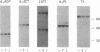Abstract
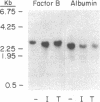
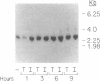
The monokine, cachectin/tumor necrosis factor (TNF) differs from interleukin 1 (IL-1) in primary structure and in recognition by a distinct cellular receptor. It does, however, encode effector functions that are similar to those of IL-1 and characteristic of the host response to inflammation or tissue injury. Accordingly, we examined the possibility that recombinant-generated human TNF regulates hepatic acute-phase gene expression. In picomolar concentrations, TNF mediated reversible, dose- and time-dependent increases in biosynthesis of complement proteins factor B and C3, alpha 1 antichymotrypsin, and decreases in biosynthesis of albumin and transferrin in human hepatoma cell lines (Hep G2, Hep 3B). Biosynthesis of complement proteins C2 and C4, and alpha 1 proteinase inhibitor were not affected by TNF. TNF also increased factor B gene expression, but had no effect on C2 gene expression, in murine fibroblasts transfected with cosmid DNA bearing the human C2 and factor B genes. The effect of TNF on acute-phase protein expression (C3, factor B, albumin) was pre-translational as shown by changes in specific messenger RNA content.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Eessalu T. E., Hass P. E. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985 Dec 19;318(6047):665–667. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- Aggarwal B. B., Kohr W. J., Hass P. E., Moffat B., Spencer S. A., Henzel W. J., Bringman T. S., Nedwin G. E., Goeddel D. V., Harkins R. N. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985 Feb 25;260(4):2345–2354. [PubMed] [Google Scholar]
- Aronsen K. F., Ekelund G., Kindmark C. O., Laurell C. B. Sequential changes of plasma proteins after surgical trauma. Scand J Clin Lab Invest Suppl. 1972;124:127–136. doi: 10.3109/00365517209102760. [DOI] [PubMed] [Google Scholar]
- Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., Dinarello C. A. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B. A., Cerami A. Recombinant interleukin 1 suppresses lipoprotein lipase activity in 3T3-L1 cells. J Immunol. 1985 Dec;135(6):3969–3971. [PubMed] [Google Scholar]
- Beutler B. A., Milsark I. W., Cerami A. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J Immunol. 1985 Dec;135(6):3972–3977. [PubMed] [Google Scholar]
- Beutler B., Greenwald D., Hulmes J. D., Chang M., Pan Y. C., Mathison J., Ulevitch R., Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985 Aug 8;316(6028):552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collins T., Lapierre L. A., Fiers W., Strominger J. L., Pober J. S. Recombinant human tumor necrosis factor increases mRNA levels and surface expression of HLA-A,B antigens in vascular endothelial cells and dermal fibroblasts in vitro. Proc Natl Acad Sci U S A. 1986 Jan;83(2):446–450. doi: 10.1073/pnas.83.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. An update on human interleukin-1: from molecular biology to clinical relevance. J Clin Immunol. 1985 Sep;5(5):287–297. doi: 10.1007/BF00918247. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and the pathogenesis of the acute-phase response. N Engl J Med. 1984 Nov 29;311(22):1413–1418. doi: 10.1056/NEJM198411293112205. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Renfer L., Wolff S. M. Human leukocytic pyrogen: purification and development of a radioimmunoassay. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4624–4627. doi: 10.1073/pnas.74.10.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B. G., Kindmark C. O., Trell E. Y., Wollheim F. A. Sequential changes of plasma proteins after myocardial infarction. Scand J Clin Lab Invest Suppl. 1972;124:117–126. doi: 10.3109/00365517209102759. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Pekala P. H., Lane M. D., Cerami A. Lipoprotein lipase suppression in 3T3-L1 cells by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1982 Feb;79(3):912–916. doi: 10.1073/pnas.79.3.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A. B., Reinke R., Feigelson P. Acute phase mediators and glucocorticoids elevate alpha 1-acid glycoprotein gene transcription. J Biol Chem. 1985 Dec 15;260(29):15386–15389. [PubMed] [Google Scholar]
- Kull F. C., Jr, Jacobs S., Cuatrecasas P. Cellular receptor for 125I-labeled tumor necrosis factor: specific binding, affinity labeling, and relationship to sensitivity. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5756–5760. doi: 10.1073/pnas.82.17.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett D., Kozan B., Hadam M., Resch K., Gemsa D. Macrophage cytotoxicity: interleukin 1 as a mediator of tumor cytostasis. J Immunol. 1986 Jan;136(1):340–347. [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Onozaki K., Matsushima K., Aggarwal B. B., Oppenheim J. J. Human interleukin 1 is a cytocidal factor for several tumor cell lines. J Immunol. 1985 Dec;135(6):3962–3968. [PubMed] [Google Scholar]
- Pekala P. H., Kawakami M., Angus C. W., Lane M. D., Cerami A. Selective inhibition of synthesis of enzymes for de novo fatty acid biosynthesis by an endotoxin-induced mediator from exudate cells. Proc Natl Acad Sci U S A. 1983 May;80(9):2743–2747. doi: 10.1073/pnas.80.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Cole F. S., Goldberger G., Colten H. R. Distinct primary translation products from human liver mRNA give rise to secreted and cell-associated forms of complement protein C2. J Biol Chem. 1984 Aug 25;259(16):10380–10385. [PubMed] [Google Scholar]
- Perlmutter D. H., Colten H. R., Grossberger D., Strominger J., Seidman J. G., Chaplin D. D. Expression of complement proteins C2 and factor B in transfected L cells. J Clin Invest. 1985 Oct;76(4):1449–1454. doi: 10.1172/JCI112123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Goldberger G., Dinarello C. A., Mizel S. B., Colten H. R. Regulation of class III major histocompatibility complex gene products by interleukin-1. Science. 1986 May 16;232(4752):850–852. doi: 10.1126/science.3010455. [DOI] [PubMed] [Google Scholar]
- Ramadori G., Sipe J. D., Dinarello C. A., Mizel S. B., Colten H. R. Pretranslational modulation of acute phase hepatic protein synthesis by murine recombinant interleukin 1 (IL-1) and purified human IL-1. J Exp Med. 1985 Sep 1;162(3):930–942. doi: 10.1084/jem.162.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby M. R., Aggarwal B. B., Rinderknecht E., Svedersky L. P., Finkle B. S., Palladino M. A., Jr Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. J Immunol. 1985 Sep;135(3):2069–2073. [PubMed] [Google Scholar]
- Strunk R. C., Whitehead A. S., Cole F. S. Pretranslational regulation of the synthesis of the third component of complement in human mononuclear phagocytes by the lipid A portion of lipopolysaccharide. J Clin Invest. 1985 Sep;76(3):985–990. doi: 10.1172/JCI112099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti F. M., Dieckmann B., Beutler B., Cerami A., Ringold G. M. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- Woloski B. M., Fuller G. M. Identification and partial characterization of hepatocyte-stimulating factor from leukemia cell lines: comparison with interleukin 1. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1443–1447. doi: 10.1073/pnas.82.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Edge M. D., Colten H. R. Isolation of a complementary DNA clone for the human complement protein C2 and its use in the identification of a restriction fragment length polymorphism. J Clin Invest. 1984 Aug;74(2):634–638. doi: 10.1172/JCI111461. [DOI] [PMC free article] [PubMed] [Google Scholar]