of ouabain on cell proliferation, the percentage of BrdU positive cells was measured 43 d following CHI in the SVZ and in the injured area. The long term administration of ouabain resulted in a 100% and 65% increase in cell proliferation in the SVZ and in the area surrounding the injury, respectively (Fig. 3).
FIG. 3.
Effect of long-term ouabain treatment on cell proliferation in mice following closed head injury (CHI). Mice following CHI were subjected to long-term ouabain treatment, as described in Figure 1. During the 10 last d of the experiment (Days 33–43), the mice received BrdU and their brains were removed (43 d after CHI), sectioned, and stained using BrdU and 4′,6-diamidino-2-phenylindole (DAPI) antibodies as described in Methods. (A) Cell proliferation was measured in the cortex surrounding the trauma and the subventricular zone (SVZ) by calculating the percentage of BrdU positive cells among the DAPI stained cells. (B) Representative images (×400) from the cortex surrounding the trauma (a,b) and the SVZ (c,d) of a control (a,c) versus an ouabain-treated mouse (b,d), showing an abundance of BrdU positive cells (green) among the total DAPI cells (blue). The values are expressed as the mean±standard error of the mean (error bars; n=45), *Significantly higher than the control; p<0.001. Color image is available online at www.liebertpub.com/neu
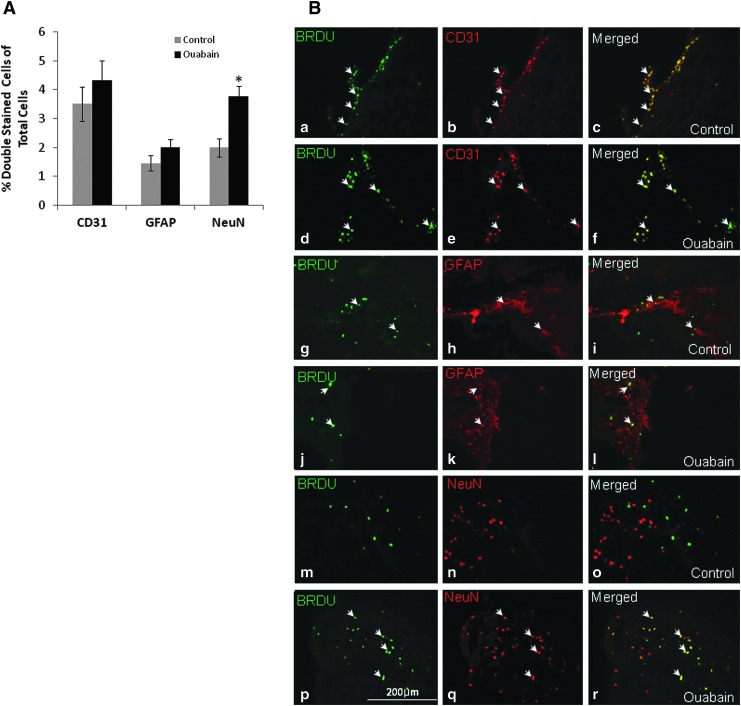
Ouabain stimulates neuronal but not astrocyte or endothelial cell proliferation
To determine the identity of the cells that underwent ouabain-stimulated proliferation in the area surrounding the injury, specific immunohistochemical labeling was used followed by microscopic analysis of colocalization. CD31, GFAP and NeuN antibodies were used as specific markers for endothelial, astrocytes and neuronal cells, respectively. As shown in Figure 4, whereas no difference was found in the proliferation of endothelial cells or astrocytes, the proliferation of neuronal cells almost doubled following long-term treatment with ouabain. This finding suggests that the improvement in mouse performance and the reduction in injury size are associated with the observed neurogenesis.
FIG. 4.
Effect of long-term ouabain treatment on the identity of the proliferating cells. Mice following closed head injury (CHI) were subjected to long-term ouabain treatment, as described in Figure 1. During the 10 last d of the experiment (Days 33–43), the mice received BrdU and their brains were removed (43 d after CHI), sectioned and stained, as described in Methods. (A) The identity of the proliferating cells was determined in the area surrounding the trauma by double labeling with BrdU and CD31, GFAP and NeuN antibodies to determine endothelial, astrocytes and neuronal cells, respectively. Total cell number was determined by 4′,6-diamidino-2-phenylindole staining. (B) Representative images (×400) of endothelial proliferation in control (a-c) versus ouabain-treated mice (d-f). Astrocytes proliferation in control (d-f) versus ouabain-treated mice (h-j) and neuronal cell proliferation in control (k-m) versus ouabain-treated mice (n-p). White arrows indicate cells that are double-positive stained with BrdU (green) and CD31, GFAP, or NeuN (red) antibodies. The values are expressed as the mean±standard error of the mean (error bars; n=15), *Significantly higher than the control; p<0.05. Color image is available online at www.liebertpub.com/neu
Discussion
The results of this study show, for the first time, that long-term treatment of mice with the cardiotonic steroid ouabain, significantly improves mouse recovery and functional outcome following CHI (Fig. 1). In addition, histological examination of the mouse brain demonstrated a much smaller lesion in the ouabain-treated animals (Fig. 2) and an elevation in cell proliferation in the SVZ and in the injured area (Fig. 3). Cell fate analysis identified an increase in neuronal cell proliferation with no change in endothelial cells or astrocyte proliferation (Fig. 4). These results suggest that the long-term administration of ouabain after CHI leads to neuroprotective effects and suggest that the compound can serve as a therapeutic agent in patients with TBI.
The beneficial effect of improving the outcome following CHI was seen in this study by treatment with 1μg/kg ouabain. Assuming that ouabain is equally distributed in the mouse circulation and tissues, this concentration is expected to result in subnanomolar levels in the animal body. These very low concentrations of the steroid have a negligible effect on pump inhibition.28 On the contrary, ouabain at these concentrations was shown to stimulate pump activity.29,30 Interestingly, it was demonstrated that ‘secondary injury’ of TBI in rats is accompanied by a reduction in Na+, K+-ATPase activity31 and it was suggested that it contributes to learning deficits observed following TBI. Stimulation of the pump activity by the administration of low levels of ouabain could reverse these alterations and improve the animal's performance.
The binding of ouabain to Na+, K+-ATPase at nanomolar concentrations also induces the activation of MAPK and the Akt signaling cascades.32 Ouabain, by activating these pathways, was shown to stimulate the viability and proliferation of various cell types, including neuronal cells.17 However, the involvement of these pathways in recovery and functional improvement following TBI is controversial. The activation of the MAPK and Akt pathways was found to facilitate animal recovery using the model presented in this study,33,34 as well as in a model of brain injury following stroke.35 However, other investigators showed that stimulation of these cascades following head injury is destructive and should be inhibited in order to obtain a positive effect.36 Further study is necessary to elucidate the molecular mechanism responsible for the effect of ouabain on mouse recovery and neuronal proliferation following TBI.
Ouabain was previously shown to stimulate the viability and proliferation of rat astrocytes,16 and neuronal cells15 and human endothelial cells19 in culture. In the present in vivo study, the administration of ouabain to mice following CHI resulted in a specific stimulation of neuronal cell proliferation but had no effect on proliferation of astrocytes or endothelial cells (Fig. 4). These differences may derive from differences in the species or in the in vitro versus in vivo experimental setup.
The specific effect of ouabain on neuronal cell proliferation merits further consideration. It is well established that neurons and glial cells in the central and peripheral nervous system are capable of synthesizing neurosteroids that regulate several neurophysiological functions. These include the regulation of excitatory and inhibitory synaptic transmission, neurotransmitter release and remyelinization, neuroprotection, and growth of axons and dendrites.37 The presence of endogenous ouabain in the brain14 and in the cerebrospinal fluid17,38 and the numerous physiological consequences of ouabain - Na+, K+-ATPase interactions on neuronal functions (see Introduction) and the in vivo neuroprotective effects of the steroid shown in this study strengthen the hypothesis that ouabain is an additional neurosteroid.
The results of the present study provide initial “proof of principal” for the development of ouabain, and, perhaps other cardiac steroids, for the treatment of TBI.
Acknowledgments
We would like to thank Mr. Yoni Levitt for his assistance in analyzing the immunostaining images. This work was supported in part by the Federico Foundation (D.L) and the Hoffman Leadership and Responsibility Fellowship Program (M.D.L).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Beauchamp K., Mutlak H., Smith W.R., Shohami E., and Stahel P.F. (2008). Pharmacology of traumatic brain injury: where is the “golden bullet”? Mol. Med. 14, 731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mustafa A.G. and Alshboul O.A. (2013). Pathophysiology of traumatic brain injury. Neurosciences (Riyadh) 18, 222–234 [PubMed] [Google Scholar]
- 3.Greve M.W. and Zink B.J. (2009). Pathophysiology of traumatic brain injury. Mt Sinai J Med 76, 97–104 [DOI] [PubMed] [Google Scholar]
- 4.Jin K. and Galvan V. (2007). Endogenous neural stem cells in the adult brain. J Neuroimmune Pharmacol. 2, 236–242 [DOI] [PubMed] [Google Scholar]
- 5.Bagrov A.Y., Shapiro J.I., and Fedorova O.V. (2009). Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol. Rev. 61, 9–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wasserstrom J.A. and Aistrup G.L. (2005). Digitalis: new actions for an old drug. Am. J. Physiol. Heart. Circ. Physiol. 289, H1781–H1793 [DOI] [PubMed] [Google Scholar]
- 7.Bauman J.L., Didomenico R.J., and Galanter W.L. (2006). Mechanisms, manifestations, and management of digoxin toxicity in the modern era. Am. J. Cardiovasc. Drugs. 6, 77–86 [DOI] [PubMed] [Google Scholar]
- 8.Furstenwerth H. (2010). Ouabain—the insulin of the heart. Int. J. Clin. Pract. 64, 1591–1594 [DOI] [PubMed] [Google Scholar]
- 9.Blanco G. and Mercer R.W. (1998). Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol. 275, F633–F650 [DOI] [PubMed] [Google Scholar]
- 10.Kaplan J.H. (2002). Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 71, 511–535 [DOI] [PubMed] [Google Scholar]
- 11.Liu J. and Xie Z.J.The sodium pump and cardiotonic steroids-induced signal transduction protein kinases and calcium-signaling microdomain in regulation of transporter trafficking. Biochim. Biophys. Acta 1802, 1237–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Z. and Askari A. (2002). Na(+)/K(+)-ATPase as a signal transducer. Eur. J. Biochem. 269, 2434–2439 [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto S., Kitagawa J., and Takeda M. (2008). The effects of ouabain on resting membrane potential and hyperpolarization-activated current in neonatal rat nodose ganglion neurons. Neurosci. Lett. 439, 241–244 [DOI] [PubMed] [Google Scholar]
- 14.Lichtstein D. and Rosen H. (2001). Endogenous digitalis-like Na+, K+-ATPase inhibitors, and brain function. Neurochem. Res. 26, 971–978 [DOI] [PubMed] [Google Scholar]
- 15.de Rezende Correa G., Araujo dos Santos A., Frederico Leite Fontes C., and Giestal de Araujo E. (2005). Ouabain induces an increase of retinal ganglion cell survival in vitro: the involvement of protein kinase C. Brain Res. 1049, 89–94 [DOI] [PubMed] [Google Scholar]
- 16.Murata Y., Matsuda T., Tamada K., Hosoi R., Asano S., Takuma K., Tanaka K., and Baba A. (1996). Ouabain-induced cell proliferation in cultured rat astrocytes. Jpn. J. Pharmacol. 72, 347–353 [DOI] [PubMed] [Google Scholar]
- 17.Dvela M., Rosen H., Ben-Ami H.C., and Lichtstein D. (2012). Endogenous ouabain regulates cell viability. Am. J. Physiol. Cell. Physiol. 302, C442–C452 [DOI] [PubMed] [Google Scholar]
- 18.Sibarov D.A., Bolshakov A.E., Abushik P.A., Krivoi II, and Antonov S.M. (2012). Na+,K-ATPase functionally interacts with the plasma membrane Na+,Ca2+ exchanger to prevent Ca2+ overload and neuronal apoptosis in excitotoxic stress. J. Pharmacol. Exp. Ther. 343, 596–607 [DOI] [PubMed] [Google Scholar]
- 19.Qiu J., Gao H.Q., Zhou R.H., Liang Y., Zhang X.H., Wang X.P., You B.A. and Cheng M. (2007). Proteomics analysis of the proliferative effect of low-dose ouabain on human endothelial cells. Biol. Pharm. Bull. 30, 247–253 [DOI] [PubMed] [Google Scholar]
- 20.Wang J.K., Portbury S., Thomas M.B., Barney S., Ricca D.J., Morris D.L., Warner D.S., and Lo D.C. (2006). Cardiac glycosides provide neuroprotection against ischemic stroke: discovery by a brain slice-based compound screening platform. Proc. Natl. Acad. Sci. U. S. A. 103, 10461–10466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oselkin M., Tian D., and Bergold P.J. (2010). Low-dose cardiotonic steroids increase sodium-potassium ATPase activity that protects hippocampal slice cultures from experimental ischemia. Neurosci. Lett. 473, 67–71 [DOI] [PubMed] [Google Scholar]
- 22.Chen Y., Constantini S., Trembovler V., Weinstock M., and Shohami E. (1996). An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J. Neurotrauma 13, 557–568 [DOI] [PubMed] [Google Scholar]
- 23.Flierl M.A., Stahel P.F., Beauchamp K.M., Morgan S.J., Smith W.R., and Shohami E. (2009). Mouse closed head injury model induced by a weight-drop device. Nat. Protoc. 4, 1328–1337 [DOI] [PubMed] [Google Scholar]
- 24.Beni-Adani L., Gozes I., Cohen Y., Assaf Y., Steingart R.A., Brenneman D.E., Eizenberg O., Trembolver V., and Shohami E. (2001). A peptide derived from activity-dependent neuroprotective protein (ADNP) ameliorates injury response in closed head injury in mice. J. Pharmacol. Exp. Ther. 296, 57–63 [PubMed] [Google Scholar]
- 25.Tsenter J., Beni-Adani L., Assaf Y., Alexandrovich A.G., Trembovler V., and Shohami E. (2008). Dynamic changes in the recovery after traumatic brain injury in mice: effect of injury severity on T2-weighted MRI abnormalities, and motor and cognitive functions. J. Neurotrauma 25, 324–333 [DOI] [PubMed] [Google Scholar]
- 26.Swanson R.A., Morton M.T., Tsao-Wu G., Savalos R.A., Davidson C., and Sharp F.R. (1990). A semiautomated method for measuring brain infarct volume. J. Cereb. Blood Flow Metab. 10, 290–293 [DOI] [PubMed] [Google Scholar]
- 27.Jin K., Minami M., Lan J.Q., Mao X.O., Batteur S., Simon R.P., and Greenberg D.A. (2001). Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc. Natl. Acad. Sci. U. S. A. 98, 4710–4715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nesher M., Shpolansky U., Rosen H., and Lichtstein D. (2007). The digitalis-like steroid hormones: new mechanisms of action and biological significance. Life Sci. 80, 2093–2107 [DOI] [PubMed] [Google Scholar]
- 29.Gao J., Wymore R.S., Wang Y., Gaudette G.R., Krukenkamp I.B., Cohen I.S., and Mathias R.T. (2002). Isoform-specific stimulation of cardiac Na/K pumps by nanomolar concentrations of glycosides. J. Gen. Physiol. 119, 297–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lichtstein D., Samuelov S., and Bourrit A. (1985). Characterization of the stimulation of neuronal Na(+), K(+)-ATPase activity by low concentrations of ouabain. Neurochem. Int. 7, 709–715 [DOI] [PubMed] [Google Scholar]
- 31.Lima F.D., Souza M.A., Furian A.F., Rambo L.M., Ribeiro L.R., Martignoni F.V., Hoffmann M.S., Fighera M.R., Royes L.F., Oliveira M.S., and de Mello C.F. (2008). Na+,K+-ATPase activity impairment after experimental traumatic brain injury: relationship to spatial learning deficits and oxidative stress. Behav. Brain Res. 193, 306–310 [DOI] [PubMed] [Google Scholar]
- 32.Liu J. and Xie Z.J. (2010). The sodium pump and cardiotonic steroids-induced signal transduction protein kinases and calcium-signaling microdomain in regulation of transporter trafficking. Biochim. Biophys. Acta 1802, 1237–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shein N.A., Tsenter J., Alexandrovich A.G., Horowitz M., and Shohami E. (2007). Akt phosphorylation is required for heat acclimation-induced neuroprotection. J. Neurochem. 103, 1523–1529 [DOI] [PubMed] [Google Scholar]
- 34.Cohen-Yeshurun A., Trembovler V., Alexandrovich A., Ryberg E., Greasley P.J., Mechoulam R., Shohami E. and Leker R.R. (2011). N-arachidonoyl-L-serine is neuroprotective after traumatic brain injury by reducing apoptosis. J. Cereb. Blood Flow Metab. 31, 1768–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shioda N., Han F. and Fukunaga K. (2009). Role of Akt and ERK signaling in the neurogenesis following brain ischemia. Int. Rev. Neurobiol. 85, 375–387 [DOI] [PubMed] [Google Scholar]
- 36.Cuny G.D. (2009). Kinase inhibitors as potential therapeutics for acute and chronic neurodegenerative conditions. Curr. Pharm. Des. 15, 3919–3939 [DOI] [PubMed] [Google Scholar]
- 37.Compagnone N.A. and Mellon S.H. (2000). Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 21, 1–56 [DOI] [PubMed] [Google Scholar]
- 38.Lichtstein D., Minc D., Bourrit A., Deutsch J., Karlish S.J., Belmaker H., Rimon R., and Palo J. (1985). Evidence for the presence of ‘ouabain like’ compound in human cerebrospinal fluid. Brain Res. 325, 13–19 [DOI] [PubMed] [Google Scholar]