Abstract
Background
Clostridium difficile (C. difficile) is the pathogen that most commonly causes nosocomial and antibiotic-associated diarrheal disease. Optimized algorithms for diagnosis, treatment, and hygiene can help lower the incidence, morbidity, and mortality of C. difficile infection (CDI).
Methods
This review is based on pertinent articles that were retrieved by a selective search in PubMed for recommendations on diagnosis and treatment (up to March 2014), with particular attention to the current epidemiological situation in Germany.
Results
The incidence of CDI in Germany is 5 to 20 cases per 100 000 persons per year. In recent years, a steady increase in severe, reportable cases of CDI has been observed, and the highly virulent epidemic strain Ribotype 027 has spread across nearly the entire country. For therapeutic and hygiene management, it is important that the diagnosis be made as early as possible with a sensitive screening test, followed by a confirmatory test for the toxigenic infection. Special disinfection measures are needed because of the formation of spores. The treatment of CDI is evidence-based; depending on the severity of the infection, it is treated orally with metronidazole, or else with vancomycin or fidaxomicin. Fulminant infections and recurrences call for specifically adapted treatment modalities. Treatment with fecal bacteria (stool transplantation) is performed in gastroenterological centers that have experience with this form of treatment after multiple failures of drug treatment for recurrent infection. For critically ill patients, treatment is administered by an interdisciplinary team and consists of early surgical intervention in combination with drug treatment. A therapeutic algorithm developed on the basis of current guidelines and recommendations enables risk-adapted, individualized treatment.
Conclusion
The growing clinical and epidemiological significance of CDI compels a robust implementation of multimodal diagnostic, therapeutic, and hygienic standards. In the years to come, anti-toxin antibodies, toxoid vaccines, and focused bacterial therapy will be developed as new treatment strategies for CDI.
Clostridium difficile is the most common pathogen in nosocomial and antibiotics-associated diarrheal diseases (1– 3). It is also responsible for diarrheal diseases in patients with no risk factors (community-acquired Clostridium difficile infection) (4, 5). The frequency of Clostridium difficile infection (CDI) and its increased morbidity, which is associated with prolonged duration of inpatient treatment and a considerable rise in the use of hygiene management, lead to a significant increase in hospital treatment costs (approx. €7200 per treated case) (6, 7). This paper aims to summarize current diagnosis and treatment guidelines and to comment on the current epidemiological situation in Germany (endemic spreading of hypervirulent strains and increase in particularly severe CDI) (1, 8– 10). This should be of help in making optimized diagnosis, treatment, and hygiene management comprehensive and in reducing disease burden in the long term.
Pathogen and etiopathogenesis
C. difficile was described as a Gram-positive, spore-forming, anaerobic bacillus in the intestinal flora of healthy neonates as early as 1935 (11). The correlation between toxin-producing CDI and pseudomembranous antibiotic-associated colitis was first described in 1977 and confirmed in animal experiments (12). Only toxigenic strains with a pathogenicity locus (PaLoc) cause disease (toxin A = enterotoxin, toxin B = cytotoxin); nontoxigenic strains are apathogenic. Hypervirulent strains, such as ribotype 027, carry characteristic mutations in the toxin repressor gene tcdC (13) which can be used in molecular diagnosis (14). They also express the binary toxin, which damages human cells by inhibiting actin polymerization (14, 15).
C. difficile infection occurs via the fecal-oral route, as a result of ingestion of spores that are resistant to their environment. During gastrointestinal passage, bile acids and other substances stimulate the germination of vegetative growth forms; these produce toxins, depending on the surrounding microflora (microbiota) (16). The main risk factors for CDI are disrupted intestinal flora following antibiotic treatment and absence of antibody response to toxins, particularly in the elderly (immunosenescence) (4, 17).
Epidemiology
The frequency of CDI is increasing worldwide (2). Data from Saxony, the only German state in which general reporting is mandatory, shows an incidence of between 5 and 20 cases per 100 000 population (17). In contrast, in some regions of North America incidence is up to 100 cases per 100 000 population (18). It is striking that the number of cases of particularly severe CDI requiring intensive care and accompanied by toxic megacolon, ileus, and perforation is increasing in Germany (mandatory reporting according to Article 6, paragraph 1, point 5a of the German Protection Against Infection Act [IfSG]) (19– 21). At the same time, the highly virulent epidemic strain ribotype 027 has taken hold in many German regions (21). This is a worrying new development, although ribotype 027 is not the only strain that gives rise to the risk of severe infections. In many German states ribotype 027 is already detected more frequently than the endemic strain ribotype 001 (20). Ribotype 027 also plays a particular role in infections in homes for the elderly and care homes (22).
Risk factors
The main risk of acquiring CDI exists in the four weeks following antibiotic treatment (accounting for 40% to 60% of cases) (23– 26). A distinction can be made here between antibiotics with high colitogenic potential (clindamycin, quinolone, cephalosporin, amoxicillin/clavulanic acid) and those with low colitogenic potential (e.g. tetracyclines). Other risk factors are age (over 65), comorbidities, hospitalization in the last three months (18, 26), and residence in a home for the elderly or care home (27). Protein pump inhibitor (PPI) treatment also increases the risk of CDI (28, 29), but enteral feeding does not play a significant role (18, 30). The possible risk groups include immunosuppressed or immunodeficient patients and those with chronic inflammatory intestinal diseases (29, 31– 33).
Clinical symptoms
It is important to distinguish between asymptomatic colonization and symptomatic CDI. Symptoms range from simple irritation of the mucosa, watery to soft diarrhea with a sweetish, foul odor (18) to the full clinical picture of pseudomembranous colitis with typical endoscopic findings, preferentially in the region of the sigma and rectum (Figure 1). CDI affecting the right colon alone is rarer (32). Stool frequency can exceed 10 times per day, so in older patients signs of exsiccosis requiring treatment can occur swiftly. If symptoms are prolonged, hypoalbuminemia and protein-losing enteropathy can occur (34). Subfebrile temperatures are common (26). On physical examination, the colon is distended in the lower left abdomen in particular. There is usually only slight local pain on palpation (18, 32). Prognostically unfavorable signs of complicated CDI with ileus, toxic megacolon, perforation, or sepsis (less than 5% of cases) include absence of colonic peristalsis, sudden-onset constipation, extreme leukocytosis, and high fever (18, 26, 32). This requires further diagnostic measures such as contrast CT of the abdomen; an experienced visceral surgeon should be consulted for this (32).
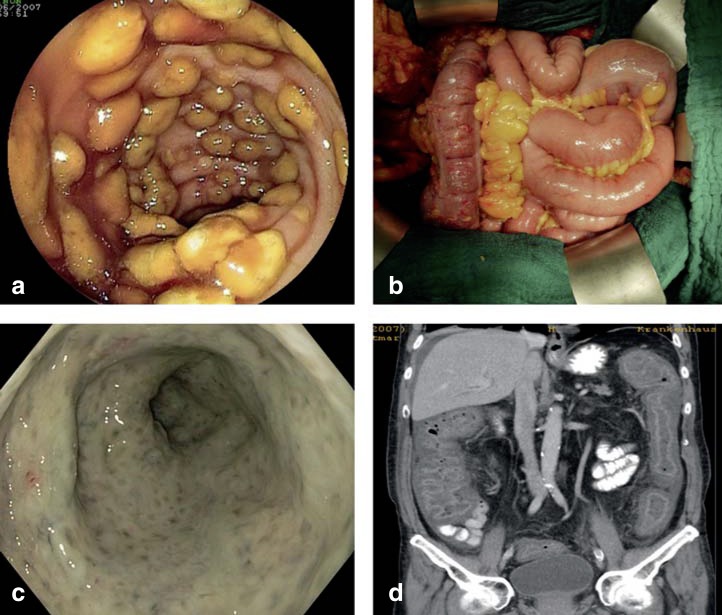
Figure 1.
Endoscopic, surgical, and radiological images of severe Clostridium difficile infection (CDI).
a) Typical endoscopic image of pseudomembranous colitis.
b) Intraoperative view of a 70-year-old male patient with acute abdomen due to fulminant CDI affecting the colon transversum. Surgery took the form of lavage, without resection of the colon. Together with concomitant conservative therapy, surgical treatment achieved complete recovery.
c) Severe C. difficile–associated pancolitis in a 71-year-old male dialysis patient. Individual pseudomembranes can no longer be distinguished in this severe case (image source: Dr. Christoph Lübbert, Leipzig).
d) CT of the same patient (coronary reconstruction) shows severe wall thickening of the entire colon area.
(Figures 1a, 1b, and 1d from [32]: Weis S. et al.: Clostridium-difficile-Infektionen (CDI) im Wandel der Zeit–ein Thema nur für den Internisten? Zentralbl Chir 2014; 139: 460–8; reproduced with the kind permission of Thieme publishers, Stuttgart, Germany)
Mortality resulting from CDI depends on the severity of symptoms, underlying diseases, and age. It ranges from 3% to 14% (18, 26). Relapses occur in approximately 20% of cases following completion of initial treatment, typically within the first 2 to 6 weeks in patients with risk factors (29, 35– 37).
Diagnosis
The international CDI diagnosis guidelines (1, 8– 10) allow evidence-based, rapid detection of toxigenic CDI from stool samples (38– 40). Multistep diagnostic procedures are recommended (14), combining a sensitive screening test with a confirmation test for the toxigenic infection (Table 1). Only symptomatic patients should be tested. Repeat stool samples are not usually required. Rapid antigen tests and nucleic acid amplification tests (NAATs) are particularly important in routine diagnosis thanks to their short turnaround time (TAT), which ranges from 15 minutes to 3 hours. The toxigenic culture, i.e. the anaerobic culture in special media, combined with evidence of the toxin in the culture supernatant, is the diagnostic gold standard. Anaerobic culture is required for further special tests such as antibiotic resistance testing and ribotype testing. Cultures are not well suited to acute diagnosis, as they have a long turnaround time (more than 72 hours).
Table 1. Microbiological diagnostic tests for Clostridium difficile and their value.
| Test | Indication |
|---|---|
| Glutamate dehydrogenase (GDH) EIA (TAT <2 hours) | Initial screening test with high sensitivity and high negative predictive value; GDH-positive samples must undergo a confirmation test for the toxigenic infection. |
| Toxin A and B EIA (TAT <2 hours) | Confirmation test for toxigenic infection in GDH-positive samples (2-step algorithm); good correlation with severe infections, limited sensitivity; NAAT (3-step algorithm) recommended if no toxin detected. |
| Cytotoxin neutralization assay (CTNA) (TAT <24 hours) | Standard test for evidence of toxins in stool; CTNA is rarely used for routine diagnosis, however, due to its longer TAT and low potential for standardization and automation. |
| NAAT of toxin genes (TAT <4 hours) | Confirmation test for toxigenic infection. NAAT (e.g. PCR) not recommended as screening test, as asymptomatic C. difficile carriers not requiring treatment or isolation may also be detected. |
| Anaerobic toxigenic culture (TAT >3 days) | Diagnostic gold standard as confirmation test for toxigenic infection; of limited use in early diagnosis of CDI due to long TAT; culture is required for ribotype testing and antibiotic resistance testing in critically ill patients and in outbreaks. |
TAT, turn-around time; EIA, enzyme immunoassay; NAAT, nucleic acid amplification test (e.g. polymerase chain reaction [PCR])
A macroscopic finding of pseudomembranous colitis is in many cases so characteristic that CDI can also be diagnosed via endoscopy or colonoscopy, though with limited sensitivity (e1, e2) (Figure 1).
Hygiene management
C. difficile spores cannot be deactivated using conventional alcohol-based disinfectants (e2, e3). CDI therefore requires isolation precautions (single rooms/cohort isolation with individual sanitation), lab coats and gloves, and sporicidal disinfection (see lists compiled by the Association for Applied Hygiene [VAH, Verbund fur Angewandte Hygiene e.V.] at www.vah-online.de) (32, e2, e3). During outbreaks and following contamination of the hands, washing with soap and water (mechanical removal of spores) is recommended. In addition to specific hygiene measures, antibiotic stewardship also contributes substantially to reducing CDI (24).
Conservative therapy
Evidence of toxigenic CDI requires rapid, risk-adapted treatment (Table 2). This usually leads to clinical improvement within 48 to 72 hours (1). If possible, the antibiotic treatment that has led to toxigenic CDI should be interrupted or switched to a less colitogenic drug such as tetracycline or tigecycline. Continued systemic antibiotic treatment increases the probability of a relapse (10). Naturally, sufficient rehydration therapy should also be administered. Motility inhibitors should be avoided and protein pump inhibitor (PPI) treatment should be discontinued if possible (28, 29).
Table 2. Clostridium difficile infection treatment recommendations by clinical presentation (according to [1, 8–10]).
| Clinical classification | Therapy | Duration | Level of evidence | Grade of recommen- dation |
|---|---|---|---|---|
| Simple | Metronidazole, 3 × 500 mg orally | 10 days | I | A |
| Vancomycin, 4 × 125 (to 250) mg orally | 10 days | I | A | |
| Interruption of antibiotic treatment that triggered infection, clinical observation, no specific treatment | II | C | ||
| Severe | Vancomycin, 4 × 125 (to 250) mg orally | 10 days | I | A |
| Fidaxomicin, 2 × 200 mg orally | 10 days | I | B | |
| Severe, complicated | If possible, vancomycin, 4 × 125 to 500 mg orally (rationale for dose escalation purely empirical) | 10 days | I | A |
| (plus) metronidazole, 3 × 500 mg IV | 10 days | II | A | |
| (plus) vancomycin retention enemas 4 × daily, intracolonically 500 mg (in 100 mL saline) | 10 days | III | B | |
| (plus) tigecyclin 2 × 50 mg IV | 10 days | III | C | |
| First recurrence | Vancomycin, 4 × 125 (to 250) mg orally | 10 days | I | B |
| Fidaxomicin, 2 × 200 mg orally | 10 days | I | B | |
| Multiple recurrences | Vancomycin, 4 × 125 (to 250) mg orally (10 days) followed by pulse schedule for at least 3 weeks (125 to 500 mg orally every 2 to 3 days) | 5 weeks | II | B |
| Vancomycin, 4 × 125 (to 250) mg orally (10 days) followed by tapering schedule (approx. 5 weeks) | 7 weeks | II | B | |
| Fidaxomicin, 2 × 200 mg orally | 10 days | II | B | |
| Rescue therapy: stool transplantation (possibly colonoscopic) in an experienced center following preliminary vancomycin treatment, 4 × 500 mg orally (4 days) | <1 week | I | A |
Oral metronidazole, vancomycin, or fidaxomicin treatment is an evidence-based recommendation (e4– e7). Only metronidazole can also be administered intravenously in exceptional cases, as a result of its pharmacokinetics. There is little data based on experience with other orally administered antibiotics such as bacitracin, nitazoxanide, fusidic acid, rifaxamin, and teicoplanin (authorized since 2013) (e8). Toxin-binding drugs such as tolevamer were inferior to standard treatment in clinical trials (e9).
There is little experience with immunotherapy using intravenously administered immunoglobulin drugs (e10). There is good data from animal experiments, however, on active and passive vaccination (e10, e11). Current research on vaccination is at the stage of Phase III clinical trials. One innovative treatment is reconstitution of protective intestinal flora via the application of vital bacteria; this is known as bacteriotherapy. The use of conventional probiotics remains controversial, as most studies into this are of poor quality. This means that no overall recommendation can be provided. In contrast, numerous observational studies and one randomized controlled trial have shown complex bacteriotherapies such as microbiome transfer to be effective (e12– e37).
., Risk-adapted treatment stratification
International treatment guidelines (1, 8– 10) distinguish between simple, severe, and complicated infections and relapses (Table 2, Figure 2). The criteria given for a diagnosis of severe infection are leukocytosis (>15 000/µL), hypoalbuminemia (<30 g/L), and increased creatinine levels (>1.5 mg/dL; alternatively, an increase by more than 1.5 times initial creatinine level). If there are additional risk factors, such as age over 65, immunosuppression, serious concomitant underlying illnesses, dialysis, or history of CDI, patients can be treated as for severe CDI. There is no need to modify therapy for initial treatment of specific highly virulent genotypes (9).
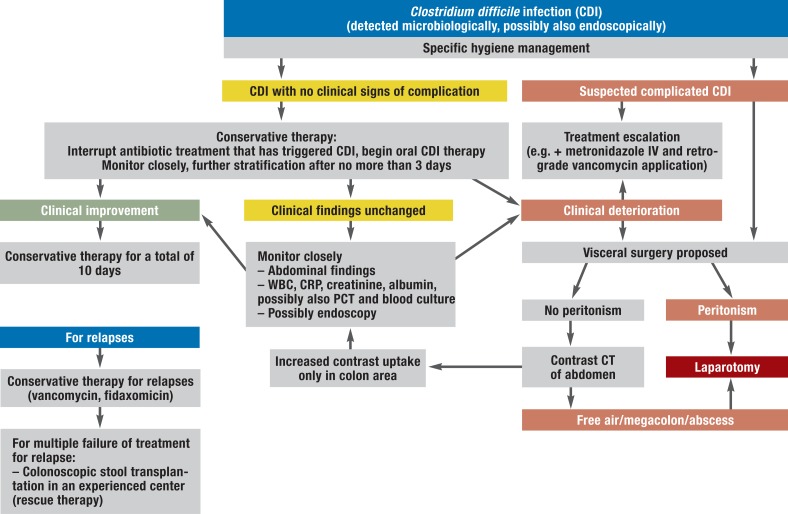
Figure 2.
Treatment pathway for patients in whom CDI is detected
WBC, white blood count; CRP, c-reactive protein; CT, computed tomography; PCT, procalcitonin
Oral metronidazole is the first-line drug for simple CDI but should not be used for severe CDI. This is because in such cases the response to treatment is lower (73% versus 81%) (e38, e39).
For initial treatment of severe CDI, oral vancomycin is the first-line drug; alternatively, oral fidaxomicin can be used (e39– e44). Treatment response was similar in pooled data from studies that compared these two options (88% versus 86%) (e43, e44). The lower relapse rate for fidaxomicin should be taken into account, particularly for patients with multiple risk factors, when treatment options are considered (e43, e44). To date there are no studies on patient groups who benefit particularly from fidaxomicin treatment (e45). Discussion of initial therapy is dominated to a great extent by cost considerations. Although fidaxomicin reduces relapses and therefore the subsequent cost of treating relapses, regular prescription of it would lead to an increase in total treatment costs (e46, e47). This is particularly true for areas in which ribotype 027 is endemic, because relapse prevention is limited for ribotype 027 infections (e46, e47).
Complicated CDI is life-threatening and requires interdisciplinary treatment by intensive care physicians and surgeons (Figure 2). A particular challenge is posed by patients in whom gastrointestinal passage, and therefore the main route of application of the appropriate medication, is disrupted (toxic megacolon, ileus). For these patients, metronidazole should be administered intravenously together with intravenous tigecycline, although the therapeutic benefit of the latter has so far only been investigated in case series (e48, e49). As far as possible, efforts should be made to continue oral vancomycin treatment in parallel even when intestinal passage is compromised, e.g. via a nasogastric tube. As an alternative, retrograde application (colonoscopy, retention enema) is possible.
A first relapse of CDI should be treated using oral vancomycin or oral fidaxomicin. This means that there is little difference between treatment recommendations for a first relapse and those for initial treatment of severe CDI.
Multiple relapses usually occur within the first 14 days following the end of treatment in patients who are particularly predisposed to CDI. Each new treatment cycle swiftly leads to an improvement in clinical findings, but it is rarely possible to ensure long-term treatment success using conventional treatment cycles (10 to 14 days). Therefore, for vancomycin, after conventional induction therapy, maintenance therapy in the form of intermittent pulse therapy or according to a tapering schedule is recommended (Table 2). As an alternative, relapses can be treated with fidaxomicin. Patients in whom relapses recur despite both vancomycin and fidaxomicin treatment are candidates for stool transplantation (synonyms: microbiome transfer, fecal bacteriotherapy).
Stool transplantation (microbiome transfer)
The principle behind stool transfer treatment for diarrheal diseases was successfully used as early as the fourth century, during the Eastern Jin Dynasty in China (e50). Since it was first described as treatment for pseudomembranous colitis in 1958 (e12), the number of original and review articles has multiplied (e13– e37). This experimental form of treatment received particular attention after a randomized controlled trial in patients with multiple relapses was successfully completed early because, after 43 patients were enrolled, stool transplantation was significantly superior to standard therapy in terms of sustained response to treatment (e35). Only patients with multiple relapses following established relapse treatment schedules should be offered stool transplantation. This selective search of the literature identified 543 cases, with a treatment response rate of 89% (Table 3). In this pooled comparison of stool transplantation (e12– e35), treatment response following colonoscopic transplantation was higher than that following application via a nasogastric or nasoduodenal tube (92% versus 82%; p = 0.005). Colonoscopic stool transfer can be recommended on the strength of better acceptance and avoidance of bacterial contamination of the small intestine with fecal microbes, in addition to its higher success rate. No more than 200 mL should be applied via the upper digestive tract (e36, e37, e51). For retrograde application, the response rate can be improved by using 500 mL or more of suspension (80% versus 97%) (e51). A highly diverse protective donor flora develops within two weeks following stool transplantation, predominantly natural Bacteroides species (e52).
Table 3. Review of the literature on stool transplantation for Clostridium difficile infections. Summary of all studies and larger case series (≥4 patients) published to date (e12– e35) and cumulative analysis.
| Reference, year | Country | Patients (n) | Successful treatments (n) | Response rate (%) | Application (method) | Study design |
|---|---|---|---|---|---|---|
| Eiseman B et al., 1958 (e12) | USA | 4 | 4 | 100% | Rectal enema | Case series |
| Bowden TA et al., 1981 (e13) | USA | 16 | 14 | 88% | Rectal enema (14 patients) Nasoduodenal tube (2 patients) |
Case series |
| Tvede M, Rask-Madsen J, 1989 (e14) | Denmark | 6 | 5 | 83% | Rectal enema | Case series |
| Paterson DL et al., 1994 (e15) | Australia | 7 | 7 | 100% | Rectal enema | Case series |
| Lund-Tønnesen S et al., 1998 (e16) | Norway | 18 | 15 | 83% | Colonoscopy | Case series |
| Gustafsson A et al., 1998 (e17) | Sweden | 9 | 9 | 100% | Rectal enema | Case series |
| Aas J et al., 2003 (e18) | USA | 18 | 15 | 83% | Nasoduodenal tube | Case series |
| Nieuwdorp M et al., 2008 (e19) | Netherlands | 7 | 7 | 100% | Colonoscopy | Case series |
| MacConnachie AA et al., 2009 (e20) | UK | 15 | 11 | 73% | Nasogastric tube | Case series |
| Rubin TA et al., 2009 (e21) | USA | 12 | 10 | 83% | Nasogastric tube | Case series |
| Rohlke F et al., 2010 (e22) | USA | 19 | 19 | 100% | Colonoscopy | Case series |
| Yoon SS, Brandt LJ., 2010 (e23) | USA | 12 | 12 | 100% | Colonoscopy | Case series |
| Garborg K et al., 2010 (e24) | Norway | 40 | 33 | 83% | Duodenoscopy (38 patients) Colonoscopy (2 patients) |
Retrospective observational study |
| Silverman MS et al., 2010 (e25) | Canada | 7 | 7 | 100% | Rectal enema | Case series |
| Polak P et al., 2011 (e26) | Czech Republic | 15 | 12 | 78% | Colonoscopy | Prospective observational study |
| Mellow MH, Kanatzar A, 2011 (e27) | USA | 13 | 11 | 85% | Colonoscopy | Case series |
| Kassam Z et al., 2012 (e28) | USA | 27 | 25 | 93% | Rectal enema | Case series |
| Brandt LJ et al., 2012 (e29) | USA | 77 | 70 | 91% | Colonoscopy | Retrospective observational study |
| Hamilton MJ et al., 2012 (e30) | USA | 43 | 37 | 86% | Colonoscopy | Retrospective observational study |
| Kelly CR et al., 2012 (e31) | USA | 26 | 24 | 92% | Colonoscopy | Retrospective observational study |
| Mattila E et al., 2012 (e32) | Finland | 70 | 66 | 94% | Colonoscopy | Retrospective observational study |
| Jorup-Rönström C et al., 2012 (e33) | Sweden | 32 | 22 | 69% | Rectal enema (27 patients) Colonoscopy (5 patients) |
Retrospective observational study |
| Maire F, 2012 (e34) | France | 34 | 34 | 100% | Colonoscopy | Prospective observational study |
| van Nood et al., 2013 (e35) | Netherlands | 16 | 15 | 94% | Nasoduodenal tube | Randomized controlled trial |
| Summary | ||||||
| Pooled data (total) | 543 | 484 | 89% | |||
| Antegrade application (nasogastric/nasoduodenal tube) | 101 | 83 | 82% | |||
| Retrograde application | 442 | 401 | 91%*1 | |||
| Colonoscopy | 341 | 313 | 92%*2 | |||
| Rectal retention enema | 101 | 88 | 87% | |||
*1p = 0.013; *2p = 0.005; statistical testing of retrograde versus antegrade application was performed using two-tailed Pearson‘s chi-square test
In a gastroenterology center with experience of selecting donors and performing the procedure, stool transplantation can be performed as an individual attempt at cure, if strictly indicated (eFigure). To do this, a protocol-based treatment schedule should be followed. Long-term risks that are as yet unknown must be monitored for in long-term follow-up and ruled out. There are, in fact, animal experiments that show a correlation between altered intestinal microbiome and the development of autoimmune diseases and obesity (e53). Overall, the legal questions regarding liability have not yet been sufficiently clarified. Although European guidelines give grade of recommendation A, funding by health insurers in Germany remains problematic.
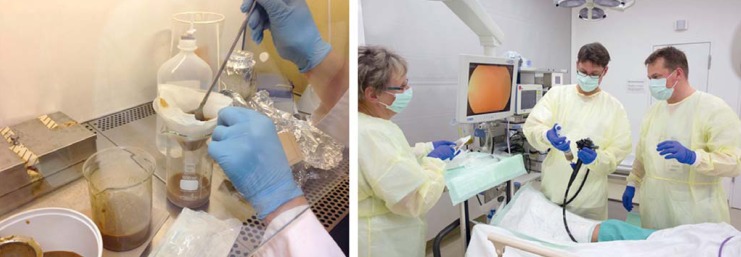
eFigure.
Stool transplantation. a) Preparation of donor stool suspension. b) Colonoscopic retrograde application (image source: Stefan Straube, Leipzig).
The concern of all treating physicians should be to perform this procedure according to a standardized protocol that guarantees patients‘ inclusion in a national stool transplantation register. Such a register, supported by the German Society for Gastroenterology, Digestive, and Metabolic Diseases (DGVS, Deutsche Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten; www.dgvs.de) is currently being developed online. Not-for-profit “stool banks” such as OpenBiome (www.openbiome.org) in North America show that the application of and access to microbiome transfer can be simplified and standardized (e54, e55). The long-term goal is oral application of standardized bacterial preparations (e14, e54, e55) that would replace stool transplantation in the future. Such preparations are already being developed (e54, e55).
Surgery
Surgery is only necessary for complicated, fulminant CDI (1% to 4%) (e56– e62). A pathophysiological correlate of surgery is reduction of the pathogen population and thus toxin production, in addition to removal of the damaged section of the intestine. It should be considered if CDI is fulminant and peritonitis, toxic megacolon, intestinal perforation, or systemic inflammation with organ failure develops despite suitable antibiotic treatment (8, 9). In these very seriously ill patients, and in CDI patients who should undergo surgery according to general criteria for visceral surgery, 30-day postoperative mortality is reported as between 24% and 80%. There is evidence that mortality following late surgery is similar to mortality without surgery (e56– e71). However, early surgery can reduce the mortality of complicated CDI (e64, e66, e68, e72– e81). Early detection of complicated CDI, before the critical stage is reached, places particular demands on clinical monitoring and ongoing diagnostics (32, e64, e65). Emergency laparotomy is performed more frequently and more rapidly in surgery departments in cases of fulminant CDI. This can reduce mortality 3.4-fold (e59, e56).
In order to provide a clinical definition of fulminant CDI requiring surgery, the criteria for systemic infection and complication can be given a risk score as an aid to classification (eTable 1). This provides a practical basis for individual clinical decisions (e56). Evidence of toxic megacolon, free air, or abscesses in contrast CT of the abdomen are clear indications for surgery. In contrast, if individual segments or even one half of the colon appear intact, this may be an indication for colon-preserving surgery (e61, e65).
eTable 1. Classification of preoperative clinical severity of Clostridium difficile infection (according to [e56]).
| Criterion | Points |
| Age >80 years | 1 |
| Severe clinical course | 1 |
| >10 episodes of diarrhea in 24 hours | 1 |
| WBC >20.000 / µL | 1 |
| CRP >150 mg/L | 1 |
| Urea >15 mg/dL | 1 |
| Albumin <20 g/l | 1 |
| Score | Mortality |
| 0 to 1 | 22% |
| 2 to 3 | 55% |
| 4 to 5 | 89% |
WBC, white blood count; CRP, c-reactive protein
Subtotal colectomy with end ileostomy remains the standard operation for fulminant, treatment-refractory CDI (e65, e68, e71, e72, e83). As this is a disease that primarily affects the luminal side of the colon, clearly externally demarcated areas of the colon that might indicate a part of the colon that could safely be preserved are rarely found intraoperatively.
Besides a colon-preserving diversion stoma (e61, e72, e87), a blow-hole colostomy or ileostomy is another interesting approach. This can be performed laparoscopically and allows for intensive antegrade colon lavage using vancomycin (e85). The selective search of the literature on case series and observational studies with more than 12 patients investigating colon-preserving surgery versus subtotal colectomy (eTable 2) shows slightly lower postoperative mortality following colon-preserving surgery (40.3% versus 30%; p = 0.078) (e63– 65, e67, e68, e84, e85, e88– e91). Overall long-term prognosis is poor even following successful surgery, with a five-year survival rate of less than 20%. Reversal of ileostomy appears to be possible in only 20% of patients (e69).
eTable 2. Review of the literature on 30-day postoperative mortality of patients with very severe, fulminant CDI and subtotal colectomy with ileostomy vs. colon-preserving surgery (case series with ≥12 patients).
| First author, no. of cases | Subtotal colectomy | Colon-preserving surgery | p-value |
|---|---|---|---|
| Perera et al. (e63), n=35 | 13/32 (40.6%) | 3/3 (100%) | N/A |
| Pepin et al. (e67), n=130 | 47/124 (40.0%) | 1/6 (16.7%) | N/A |
| Ali et al. (e68), n=36 | 14/28 (50.0%) | 3/8 (37.5%) | N/A |
| Koss et al. (e65), n=14 | 1/9 (11.1%) | 4/5 (80.0%) | N/A |
| Byrn et al. (e64), n=73 | 24/64 (37.5%) | 1/9 (11.1%) | N/A |
| Seder et al. (e84), n=69 | 29/68 (42.6%) | 0/1 (0%) | N/A |
| Medich et al. (e88), n=12 | 0/5 (0%) | 4/7 (57.1%) | N/A |
| Hall et al. (e89), n=36 | 13/34 (38.2%) | 0/2 (0%) | N/A |
| Dudukgian et al. (e90), n=14 | 4/11 (36.4%) | 1/3 (33.3%) | N/A |
| Chan et al. (e91), n=15 | 7/12 (58.3%) | 2/3 (66.7%) | N/A |
| Neal et al. (e85), n=84 | 21/42 (50.0%) | 8/42 (19.0%) | N/A |
| Total | 173/429 (40.3%) | 27/89 (30.3%) | 0.078 |
N/A: Not available. Statistical testing performed using two-tailed Pearson‘s chi-square test.
CDI: Clostridium difficile infection
Key Messages.
Hypervirulent epidemic strains of ribotype 027 have spread to cover almost all of Germany. The frequency of severe Clostridium difficile infections that are subject to mandatory reporting is increasing steadily.
Multistep diagnosis and risk-adapted treatment stratification according to the European guidelines updated in 2014 allow for rapid, effective, standardized action in patients with CDI. The combination of a sensitive screening test followed by a confirmation test for the toxigenic infection is recommended for diagnosis.
Conservative therapy primarily involves oral administration of metronidazole, vancomycin, or fidaxomicin. Special challenges are posed by patients in whom oral therapy is impossible, in relapses, and in complicated, fulminant cases.
As individual attempts at cure, patients with multiple relapses can be treated very successfully using “stool transplantation” in centers with experience in this area. Colonoscopic stool transplantation should be preferred over administration via gastrointestinal tube.
Surgery is necessary only for patients with very severe, fulminant CDI. Standard surgery is subtotal colectomy with end ileostomy. Colon-preserving surgery is only possible when treatment is initiated in good time.
Acknowledgments
Translated from the original German by Caroline Devitt, M.A.
The authors would like to thank Dr. rer. nat. Thilo Busch (Leipzig University Hospital) for his help in statistical evaluation of stool transplantation. The work of the Clostridium difficile Advisory Laboratory is funded by the Robert Koch Institute.
Footnotes
Conflict of interest statement
Dr. Lübbert has received reimbursement of conference fees from Novartis, MSD, and Astellas. Novartis and Astellas have paid travel expenses for him. He has received lecture fees from Novartis, InfectoPharm, MSD, and Astellas.
Prof. von Müller has received conference fees and reimbursement of travel expenses from Novartis and Astellas. He has received lecture fees from Astellas, Pfizer, Novartis, and Diasorin. He has received trial funding (third-party funds) from Astellas, Diasorin, BD, and Great Basin.
Dr. John declares that no conflict of interest exists.
References
- 1.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 pdate by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 2.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nature Rev Microbiol. 2009;7:526–536. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 3.Behnke M, Hansen S, Leistner R, et al. Nosocomial infection and antibiotic use: a second national prevalence study in Germany. Dtsch Arztebl Int. 2013;110:627–633. doi: 10.3238/arztebl.2013.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deshpande A, Pasupuleti V, Thota P, et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother. 2013;68:1951–1961. doi: 10.1093/jac/dkt129. [DOI] [PubMed] [Google Scholar]
- 5.Ott E, Saathoff S, Graf K, Schwab F, Chaberny IF. The prevalence of nosocomial and community acquired infections in a university hospital: an observational study. Dtsch Arztebl Int. 2013;110:533–540. doi: 10.3238/arztebl.2013.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vonberg RP, Reichardt C, Behnke M, Schwab F, Zindler S, Gastmeier P. Costs of nosocomial Clostridium difficile-associated diarrhoea. J Hosp Infect. 2008;70:15–20. doi: 10.1016/j.jhin.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care-associated infections. New Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crobach MJ, Dekkers OM, Wilcox MH, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): data review and recommendations for diagnosing Clostridium difficile-infection (CDI) Clin Microbiol Infect. 2009;15:1053–1066. doi: 10.1111/j.1469-0691.2009.03098.x. [DOI] [PubMed] [Google Scholar]
- 9.Debast SB, Bauer MP, Kuijper EJ. The Committee European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(Suppl 2):1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 10.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–498. doi: 10.1038/ajg.2013.4. quiz 99. [DOI] [PubMed] [Google Scholar]
- 11.Hall IC, O'Toole E. Intestinal flora in newborn infants with description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child. 1935;49:390–402. [Google Scholar]
- 12.Bartlett JG, Onderdonk AB, Cisneros RL, Kasper DL. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J Infect Dis. 1977;136:701–705. doi: 10.1093/infdis/136.5.701. [DOI] [PubMed] [Google Scholar]
- 13.Joost I, Speck K, Herrmann M, von Muller L. Characterisation of Clostridium difficile isolates by slpA and tcdC gene sequencing. Int J Antimicrob Agents. 2009;33(Suppl 1):13–18. doi: 10.1016/S0924-8579(09)70010-X. [DOI] [PubMed] [Google Scholar]
- 14.Stahlmann J, Schonberg M, Herrmann M, von Müller L. Detection of nosocomial Clostridium difficile infections with toxigenic strains despite negative toxin A/B testing on stool samples. Clin Microbiol Infect. 2014 doi: 10.1111/1469-0691.12558. doi: 10.1111/1469-0691.12558 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 15.Gerding DN, Johnson S, Rupnik M, Aktories K. Binary toxin CDT: Mechanism, epidemiology, and potential clinical importance. Gut Microbes. 2013;5:15–27. doi: 10.4161/gmic.26854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heeg D, Burns DA, Cartman ST, Minton NP. Spores of Clostridium difficile clinical isolates display a diverse germination response to bile salts. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burckhardt F, Friedrich A, Beier D, Eckmanns T. Clostridium difficile surveillance trends, Saxony, Germany. Emerg Infect Dis. 2008;14:691–692. doi: 10.3201/eid1404.071023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grünewald T, Kist M, Mutters R, Ruf BR, Kern WV. Clostridium-difficile-Infektion [Clostridium difficile infection] Dtsch Med Wochenschr. 2010;135:699–703. doi: 10.1055/s-0030-1251918. [DOI] [PubMed] [Google Scholar]
- 19.Weiss B. Schwer verlaufende Clostridium-difficile-Infektionen: IfSG-Surveillancedaten von 2011 und 2012. Epidemiologisches Bulletin. 2013 [Google Scholar]
- 20.Mock M, Halfmann A, Herrmann M, von Müller L. Aktuelles zur Epidemiologie von Clostridium difficile - Bericht aus dem Konsiliarlabor C. difficile. Epidemiologisches Bulletin. 2013;26:241–244. [Google Scholar]
- 21.Zaiss NH, Weile J, Ackermann G, Kuijper E, Witte W, Nuebel U. A case of Clostridium difficile-associated disease due to the highly virulent clone of Clostridium difficile PCR ribotype 027, March 2007 in Germany. Eurosurveillance. 2007;12 doi: 10.2807/esw.12.46.03306-en. E071115.1. [DOI] [PubMed] [Google Scholar]
- 22.Arvand M, Vollandt D, Bettge-Weller G, Harmanus C, Kuijper E. Clostridium difficile study group H: Increased incidence of Clostridium difficile PCR ribotype 027 in Hessen, Germany, 2011 to 2013. Eurosurveillance. 2014;19 doi: 10.2807/1560-7917.es2014.19.10.20732. [DOI] [PubMed] [Google Scholar]
- 23.Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. New Engl J Med. 2008;359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 24.Talpaert MJ, Gopal Rao G, Cooper BS, Wade P. Impact of guidelines and enhanced antibiotic stewardship on reducing broad-spectrum antibiotic usage and its effect on incidence of Clostridium difficile infection. J Antimicrob Chemother. 2011;66:2168–2174. doi: 10.1093/jac/dkr253. [DOI] [PubMed] [Google Scholar]
- 25.Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother. 2012;67:742–748. doi: 10.1093/jac/dkr508. [DOI] [PubMed] [Google Scholar]
- 26.Bauer MP, Notermans DW, van Benthem BH, et al. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;377:63–73. doi: 10.1016/S0140-6736(10)61266-4. [DOI] [PubMed] [Google Scholar]
- 27.Garg S, Mirza YR, Girotra M, et al. Epidemiology of Clostridium difficile-associated disease (CDAD): a shift from hospital-acquired infection to long-term care facility-based infection. Dig Dis Sci. 2013;58:3407–3412. doi: 10.1007/s10620-013-2848-x. [DOI] [PubMed] [Google Scholar]
- 28.Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989–2995. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- 29.Lübbert C, Johann C, Kekulé AS, et al. Immunsuppressive Behandlung als Risikofaktor für das Auftreten einer Clostridium difficile-Infektion (CDI) Z Gastroenterol. 2013;51:1251–1258. doi: 10.1055/s-0033-1335505. [DOI] [PubMed] [Google Scholar]
- 30.Bliss DZ, Johnson S, Savik K, et al. Acquisition of Clostridium difficile and Clostridium difficile-associated diarrhea in hospitalized patients receiving tube feeding. Ann Intern Med. 1998;129:1012–1019. doi: 10.7326/0003-4819-129-12-199812150-00004. [DOI] [PubMed] [Google Scholar]
- 31.Collini PJ, Bauer M, Kuijper E, Dockrell DH. Clostridium difficile infection in HIV-seropositive individuals and transplant recipients. J Infect Dis. 2012;64:131–147. doi: 10.1016/j.jinf.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Weis S, John E, Lippmann N, Mössner J, Lübbert C. Clostridium difficile-Infektionen (CDI) im Wandel der Zeit - ein Thema nur für den Internisten? Zentralbl Chir. 2014;139:460–468. doi: 10.1055/s-0032-1328623. [DOI] [PubMed] [Google Scholar]
- 33.Das R, Feuerstadt P, Brandt LJ. Glucocorticoids are associated with increased risk of short-term mortality in hospitalized patients with clostridium difficile-associated disease. Am J Gastroenterol. 2010;105:2040–2049. doi: 10.1038/ajg.2010.142. [DOI] [PubMed] [Google Scholar]
- 34.Dansinger ML, Johnson S, Jansen PC, et al. Protein-losing enteropathy is associated with Clostridium difficile diarrhea but not with asymptomatic colonization: a prospective, case-control study. Clin Infect Dis. 1996;22:932–937. doi: 10.1093/clinids/22.6.932. [DOI] [PubMed] [Google Scholar]
- 35.DuPont HL. The search for effective treatment of Clostridium difficile infection. N Engl J Med. 2011;364:473–475. doi: 10.1056/NEJMe1013236. [DOI] [PubMed] [Google Scholar]
- 36.Lo Vecchio A, Zacur GM. Clostridium difficile infection: an update on epidemiology, risk factors, and therapeutic options. Curr Opin Gastroenterol. 2012;28:1–9. doi: 10.1097/MOG.0b013e32834bc9a9. [DOI] [PubMed] [Google Scholar]
- 37.Kuijper EJ, Coignard B, Tüll P. ESCMID Study Group for Clostridium difficile; EU Member States; European Centre for Disease Prevention and Control: Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect. 2006;12(Suppl 6):2–18. doi: 10.1111/j.1469-0691.2006.01580.x. [DOI] [PubMed] [Google Scholar]
- 38.Lanzas C, Dubberke ER, Lu Z, Reske KA, Grohn YT. Epidemiological model for Clostridium difficile transmission in healthcare settings. Infect Control Hosp Epidemiol. 2011;32:553–561. doi: 10.1086/660013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Planche TD, Davies KA, Coen PG, et al. Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C difficile infection. Lancet Infect Dis. 2013;13:936–945. doi: 10.1016/S1473-3099(13)70200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubberke ER, Han Z, Bobo L, et al. Impact of clinical symptoms on interpretation of diagnostic assays for Clostridium difficile infections. J Clin Microbiol. 2011;49:2887–2893. doi: 10.1128/JCM.00891-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e1.Berdichevski T, Keller N, Rahav G, et al. The impact of pseudomembrane formation on the outcome of Clostridium difficile-associated disease. Infection. 2013;41:969–977. doi: 10.1007/s15010-013-0473-4. [DOI] [PubMed] [Google Scholar]
- e2.Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):12–18. doi: 10.1086/521863. [DOI] [PubMed] [Google Scholar]
- e3.Boyce JM, Ligi C, Kohan C, Dumigan D, Havill NL. Lack of association between the increased incidence of Clostridium difficile-associated disease and the increasing use of alcohol-based hand rubs. Infect Control Hosp Epidemiol. 2006;27:479–483. doi: 10.1086/504362. [DOI] [PubMed] [Google Scholar]
- e4.Surawicz CM, Alexander J. Treatment of refractory and recurrent Clostridium difficile infection. Nature Rev Gastroenterol Hepatol. 2011;8:330–339. doi: 10.1038/nrgastro.2011.59. [DOI] [PubMed] [Google Scholar]
- e5.Goldstein EJ, Babakhani F, Citron DM. Antimicrobial activities of fidaxomicin. Clin Infect Dis. 2012;55(Suppl 2):143–148. doi: 10.1093/cid/cis339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e6.Bolton RP, Culshaw MA. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut. 1986;27:1169–1172. doi: 10.1136/gut.27.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e7.Tedesco F, Markham R, Gurwith M, Christie D, Bartlett JG. Oral vancomycin for antibiotic-associated pseudomembranous colitis. Lancet. 1978;2:226–228. doi: 10.1016/s0140-6736(78)91741-5. [DOI] [PubMed] [Google Scholar]
- e8.Wenisch C, Parschalk B, Hasenhundl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813–818. doi: 10.1093/clinids/22.5.813. [DOI] [PubMed] [Google Scholar]
- e9.Johnson S, Louie TJ, Gerding DN, Cornely OA, Chasan-Taber S, Fitts D, et al. Polymer Alternative for CDI Treatment (PACT) investigators. Vancomycin, Metronidazole, or Tolevamer for Clostridium difficile Infection: Results From Two Multinational, Randomized, Controlled Trials. Clin Infect Dis. 2014;59:345–354. doi: 10.1093/cid/ciu313. [DOI] [PubMed] [Google Scholar]
- e10.Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362:197–205. doi: 10.1056/NEJMoa0907635. [DOI] [PubMed] [Google Scholar]
- e11.Anosova NG, Brown AM, Li L, et al. Systemic antibody responses induced by a two-component Clostridium difficile toxoid vaccine protect against C. difficile-associated disease in hamsters. J Med Microbiol. 2013;62:1394–1404. doi: 10.1099/jmm.0.056796-0. [DOI] [PubMed] [Google Scholar]
- e12.Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44:854–859. [PubMed] [Google Scholar]
- e13.Bowden TA, Jr, Mansberger AR, Jr, Lykins LE. Pseudomembranous enterocolitis: mechanism for restoring floral homeostasis. Am Surg. 1981;47:178–183. [PubMed] [Google Scholar]
- e14.Twede M, Rask-Madsen J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet. 1989;1:1156–1160. doi: 10.1016/s0140-6736(89)92749-9. [DOI] [PubMed] [Google Scholar]
- e15.Paterson DL, Iredell J, Whitby M. Putting back the bugs: bacterial treatment relieves chronic diarrhoea. Med J Aust. 1994;160:232–233. [PubMed] [Google Scholar]
- e16.Lund-Tønnesen S, Berstad A, Schreiner A, Midtvedt T. [Clostridium difficile-associated diarrhea treated with homologous feces] Tidsskr Nor Laegeforen. 1998;118:1027–1030. [PubMed] [Google Scholar]
- e17.Gustafsson A, Lund-Tønnesen S, Berstad A, et al. Faecal short-chain fatty acids in patients with antibiotic-associated diarrhoea, before and after faecal enema treatment. Scand J Gastroenterol. 1998;33:721–727. doi: 10.1080/00365529850171666. [DOI] [PubMed] [Google Scholar]
- e18.Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: a case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36:580–585. doi: 10.1086/367657. [DOI] [PubMed] [Google Scholar]
- e19.Nieuwdorp M, van Nood E, Speelman P, et al. [Treatment of recurrent Clostridium difficile-associated diarrhoea with a suspension of donor faeces.] Ned Tijdschr Geneeskd. 2008;152:1927–1932. [PubMed] [Google Scholar]
- e20.MacConnachie AA, Fox R, Kennedy DR, Seaton RA. Faecal transplant for recurrent Clostridium difficile-associated diarrhoea: a UK case series. QJM. 2009;102:781–784. doi: 10.1093/qjmed/hcp118. [DOI] [PubMed] [Google Scholar]
- e21.Rubin TA, Gessert CE, Aas J. Stool transplantation for older patients with Clostridium difficile infection. J Am Geriatr Soc. 2009;57 doi: 10.1111/j.1532-5415.2009.02600.x. [DOI] [PubMed] [Google Scholar]
- e22.Rohlke F, Surawicz CM, Stollman N. Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol. 2010;44:567–570. doi: 10.1097/MCG.0b013e3181dadb10. [DOI] [PubMed] [Google Scholar]
- e23.Yoon SS, Brandt LJ. Treatment of refractory/recurrent C. difficile-associated disease by donated stool transplanted via colonoscopy: a case series of 12 patients. J Clin Gastroenterol. 2010;44:562–566. doi: 10.1097/MCG.0b013e3181dac035. [DOI] [PubMed] [Google Scholar]
- e24.Garborg K, Waagsbø B, Stallemo A, Matre J, Sundøy A. Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scand J Infect Dis. 2010;42:857–861. doi: 10.3109/00365548.2010.499541. [DOI] [PubMed] [Google Scholar]
- e25.Silverman MS, Davis I, Pillai DR. Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol. 2010;8:471–473. doi: 10.1016/j.cgh.2010.01.007. [DOI] [PubMed] [Google Scholar]
- e26.Polak P, Freibergerova M, Jurankova J, et al. [First experiences with faecal bacteriotherapy in the treatment of relapsing pseudomembranous colitis due to Clostridium difficile] Klein Mikrobiol Infekc Lek. 2011;17:214–217. [PubMed] [Google Scholar]
- e27.Mellow MH, Kanatzar A. Colonoscopic fecal bacteriotherapy in the treatment of recurrent Clostridium difficile infection—results and follow-up. J Okla State Med Assoc. 2011;104:89–91. [PubMed] [Google Scholar]
- e28.Kassam Z, Hundal R, Marshall JK, Lee CH. Fecal transplant via retention enema for refractory or recurrent Clostridium difficile infection. Arch Intern Med. 2012;172:191–193. doi: 10.1001/archinte.172.2.191. [DOI] [PubMed] [Google Scholar]
- e29.Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:1079–1087. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- e30.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:761–767. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- e31.Kelly CR, de Leon L, Jasutkar N. Fecal microbiota transplantation for relapsing Clostridium difficile infection in 26 patients: methodology and results. J Clin Gastroenterol. 2012;46:145–149. doi: 10.1097/MCG.0b013e318234570b. [DOI] [PubMed] [Google Scholar]
- e32.Mattila E, Uusitalo-Seppala R, Wuorela M, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012;142:490–496. doi: 10.1053/j.gastro.2011.11.037. [DOI] [PubMed] [Google Scholar]
- e33.Jorup-Rönström C, Hakanson A, Sandell S, et al. Fecal transplant against relapsing Clostridium difficile-associated diarrhea in 32 patients. Scand J Gastroenterol. 2012;47:548–552. doi: 10.3109/00365521.2012.672587. [DOI] [PubMed] [Google Scholar]
- e34.Maire F. [Fecal transplantation: new therapy for recurrent Clostridium difficile infection?] Hepato-Gastro. 2012;19:285–288. [Google Scholar]
- e35.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- e36.Kleger A, Schnell J, Essig A, et al. Fecal transplant in refractory Clostridium difficile colitis. Dtsch Arztebl Int. 2013;110:108–115. doi: 10.3238/arztebl.2013.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e37.Gross M, Meyer C. Stuhlübertragung bei rezidivierender Clostridium-difficile-Infektion [Stool transplantation for relapsing Clostridium difficile colitis] Z Gastroenterol. 2013;51:1441–1443. doi: 10.1055/s-0033-1350583. [DOI] [PubMed] [Google Scholar]
- e38.Baines SD, O‘Connor R, Freeman J, et al. Emergence of reduced susceptibility to metronidazole in Clostridium difficile. J Antimicrob Chemother. 2008;62:1046–1052. doi: 10.1093/jac/dkn313. [DOI] [PubMed] [Google Scholar]
- e39.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–307. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- e40.von Müller L, Halfmann A, Herrmann M. Aktuelle Daten und Trends zur Antibiotikaresistenzentwicklung von Clostridium difficile [Current data and trends on the development of antibiotic resistance of Clostridium difficile] Bundesgesundheitsblatt. 2012;55:1410–1417. doi: 10.1007/s00103-012-1556-6. [DOI] [PubMed] [Google Scholar]
- e41.Babakhani F, Bouillaut L, Sears P, Sims C, Gomez A, Sonenshein AL. Fidaxomicin inhibits toxin production in Clostridium difficile. J Antimicrob Chemother. 2013;68:515–522. doi: 10.1093/jac/dks450. [DOI] [PubMed] [Google Scholar]
- e42.Babakhani F, Bouillaut L, Gomez A, Sears P, Nguyen L, Sonenshein AL. Fidaxomicin inhibits spore production in Clostridium difficile. Clin Infect Dis. 2012;55(Suppl 2):162–129. doi: 10.1093/cid/cis453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e43.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. New Engl J Med. 2011;364:422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- e44.Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12 doi: 10.1016/S1473-3099(11)70374-7. [DOI] [PubMed] [Google Scholar]
- e45.Johnson S, Gerding DN, Louie TJ, Ruiz NM, Gorbach SL. Sustained clinical response as an endpoint in treatment trials of Clostridium difficile-associated diarrhea. Antimicrob Agents Chemother. 2012;56:4043–4045. doi: 10.1128/AAC.00605-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e46.Wagner M, Lavoie L, Goetghebeur M. Clinical and economic consequences of vancomycin and fidaxomicin for the treatment of Clostridium difficile infection in Canada. Can J Infect Dis Med Microbiol. 2014;25:87–94. doi: 10.1155/2014/793532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e47.Bartsch SM, Curry SR, Harrison LH, Lee BY. The potential economic value of screening hospital admissions for Clostridium difficile. Eur J Clin Microbiol Infect Dis. 2012;31:3163–3171. doi: 10.1007/s10096-012-1681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e48.Herpers BL, Vlaminckx B, Burkhardt O, et al. Intravenous tigecycline as adjunctive or alternative therapy for severe refractory Clostridium difficile infection. Clin Infect Dis. 2009;48:1732–1735. doi: 10.1086/599224. [DOI] [PubMed] [Google Scholar]
- e49.Baines SD, Saxton K, Freeman J, Wilcox MH. Tigecycline does not induce proliferation or cytotoxin production by epidemic Clostridium difficile strains in a human gut model. J Antimicrob Chemother. 2006;58:1062–1065. doi: 10.1093/jac/dkl364. [DOI] [PubMed] [Google Scholar]
- e50.Zhang F, Lhuo W, Shi Y, Fan Z, Ji G. Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol. 2012;107:1755–1756. doi: 10.1038/ajg.2012.251. 1755, author reply. [DOI] [PubMed] [Google Scholar]
- e51.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- e52.Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2010;44:354–360. doi: 10.1097/MCG.0b013e3181c87e02. [DOI] [PubMed] [Google Scholar]
- e53.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- e54.Petrof EO, Khoruts A. From stool transplants to next-generation microbiota therapeutics. Gastroenterology. 2014;146:1573–1582. doi: 10.1053/j.gastro.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e55.Smith MB, Kelly C. How to regulate faecal transplants. Nature. 2014;506:290–291. doi: 10.1038/506290a. [DOI] [PubMed] [Google Scholar]
- e56.Bhangu A, Nepogodiev D, Gupta A, Torrance A, Singh P. Systematic review and meta-analysis of outcomes following emergency surgery for Clostridium difficile colitis. Br J Surg. 2012;99:1501–1513. doi: 10.1002/bjs.8868. [DOI] [PubMed] [Google Scholar]
- e57.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235:363–372. doi: 10.1097/00000658-200203000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e58.Longo WE, Mazuski JE, Virgo KS, et al. Outcome after colectomy for Clostridium difficile colitis. Dis Colon Rectum. 2004;47:1620–1626. doi: 10.1007/s10350-004-0672-2. [DOI] [PubMed] [Google Scholar]
- e59.Sailhamer EA, Carson K, Chang Y, et al. Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality. Arch Surg. 2009;144:433–439. doi: 10.1001/archsurg.2009.51. discussion 439-40. [DOI] [PubMed] [Google Scholar]
- e60.Wysowski DK. Increase in deaths related to enterocolitis due to Clostridium difficile in the United States, 1999-2002. Public Health Rep. 2006;121:361–362. [PMC free article] [PubMed] [Google Scholar]
- e61.Wang MF, Ding Z, Zhao J, Jiang CQ, Liu ZS, Qian Q. Current role of surgery for the treatment of fulminant Clostridium difficile colitis. Chin Med J. 2013;126:949–956. [PubMed] [Google Scholar]
- e62.Carchman EH, Peitzman AB, Simmons RL, Zuckerbraun BS. The role of acute care surgery in the treatment of severe, complicated Clostridium difficile-associated disease. J Trauma Acute Care Surg. 2012;73:789–800. doi: 10.1097/TA.0b013e318265d19f. [DOI] [PubMed] [Google Scholar]
- e63.Perera AD, Akbari RP, Cowher MS, Read TE, McCormick JT, Medich DS, et al. Colectomy for fulminant Clostridium difficile colitis: predictors of mortality. Am Surg. 2010;76:418–421. doi: 10.1177/000313481007600421. [DOI] [PubMed] [Google Scholar]
- e64.Byrn JC, Maun DC, Gingold DS, et al. Predictors of mortality after colectomy for fulminant Clostridium difficile colitis. Arch Surg. 2008;143:150–154. doi: 10.1001/archsurg.2007.46. discussion 155. [DOI] [PubMed] [Google Scholar]
- e65.Koss K, Clark MA, Sanders DS, et al. The outcome of surgery in fulminant Clostridium difficile colitis. Colorectal Dis. 2006;8:149–154. doi: 10.1111/j.1463-1318.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- e66.Lamontagne F, Labbe AC, Haeck O, et al. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg. 2007;245:267–272. doi: 10.1097/01.sla.0000236628.79550.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e67.Pepin J, Vo TT, Boutros M, et al. Risk factors for mortality following emergency colectomy for fulminant Clostridium difficile infection. Dis Colon Rectum. 2009;52:400–405. doi: 10.1007/DCR.0b013e31819a69aa. [DOI] [PubMed] [Google Scholar]
- e68.Ali SO, Welch JP, Dring RJ. Early surgical intervention for fulminant pseudomembranous colitis. Am Surg. 2008;74:20–26. doi: 10.1177/000313480807400105. [DOI] [PubMed] [Google Scholar]
- e69.Miller AT, Tabrizian P, Greenstein AJ, et al. Long-term follow-up of patients with fulminant Clostridium difficile colitis. J Gastrointest Surg. 2009;13:956–959. doi: 10.1007/s11605-009-0819-5. [DOI] [PubMed] [Google Scholar]
- e70.Greenstein AJ, Byrn JC, Zhang LP, et al. Risk factors for the development of fulminant Clostridium difficile colitis. Surgery. 2008;143:623–629. doi: 10.1016/j.surg.2007.12.008. [DOI] [PubMed] [Google Scholar]
- e71.Butala P, Divino CM. Surgical aspects of fulminant Clostridium difficile colitis. Am J Surg. 2010;200:131–135. doi: 10.1016/j.amjsurg.2009.07.040. [DOI] [PubMed] [Google Scholar]
- e72.Olivas AD, Umanskiy K, Zuckerbraun B, Alverdy JC. Avoiding colectomy during surgical management of fulminant Clostridium difficile colitis. Surg Infect. 2010;11:299–305. doi: 10.1089/sur.2010.026. [DOI] [PubMed] [Google Scholar]
- e73.Markelov A, Livert D, Kohli H. Predictors of fatal outcome after colectomy for fulminant Clostridium difficile Colitis: a 10-year experience. Am Surg. 2011;77:977–980. doi: 10.1177/000313481107700813. [DOI] [PubMed] [Google Scholar]
- e74.Synnott K, Mealy K, Merry C, et al. Timing of surgery for fulminating pseudomembranous colitis. Br J Surg. 1998;85:229–231. doi: 10.1046/j.1365-2168.1998.00519.x. [DOI] [PubMed] [Google Scholar]
- e75.Stanley JD, Burns RP. Clostridium difficile and the surgeon. Am Surg. 2010;76:235–244. [PubMed] [Google Scholar]
- e76.Henrich TJ, Krakower D, Bitton A, Yokoe DS. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis. 2009;15:415–422. doi: 10.3201/eid1503.080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e77.Ricciardi R, Harriman K, Baxter NN, et al. Predictors of Clostridium difficile colitis infections in hospitals. Epidemiol Infect. 2008;136:913–921. doi: 10.1017/S0950268807009387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e78.Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- e79.Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365:1693–1703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- e80.Chu EW, Ecker BL, Garg M, Divino CM. The surgical management of active ulcerative colitis complicated by Clostridium difficile infection. J Gastrointest Surg. 2013;17:392–396. doi: 10.1007/s11605-012-2031-2. [DOI] [PubMed] [Google Scholar]
- e81.Drew RJ, Boyle B. RUWA scoring system: a novel predictive tool for the identification of patients at high risk for complications from Clostridium difficile infection. J Hosp Infect. 2009;71:93–94. 94–95. doi: 10.1016/j.jhin.2008.09.020. author reply. [DOI] [PubMed] [Google Scholar]
- e82.Noblett SE, Welfare M, Seymour K. The role of surgery in Clostridium difficile colitis. BMJ. 2009;338 doi: 10.1136/bmj.b1563. [DOI] [PubMed] [Google Scholar]
- e83.Musher DM, Aslam S. Treatment of clostridium difficile colitis in the critical care setting. Crit Care Clin. 2008;24:279–291. doi: 10.1016/j.ccc.2007.12.006. [DOI] [PubMed] [Google Scholar]
- e84.Seder CW, Villalba MR, Jr, Robbins J, et al. Early colectomy may be associated with improved survival in fulminant Clostridium difficile colitis: an 8-year experience. Am J Surg. 2009;197:302–307. doi: 10.1016/j.amjsurg.2008.11.001. [DOI] [PubMed] [Google Scholar]
- e85.Neal MD, Alverdy JC, Hall DE, Simmons RL, Zuckerbraun BS. Diverting loop ileostomy and colonic lavage: an alternative to total abdominal colectomy for the treatment of severe, complicated Clostridium difficile associated disease. Ann Surg. 2011;254:423–427. doi: 10.1097/SLA.0b013e31822ade48. discussion 427-9. [DOI] [PubMed] [Google Scholar]
- e86.Nassour I, Carchman EH, Simmons RL, Zuckerbraun BS. Novel management strategies in the treatment of severe Clostridium difficile infection. Adv Surg. 2012;46:111–135. doi: 10.1016/j.yasu.2012.03.009. [DOI] [PubMed] [Google Scholar]
- e87.Al-Abed YA, Gray EA, Rothnie ND. Outcomes of emergency colectomy for fulminant Clostridium difficile colitis. Surgeon. 2010;8:330–333. doi: 10.1016/j.surge.2010.06.003. [DOI] [PubMed] [Google Scholar]
- e88.Medich DS, Lee KK, Simmons RL, Grubbs PE, Yang HC, Showalter DP. Laparotomy for fulminant pseudomembranous colitis. Arch Surg. 1992;127:847–852. doi: 10.1001/archsurg.1992.01420070111020. [DOI] [PubMed] [Google Scholar]
- e89.Hall JF, Berger D. Outcome of colectomy for Clostridium difficile colitis: a plea for early surgical management. Am J Surg. 2008;196:384–388. doi: 10.1016/j.amjsurg.2007.11.017. [DOI] [PubMed] [Google Scholar]
- e90.Dudukgian H, Sie E, Gonzalez-Ruiz C, Etzioni DA, Kaiser AM. C. difficile colitis—predictors of fatal outcome. J Gastrointest Surg. 2010;14:315–322. doi: 10.1007/s11605-009-1093-2. [DOI] [PubMed] [Google Scholar]
- e91.Chan S, Kelly M, Helme S, Gossage J, Modarai B, Forshaw M. Outcomes following colectomy for Clostridium difficile colitis. Int J Surg. 2009;7:78–81. doi: 10.1016/j.ijsu.2008.11.002. [DOI] [PubMed] [Google Scholar]