Abstract
Background
The long-term use of opioid analgesic drugs to treat chronic non-cancer pain (CNCP) is a major component of pain pharmacotherapy. The interpretation of the evidence concerning its efficacy and risks is currently debated.
Methods
An interdisciplinary evidence- and consensus-based S3 guideline was updated on the basis of a systematic literature search (CENTRAL, Medline, and Scopus databases, from October 2008 to October 2013); meta-analyses of randomized controlled trials (≥ 4 weeks); and a consensus procedure, as specified by the AWMF regulations, including 22 medical and psychological societies and 2 patient self-help organizations.
Results
119 publications were used to update the guideline, and 6 systematic reviews with meta-analyses were performed. A nominal group process was used to formulate recommendations concerning the indications and contraindications for the treatment of CNCP with opioid analgesics and the manner in which such treatments should be carried out. Opioid analgesics are an option for the short-term treatment (4–12 weeks) of chronic pain due to osteoarthritis (pain intensity, standardized mean difference [SMD]: –0.22 and –0.26), diabetic polyneuropathy (SMD –0.74), post-herpetic neuralgia (SMD –0.58), and chronic low back pain (SMD: –0.29 and –0.74). Long-term opioid treatment (≥26 weeks) for these diseases benefits only about 25% of patients. For other conditions, either short- or long-term treatment with opioid analgesics should be considered an individual therapeutic trial. Opioid treatment for pain is contraindicated by primary headaches and by any functional or mental disorder of which pain is a leading manifestation.
Conclusion
To minimize the risks of opioid analgesic treatment, physicians must be aware of its contraindications and must regularly reassess its efficacy and side effects. Pharmacotherapy should be combined with other types of treatment.
In a representative survey of persons in Germany aged 14 and older, carried out in 2013, 7.4% of respondents met the criteria for disabling chronic non-cancer pain (CNCP) (1). CNCP is associated with high costs, both direct and indirect (2). In Germany, opioid analgesics are often used to treat CNCP over the long term (i.e., for 3 months or longer) (e1).
Routine data from German health insurance carriers have revealed an increase in recent years in the short- and long-term prescribing of opioid analgesics to treat CNCP. The number of prescriptions of weak and strong opioid analgesics for longer than three months among BEK insurees who had non-malignant diagnoses rose from 1.9% in 2006 to 2.1% in 2009 (3). In 2000, 5.3% of the insurees of the AOK and KV Hesse insurance carriers who did not have cancer received at least one prescription for an opioid analgesic; in 2010, the comparable figure was 6.9%. Among insurees receiving at least one prescription for an opioid analgesic, the percentage under long-term opioid treatment (>90 days) was 4.3% in 2001 and 7.5% in 2009 (4).
The long-term use of opioid analgesics to treat CNCP is controversial in Germany, as in other countries, because of the discrepancy between clinical practice and the extant evidence base (5– 7, e1, e2). Critical attitudes are sometimes said to reflect an “opioid phobia” that can harm both patients and their physicians (e3– e5). On the other hand, recent review articles and editorials from the United States have highlighted a marked rise in opioid prescriptions and in opioid-related deaths, referring to a so-called “opioid epidemic.” The long-term efficacy and safety of opioid analgesics are now being called in question (6, 7, e1).
The guideline takes positions on the indications and contraindications for opioid analgesic treatment for 4 weeks or more and the manner in which such treatment should be carried out, based on detailed analysis of the evidence and structured consensus formation. For the basic question of the utility of opioid analgesics in the treatment of chronic pain syndromes, compared to non-opioid drugs and other treatments, the reader is referred to the German S3 guidelines for the treatment of the respective conditions.
Methods
Updating of the guideline
The first version of this guideline was prepared according to the then-current methodological standards for S3 guidelines and published in 2008 (5). The large amount of new scientific evidence that has emerged since then has made it necessary for this guideline to be updated.
Guideline-writing group
The directors of the German Pain Society named 17 persons (clinicians, experts on guideline preparation, patient representatives) to the steering committee for the creation of the updated guideline, on the basis of their clinical and/or scientific expertise. These persons included practitioners of primary care medicine, anesthesiology, internal medicine (with geriatrics), neurology, orthopedics/trauma surgery, psychosomatic medicine, palliative-care medicine, and clinical psychology (eBox 1).
eBox 1. The guideline-creating group.
Members of the steering committee and their membership in medical societies representing areas in which physicians undergo continuing medical education, or in psychological societies or patient self-help groups
Spokesman:
PD Dr. med. Winfried Häuser, German Society for Internal Medicine (Deutsche Gesellschaft für Innere Medizin, DGIM), German Society for Psychosomatic Medicine and Psychotherapy (Deutsche Gesellschaft für Psychosomatische Medizin und Psychotherapie, DGPM)
Members:
Dr. med. Fritjof Bock, German Society for Orthopedics and Orthopedic Surgery (Deutsche Gesellschaft für Orthopädie und orthopädische Chirurgie, DGOOC)
Dr. med. Peter Engeser, German Society of General Practice and Family Medicine (Deutsche Gesellschaft für Allgemein- und Familienmedizin, DEGAM)
Dr. med. Gerhard Hege-Scheuing, German Society for Anesthesiology and Intensive Care Medicine (Deutsche Gesellschaft für Anästhesie und Intensivmedizin, DGAI)
Prof. Dr. phil. Michael Hüppe, German Society for Psychological Pain Therapy and Research (Deutsche Gesellschaft für psychologische Schmerztherapie und -forschung, DGPSF)
Dr. rer. nat. Gabriele Lindena
Prof. Dr. med. Christoph Maier, DGAI
Heike Norda, SchmerzLOS
Prof. Dr. med. Frank Petzke, DGAI
Prof. Dr. med. Lukas Radbruch, DGAI
Prof. Dr. med. Rainer Sabatowski, DGAI
Prof. Dr. med. Michael Schäfer, DGAI
Prof. Dr. med. Marcus Schiltenwolf, DGOOC
PD Dr. med. Matthias Schuler, DGIM
Prof. Dr. phil. Hardo Sorgatz, DGPSF
Prof. Dr. med. Dr. rer. nat. Thomas Tölle, German Neurological Society (Deutsche Gesellschaft für Neurologie, DGN)
Dipl. psych. Anne Willweber-Strumpf, DGPSF
All societies representing a medical specialty in which physicians caring for adult patients must undergo continuing medical education were invited to participate in the consensus group. The following were also invited:
specialty societies that had participated in the creation of the original version of the guideline,
the German Society for Pain Medicine (Deutsche Gesellschaft für Schmerzmedizin),
three patient self-help organizations (eTable 1).
eTable 1. Participating societies and their representatives.
| Specialty societies | Delegates to the consensus conference |
|---|---|
| Medical | |
| German Diabetes Society (DDG) | Prof. Dr. med. Dan Ziegler, Deutsches Diabeteszentrum Düsseldorf, Auf'm Hennekamp 65, 40225 Düsseldorf |
| German Society of General Practice and Family Medicine (DEGAM) | Dr. med. Peter Engeser, FA f. Allgemeinmed., Hohenzollernstr. 36, 75177 Pforzheim |
| German Society for Anesthesiology and Intensive Care Medicine (DGAI) | Prof. Dr. med. Michael Schäfer, Klinik für Anästhesiologie mit Schwerpunkt operative Intensivmedizin der Charité, Universitätsmedizin Berlin, Charitéplatz 1, 10117 Berlin |
| German Society for Occupational and Environmental Medicine (DGAUM) | Dr. med. Kristin Hupfer, BASF—The Chemical Company, Carl-Bosch-Str. 38, 67056 Ludwigshafen |
| Geman Surgical Society (DGCH) | Prof. Dr. med. Stephan Freys, Chirurgische Klinik, Klinikleitung, DIAKO e. V. Diakonie-Krankenhaus gGmbH, Gröpelinger Heerstr. 406–408, 28239 Bremen |
| German Society for Geriatric Psychiatry and Psychotherapy (DGGPP) | Dr. med. Lutz M. Drach, C. F. Flemming-Klinik, Helios-Kliniken Schwerin, Wismarsche Str. 393, 19049 Schwerin |
| German Society for Gynecology and Obstetrics (DGGG) | Prof. Dr. med. Achim Rody, Klinik für Gyn/Geb., Univ.klinikum Schleswig-Holstein, Campus Lübeck, Ratzeburger Allee 160, 23538 Lübeck |
| German Society for Otorhinolaryngology and Head and Neck Surgery (DGHNOKHC) | supporting society |
| German Society for Internal Medicine (DGIM) | Prof. Dr. med. Gerhard Müller, Universitätsmedizin Göttingen, Georg-August-Univ. Göttingen, Direktor der Abt. Nephrologie und Rheumatologie, Robert-Koch-Str. 40, 37075 Göttingen |
| German Society for Oral and Maxillofacial Surgery (DGMKG) | Dr. Dr. med. habil. Volker Thieme, MKG-Chirurg, Plastische Operationen, Spez. Schmerzther., Justus-Liebig-Str. 56, 28357 Bremen |
| German Society for Neurosurgery (DGNC) | Prof. Dr. med. Volker Tronnier, Direktor der Klinik und Poliklinik für Neurochirurgie der Univ. Lübeck, Ratzeburger Allee 160, Haus 40, 23538 Lübeck |
| German Neurological Society (DGN) | Prof. Dr. med. Thomas R. Tölle, Klinik für Neurologie, Technische Universität München, Ismaninger Str. 22, 81675 Munich |
| German Society for Orthopedics and Orthopedic Surgery (DGOOC) | Prof. Dr. med. Marcus Schiltenwolf, Universität Heidelberg, Orthopädische Klinik, Schlierbacher Landstr. 200a, 69118 Heidelberg |
| German Society for Osteology (DGO) | Dr. med. Dieter Schöffel, Privatpraxis für Rheumatologie und Schmerztherapie Mannheim, Kaiserring 36, 68161 Mannheim |
| German Society for Physical Medicine and Rehabilitation (DGPMR) | supporting society |
| German Society for Psychiatry, Psychotherapy, Psychosomatic Medicine, and Neurology (DGPPN) | Prof. Dr. med. Karl-Jürgen Bär, Klinik für Psychiatrie und Psychotherapie, Universitätsklinikum Jena, Philosophenweg 3, 07743 Jena |
| German Society for Psychosomatic Medicine and Medical Psychotherapy (DGPM) | Prof. Dr. med. Ralf Nickel, Klinik f. Psychosom. Med. u. Psychother., Dr. Horst Schmidt Kliniken Wiesbaden, Rheingauerstr. 35, 65388 Schlangenbad |
| German Society for Radio-Oncology (DEGRO) | supporting society |
| German Society for Rheumatology (DGRh) | Prof. Dr. med. Christoph G. O. Baerwald, Universitätsklinikum Leipzig, Klinik und Poliklinik f. Gastroenterolo. u. Rheumatolo., Sektion Rheumatolo./Gerontolo., Liebigstr. 20, 04103 Leipzig |
| German Society for Addiction Research and Treatment (DG-Sucht) | Prof. Dr. med. Ursula Havemann-Reinecke, Bereich Suchtmedizin, Univ.medizin Göttingen, Abt. Psychiatrie u. Psychotherapie, Von-Siebold-Str. 5, 37075 Göttingen |
| German Society for Thoracic and Cardiovascular Surgery (DGTHG) | Dr. med. Bernhard Gohrbandt, Univ.medizin Mainz, Klinik und Poliklinik für Herz-/Thorax- u. Gefäßchirurgie, Langenbeckstr. 1, 55131 Mainz |
| German Society for Urology (DGU) | Prof. Dr. med. Dirk-Henrik Zermann, Vogtland-Klinik Bad Elster, Forststr. 3, 08645 Bad Elster |
| German Migraine and Headache Society (DMKG) | Prof. Dr. med. Martin Marziniak, Neurologie des kbo-Isar-Amper-Klinikums gGmbH, Vockestr. 72, 85540 Haar b. München |
| German Ophthalmological Society (DOG) | Prof. Dr. med. Ulrich Kellner, Augen-Zentrum Siegburg, Europaplatz 3, 53721 Siegburg |
| Interdisciplinary Society for Orthopedic, Trauma Surgical, and General Pain Therapy (IGOST) | Dr. med. Stefan Middeldorf, Orthopädische Klinik, Schön Klinik Bad Staffelstein, Am Kurpark 11, 96231 Bad Staffelstein |
| Psychological | |
| German Society for Psychological Pain Therapy and Research (DGPSF) | Dr. phil. Dipl.-Psych. Jule Frettlöh, BG-Uniklinikum Bergmannsheil, Neurolog. Klinik und Poliklinik, Bürkle-de-la-Camp-Platz 1, 44789 Bochum |
| Patient organizations | |
| German Rheumatology Patients‘ League | Helga Germakowski, Lerchenweg 43, 44807 Bochum |
| SchmerzLos, Lübeck | Heike Norda, Röntgenstr. 74, 24537 Neumünster |
Die German Dermatological Society (Deutsche Dermatologische Gesellschaft), the German Society for Pain Medicine, and the German Pain League (Deutsche Schmerzliga) declined to participate.
The conflicts of interest declared by the members of the guideline group are documented in the guideline‘s Methods section. They were evaluated by two directors of the German Pain Society (a psychologist and a physician) who did not participate in the creation of the guideline, with the aid of a representative of the Association of Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften, AWMF).
Literature search
The search strategy was developed on the basis of current Cochrane reviews regarding the use of opioid analgesics in the treatment of CNCP (8, 9) and the protocol of a systematic review of pertinent randomized controlled trials (RCTs) (10). For the creation of the guideline, a search was carried out for randomized trials of at least 4 weeks‘ duration in which opioid analgesics were compared with placebo and/or other analgesics for the treatment of CNCP. Open-label extension studies of RCTs and cohort studies were also assessed for the information they contained regarding long-term efficacy and risks. The search was performed in the CENTRAL, Medline, and Scopus databases for the period October 2008 to October 2013.
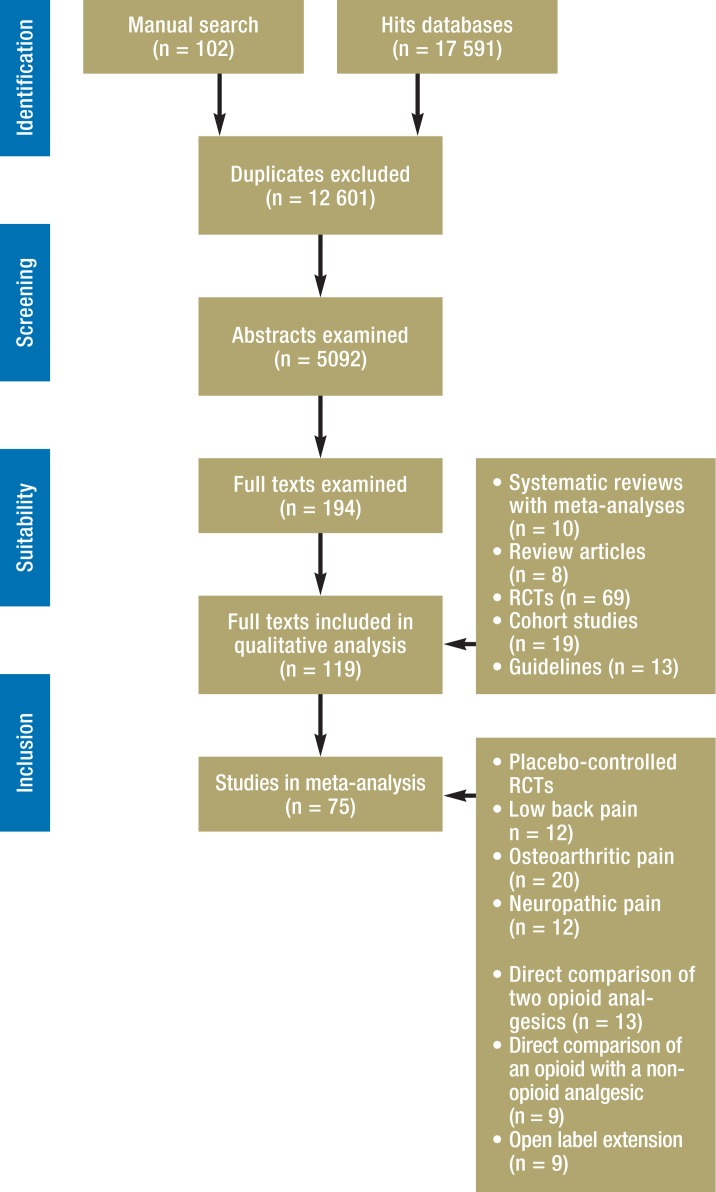
The guideline creation process also made use of a systematic review (12) of earlier guidelines (published up to July 2013) on the treatment of CNCP with opioid analgesics, which were assessed with the AGREE instrument (Appraisal of Guidelines for Research and Evaluation) (11). A total of 119 publications were used in the creation of the updated guideline (Figure).
Figure.
Results of systematic literature search; RCT, randomized controlled trial
Meta-analyses
Meta-analyses on the following topics were carried out by teams consisting of members of the steering committee along with other, external team members:
placebo-controlled trials of opioid analgesics for osteoarthritis pain (13), neuropathic pain (14), and low back pain (15);
direct comparisons of opioid analgesics with other analgesics for the treatment of CNCP (16);
direct comparisons of different types of opioid analgesics for the treatment of CNCP (17);
open-label extension studies of at least 26 weeks‘ duration, in the aftermath of RCTs of at least 2 weeks‘ duration for the treatment of CNCP (18).
The following variables were quantitatively evaluated:
Efficacy: pain intensity, percentage of patients obtaining at least 50% pain relief, global improvement (percentage of patients who reported to be much or very much improved), subjective physical impairment.
Tolerability: the percentage of patients who dropped out of the trial because of adverse effects.
Safety: the percentage of patients with severe adverse effects, and the percentage of patients who died.
Quantitative data analysis was performed with the Revman software package (e6). The effect measures were the absolute risk differences for dichotomous variables and standardized mean differences (SMD) for continuous variables, which were calculated with a random-effect model (method of inverse variance).
Uncertainty was expressed in the form of 95% confidence intervals (CI). For dichotomous variables, the threshold value for a relative benefit or relative harm was set at a 10% decrease or increase of relative risk (8). Effect strengths expressed as standardized mean differences were classified in the scheme of Cohen:
0–0.2: not substantial
0.2–0.5: weak
0.5–0.8: moderate
>0.8: strong (e7).
The minimal important difference was set at SMD ≥ 0.2 (e8). Software tools of the Cochrane Musculoskeletal Group were used to calculate the number needed to benefit (NNTB) and number needed to harm (NNTH) for dichotomous variables, and to convert SMD values into NNTB and NNTH values. For SMD-to-NNT (number needed to treat) conversions, the minimal clinically important difference (MCID) between opioid analgesics and placebo was set at 15%. The methodological quality of published studies was assessed with the GRADE scheme (Grading of Recommendations Assessment, Development and Evaluation) (e9).
Consensus-finding procedure
The key questions and recommendations of the guideline were developed by the steering committee in 14 Delphi rounds. The consensus group then voted on the recommendations over the Internet from 22 May to 11 June 2014. The consensus group held a final consensus conference, moderated by a representative of the AWMF, on 4 July 2014. The wider public was given the opportunity to comment on the guideline from 15 July to 31 August 2014; in response to these comments, a few of the recommendations and/or the accompanying explanations were modified in a further four Delphi rounds by the steering committee and the consensus group.
Recommendation strengths
The recommendation strengths were formulated as specified in the AWMF regulations (19). The evidence levels (according to the Oxford scheme) (e10) were of prime importance for the derivation of recommendation grades: the higher the evidence level, the stronger the recommendation. In general, a grade A (strong) recommendation was issued on the basis of grade 1 evidence, a grade B recommendation on the basis of grade 2 evidence, and a discretionary recommendation on the basis of evidence of grade 3, 4, or 5 (e11).
Aside from the level of evidence, the assignment of recommendation grades also took the following aspects into account: physicians‘ ethical responsibilities, the clinical relevance of the efficacy measures used in the trials, the applicability of the trial findings to the target group of patients, patients‘ wishes, and the practicality of implementation. A consideration of any of these aspects could result in a recommendation becoming stronger or weaker than it would have been on the basis of the evidence grade alone (19). To make such changes transparent, the steering committee used a Delphi procedure to determine a priori what criteria would be considered permissible for strengthening or weakening the recommendations (e11). The clinical consensus point (CCP), a further category of recommendation, was adopted as used in the German National Disease Management Guidelines: a recommendation of this type indicates a consensus in the guideline-creating group that the intervention in question is good clinical practice, i.e., a standard of care for which no scientific or experimental justification is possible or desired (e12). The recommendations do not explicitly take any considerations of health economics into account. The final guideline conference also determined the strength of each consensus that was reached (e13).
External assessment
The guideline was externally assessed by the Drug Commission of the German Medical Association and by the Swiss and Austrian Pain Societies.
Guideline contents and recommendations concerning the administration of opioid analgesics to treat CNCP
Short-term efficacy and risks (trial duration: 4–16 weeks)
Two or more RCTs on the treatment of specific types of pain with opioids are available only for chronic osteoarthritis, chronic low back pain, diabetic polyneuropathy, and post-herpetic neuralgia. For patients with these conditions, it has been shown that opioid analgesics relieve pain and subjective physical impairment better than placebo. Opioids are as safe as placebo, but less well tolerated (Table 1 and eTable 2).
Table 1. The efficacy, tolerability, and safety of opioid analgesics compared to placebo at the end of treatment (randomized, double-blind trials with parallel and crossover design, duration ≥ 4 weeks) (13, 15).
| Chronic low back pain | |||||
| Number of trials / patients | Target variable | Opioid*1 vs. placebo (%) | Statistical measures of efficacy (95% confidence interval) | Number needed to benefit or harm (95% confidence interval) | |
| 6/2869 | Pain intensity | SMD –
0.29 (–0.37; –0.21); p<0.00001; i2 = 0% |
11 (9–14) | ||
| 2/1492 | At least 50% relief of pain | 26.2 vs. 21.0 | RD 0.05 (0.01; 0.10); p = 0.01; I2 = 0% |
19 (10–107) | |
| 2/1153 | Marked or very marked global improvement | 48.6 vs. 29.0 | RD 0.16 (–0.01; 0.34); p = 0.07; I2 = 92% |
Not calculated because of lack of significance | |
| 4/1895 | Subjective physical impairment | SMD –0.22 (–0.31; –0.12); p<0.0001; i2 = 0% |
13 (10–17) | ||
| 6/2910 | Rate of termination due to adverse effects | 21.2 vs. 6.0 | RD 0.12 (0.05; 0.19); p = 0.0007; I2 = 88% |
7 (6–8) | |
| 5/2509 | Severe adverse effects | 1.4 vs. 0.8 | RD –0.01 (–0.00; 0.02); p = 0.08; I2 = 0% |
Not calculated because of lack of significance | |
| Pain due to chronic osteoarthritis | |||||
| Number of trials / patients | Target variable | Opioid*2 vs. placebo (%) | Statistical measures of efficacy (95% confidence interval) | Number needed to benefit or harm (95% confidence interval) | |
| 16/6743 | Pain intensity | SMD –0.22 (–0.28; –0.17); p<0.00001; i2 = 21% |
13 (10–17) | ||
| 2/2709 | At least 50% relief of pain | 25.1 vs. 25.7 | RD –0.00 (–0.07; 0.07); p = 0.96; I2 = 78% |
Not calculated because of lack of significance | |
| 3/2251 | Marked or very marked global improvement | 50.0 vs. 37.8 | RD 0.13 (0.05; 0.21); p = 0.002; I2 = 74% |
8 (6–12) | |
| 14/5887 | Subjective physical impairment | SMD –0.22 (–0.28; –0.17); p<0.00001; i2 = 0% |
11 (9–14) | ||
| 14/6457 | Rate of termination due to adverse effects | 25.6 vs. 7.0 | RD 0.17 (0.14; 0.21); p<0.00001; i2 = 77% |
5 (4–6) | |
| 11/5520 | Severe adverse effects | 2.4 vs. 1.8 | RD 0.00 (–0.00; 0.01); p = 0.37; I2 = 2% |
Not calculated because of lack of significance | |
*1Drugs tested: buprenorphine, oxycodone, tapentadol, tramadol
*2Grugs tested: buprenorphine, codeine, fentanyl, hydromorphone, morphine, oxycodone, oxymorphone, tapentadol, tramadol
Is A statistical measure of the homogeneity of effect; RD, risk difference; SMD, standardized mean difference
eTable 2. The efficacy, tolerability, and safety of opioid analgesics compared to placebo at the end of treatment (randomized, double-blind trials, duration ≥ 4 weeks) (13–15).
| Chronic low back pain—enriched enrollment randomized withdrawal (EERW) design*1 | ||||
| Number of trials/ patients | Target variable | Opioid*2 vs. placebo (%) | Statistical measures of efficacy (95% confidence interval) | Number needed to benefit or harm (95% confidence interval) |
| 4/1254 | Pain intensity | SMD –0.74 (–1.25; –0.22); p = 0.005; I2 = 94% |
3 (2–9) | |
| 1/498 | At least 50% relief of pain | 49.9 vs. 34.5 | RD 0.17 (0.05; 0.29); p = 0.004; I2 = 69% |
6 (5–11) |
| 1/237 | Marked or very marked global improvement | 25.7 vs. 16.1 | RD 0.10 (0.03; 0.17); p = 0.008 |
10 (6–40) |
| 2/825 | Subjective physical impairment | SMD –0.23 (–0.37; – 0.10); p = 0.0009 I2 = 0% |
9 (6–21) | |
| 6/1872 | Rate of termination due to adverse effects | 8.2 vs. 6.7 | RD 0.01 (–0.03; 0.04); p = 0.69; I2 = 57% |
Not calculated because of lack of significance |
| 6/1875 | Severe adverse effects | 1.8 vs. 1.5 | RD –0.01 (–0.00; 0.02); p = 0.64; I2 = 25% |
Not calculated because of lack of significance |
| Chronic pain due to osteoarthritis—EERW design | ||||
| Number of trials/ patients | Target variable | Opioid*3 vs. placebo (%) | Statistical measures of efficacy (95% confidence interval) | Number needed to benefit or harm (95% confidence interval) |
| 3/823 | Pain intensity | SMD –0.26 (–0.49; –0.03); p = 0.03; I2 = 57% |
8 (4–69) | |
| 1/498 | At least 50% relief of pain | 56.7 vs. 47.4 | RD 0.09 (–0.01; 0.20); p = 0.08 |
Not calculated because of lack of significance |
| 0/0 | Marked or very marked global improvement | Not assessed | ||
| 1/171 | Subjective physical impairment | SMD –0.13 (–0.34; 0.08); p = 0.24 |
Not calculated because of lack of significance | |
| 3/826 | Rate of termination due to adverse effects | 15.6 vs. 9.1 | RD 0.05 (–0.00; 0.11); p = 0.06; I2 = 35% |
Not calculated because of lack of significance |
| 2/756 | Severe adverse effects | 3.7 vs. 3.4 | RD 0.01 (–0.01; 0.03); p = 0.40; I2 = 0% |
Not calculated because of lack of significance |
| Painful diabetic polyneuropathy—parallel or crossover design | ||||
| Number of trials/ patients | Target variable | Opioid*4 vs. placebo (%) | Statistical measures of efficacy (95% confidence interval) | Number needed to benefit or harm (95% confidence interval) |
| 3/380 | Pain intensity | SMD –0.74 (–1.06; –0.43); p < 0.0001; i2 = 55% |
3 (2–5) | |
| 0/0 | At least 50% relief of pain | Not assessed | ||
| 0/0 | Marked or very marked global improvement | Not assessed | ||
| 3/380 | Subjective physical impairment | SMD –0.31 (–0.51; –0.11); p = 0.003; I2 = 0% |
7 (4–20) | |
| 3/380 | Rate of termination due to adverse effects | 12.0 vs. 5.0 | RD 0.07 (0.01; 0.13); p = 0.01; I2 = 10% |
14 (8–60) |
| 2/249 | Severe adverse effects | 4.7 vs. 9.8 | RD –0.05 (–0.11; 0.01); p = 0.11; I2 = 0% |
Not calculated because of lack of significance |
| Painful diabetic polyneuropathy—EERW design | ||||
| Number of trials/ patients | Target variable | Opioid*5 vs. placebo (%) | Statistical measures of efficacy (95% confidence interval) | Number needed to benefit or harm (95% confidence interval) |
| 0/0 | Pain intensity | Not assessed | ||
| 1/200 | At least 50% relief of pain | 59.1 vs. 36.4 | RD 0.23(0.09; 0.36); p = 0.001 | 4 (3–11) |
| 1/357 | Marked or very marked global improvement | 64.4 vs. 38.4 | RD 0.26(0.16; 0.36); p < 0.0001 | 4 (3–6) |
| 0/0 | Subjective physical impairment | Not assessed | ||
| 1/389 | Rate of termination due to adverse effects | 14.8 vs. 10.5 | RD 0.07(0.01; 0.13); p = 0.03 | 15 (6–128) |
| 1/389 | Severe adverse effects | 5.3 vs. 1.6 | RD 0.04(0.01; 0.07); p = 0.05 | 28 (14–14 166) |
| Post-herpetic neuralgia—parallel or crossover design | ||||
| Number of trials/ patients | Target variable | Opioid*6 vs. placebo (%) | Statistical measures of efficacy (95% confidence interval) | Number needed to benefit or harm (95% confidence interval) |
| 3/323 | Pain intensity | SMD –0.58(–0.85; –0.31); p < 0.0001; i2 = 29% | 4 (3–7) | |
| 0/0 | At least 50% relief of pain | Not assessed | ||
| 0/0 | Marked or very marked global improvement | Not assessed | ||
| 1/122 | Subjective physical impairment | SMD –0.13(–0.49; 0.22); p = 0.47 | Not calculated because of lack of significance | |
*1In an EERW design, the initial phase of the trial is conducted without blinding of the patient or the trial physician. Only responders, i.e., patients meeting the a priori defined criteria for a response (e.g., 50% pain reduction) in the open phase and who do not decide to stop taking the drug because of adverse effects, are admitted into the second, double-blind phase. Some of the responders continue to receive the study drug, while others receive placebo. Thus, the RCT is performed exclusively on responders; this selection impairs the generalizability of the findings to the overall population of patients with the condition in question. EERW is nonetheless considered an appropriate type of design for trials of drugs for chronic pain, because the procedure followed in the trial (treating only responders) resembles the clinical situation.
*2Tested drugs: buprenorphine, oxycodone, tapentadol, tramadol
*3Tested drugs: morphine, oxycodone
*4Tested drugs: oxycodone, tramadol
*5Tested drug: tapentadol
*6Tested drugs: morphine, methadone, oxycodone
I2, a statistical measure of effect homogeneity; RD, risk difference; SMD, standardized mean difference
Long-term efficacy and risks (trial duration: 26–108 weeks)
In a randomized trial of 6 months‘ duration involving 199 patients with chronic pain due to osteoarthritis, transdermal buprenorphine was not found to be significantly better than placebo for the reduction of pain or subjective physical impairment (p = 0.06 for both) (e14).
In an open, controlled trial, 675 patients with chronic low back pain (nociceptive, neuropathic, or mixed) were treated with either transdermal fentanyl or oral morphine for 13 months. 37% of the patients in the fentanyl group and 37% in the morphine group stated, at the end of the treatment, that their pain at rest had improved by at least 50%; for pain during movement, the corresponding figures were 40% and 50%, respectively. Average physical functional ability improved significantly (p<0.0001), from 29 to 37 on a 0–100 scale, in both groups. The rate of premature termination of treatment was 37% in the fentanyl group and 31% in the morphine group. Deaths or behavior typical of addiction were not observed (e15).
In an open, controlled trial of 52 weeks‘ duration, 1117 patients with chronic low back pain or osteoarthritis pain were treated with either tapentadol or oxycodone. The mean (with standard error) of pain intensity declined, from the beginning to the end of the trial, from 7.6 (0.05) to 4.4 (0.09) in the tapentadol group and from 7.6 (0.11) to 4.5 (0.17) in the oxycodone group. 48.1% (394/819) of the patients in the tapentadol group and 41.2% (73/177) of those in the oxycodone group reported to be much or very much improved. The rate of termination of treatment because of adverse effects was 23% for tapentadol and 37% for oxycodone. Deaths or behavior typical of addiction were not observed (e16).
Eleven open-label extension studies of placebo-controlled RCTs with a total of 2445 subjects with nociceptive pain (back pain, osteoarthritis pain) and neuropathic pain (radiculopathy, polyneuropathy) were included in the meta-analysis. The median study duration was 26 weeks (range, 26 to 108 weeks). There were four studies of oxycodone, two of tramadol, and one each of buprenorphine, hydromorphone, morphine, oxymorphone, and tapentadol. 28.5% of the initially randomized patients completed the open-label phase; 4.9% terminated it prematurely because of inadequate pain relief, and 16.8% did so because of adverse effects. 0.08% of the patients died during the open-label phase. Only a single study, from the United States, systematically investigated opioid abuse: 5.7% of the patients met the criteria for opioid abuse as assessed by the study directors, and 2.6% did so as assessed by independent experts (18).
Risks of opioid analgesics in cohort studies
In a systematic review of 67 studies, mainly from the USA, the prevalence of abuse of prescribed opioids ranged from 0.2% to 3.3% (20). Case series from German pain centers revealed no evidence of problematic drug-taking behavior among highly selected patients (21, 22). A panel of experts convened by the German federal government examined this question with the aid of data from various sources (inquiries to pharmacists and addiction clinics, analyses of routine data from health-insurance carriers, information from the Federal Chamber of Pharmacists and the Federal Criminal Police Office) and found no evidence of any numerically significant degree of abuse of tilidine or tramadol (23).
Cohort studies from the USA have shown higher mortality among elderly persons treated with opioids for painful osteoarthritis and rheumatoid arthritis than among those treated with non-steroidal anti-inflammatory drugs (24). The following have been discussed as potential reasons for this finding: inadvertent overdose, self-medication, worsening of sleep apnea, and fractures due to falls (24, 25).
High prevalences of loss of libido, impotence, and amenorrhea have been described in case series from pain centers in the USA (e17, e18).
Potential indications for short-term treatment (4–12 weeks)
Quantitatively and qualitatively adequate evidence for treatment with opioid analgesics for 4 to 12 weeks exists for chronic pain due to osteoarthritis, diabetic polyneuropathy, and post-herpetic neuralgia, and for chronic low back pain. Short-term opioid analgesic treatment for other indications should be viewed as an individual therapeutic trial (Table 2).
Table 2. Possible indications for treatment with an opioid analgesic drug for 4 to 12 weeks.
| Condition | Evidence level (Oxford) | Recommendation strength | Consensus strength |
|---|---|---|---|
| Chronic pain due to diabetic polyneuropathy | 1a | Strong | Strong consensus |
| Post-herpetic neuralgia (PHN) | 1a | Discretionary | Strong consensus |
| Chronic pain due to osteoarthritis | 1a | Discretionary | Strong consensus |
| Chronic low back pain | 1a | Discretionary | Strong consensus |
| Chronic phantom pain | 2b | Discretionary | Strong consensus |
| Chronic pain after spinal cord injury | 2b | Discretionary | Strong consensus |
| Chronic pain due to radiculopathy | 2b | Discretionary | Strong consensus |
| Chronic pain due to rheumatoid arthritis | 2b | Discretionary | Consensus |
| Chronic pain due to brain lesions(e.g., status post thalamic stroke, multiple sclerosis) | 5 | CCP: individual therapeutic trial | Strong consensus |
| Chronic pain due to complex regional pain syndrome (CRPS), types I and II | 5 | CCP: individual therapeutic trial | Strong consensus |
| Chronic pain due to polyneuropathy other than diabetic polyneuropathy and PHN (e.g., HIV, drug-induced, alcohol-induced) | 5 | CCP: individual therapeutic trial | Strong consensus |
| Chronic secondary headache (e.g., after subarachnoid hemorrhage) | 5 | CCP: individual therapeutic trial | Strong consensus |
| Chronic pain due to manifest osteoporosis (vertebral body fractures) | 5 | CCP: individual therapeutic trial | Strong consensus |
| Chronic pain due to inflammatory rheumatic diseases other than rheumatoid arthritis (e.g., systemic lupus erythematosus, seronegative spondyloarthropathy) | 5 | CCP: individual therapeutic trial | Strong consensus |
| Chronic postoperative pain (e.g., post-thoracotomy, post-sternotomy, and post-mastectomy syndrome, and after abdominal, facial, or hernia surgery) | 5 | CCP: individual therapeutic trial | Strong consensus |
| Chronic pain due to ischemic or inflammatory arterial occlusive disease | 5 | CCP: individual therapeutic trial | Strong consensus |
| Chronic pain due to grade 3 and 4 decubitus ulcers | Strong consensus | ||
| Chronic pain due to fixed contractures in nursing-dependent patients | Consensus |
CCP, clinical consensus point
Potential indications for long-term treatment (>26 weeks)
Quantitatively adequate evidence exists for chronic pain due to osteoarthritis, diabetic polyneuropathy, and post-herpetic neuralgia, and for chronic low back pain. The percentage of patients whose symptoms improved spontaneously cannot be determined from the studies that were analyzed, as they lacked control groups; nor can it be determined how many patients additionally received uncontrolled accompanying treatments. Long-term opioid analgesic treatment for other indications should be viewed as an individual therapeutic trial.
Contraindications for treatment
Contraindications for treatment with opioid analgesics are listed in Table 3.
Table 3. Contraindications to treatment with opioid analgesics.
| Condition | Evidence level (Oxford) | Recommendation strength | Consensus strength |
|---|---|---|---|
| Primary headaches | 3b | CCP | Strong consensus |
| Functional disorders | 5 | CCP | Strong consensus |
| Fibromyalgia syndrome*1 | 4b | Negative | Strong consensus |
| Chronic pain as a (major) manifestation of a mental disorder (atypical depression, persistent somatoform pain disorder, generalized anxiety disorder, post-traumatic stress disorder) | 5 | CCP | Consensus |
| Chronic pancreatitis*2 | 2b | Negative | Strong consensus |
| Chronic inflammatory bowel disease*2 | 3b | Negative | Strong consensus |
| Comorbid severe affective disorder and/or suicidality | 5 | CCP | Strong consensus |
| Current medication abuse or passing on of medications to unauthorized persons, and/or serious doubt concerning responsible use of opioid analgesics (e.g., uncontrolled taking of medications and/or unwillingness or inability to adhere to the dosing schedule) | 5 | CCP | Strong consensus |
| Current or planned pregnancy | 5 | CCP | Strong consensus |
*1Exception: tramadol (also inhibits serotonin and norepinephrine reuptake); evidence level 2b, open recommendation (can be considered as a treatment option).
*2Treatment for a limited time (< 4 weeks) is possible during an acute episode.
CCP, clinical consensus point
Treatment with opioid analgesics in practice
The manner of treatment with opioid analgesics in clinical practice is explained in Boxes 1 and 2.
Box 1. Key recommendations on measures to be taken before initiating opioid analgesic treatment (clinical consensus points).
1. Participative decision-making: The physician and the patient should discuss the potential benefits and harms of opioid analgesics compared to those of other drugs and non-pharmacological treatments. Strong consensus
2. The choice of drug: This should take account of the type of chronic pain syndrome, any accompanying medical conditions or contraindications, the patient‘s preferences, the benefits and harms of earlier treatments, and the risk–benefit profile of alternative drugs and non-pharmacological treatments. Strong consensus
3. Monotherapy with opioid analgesics: Opioid analgesics should not be the sole treatment for CNCP. Self-help resources and physical, physiotherapeutic, and/or psychotherapeutic techniques (including patient education), and/or lifestyle modification, should be used as complements to drug treatment for pain. Strong consensus
4. Screening for mental disorders: The treating physician should take a psychosocial history and screen the patient for current or past mental illness. Consensus
5. Treatment goals: Individual and realistic treatment goals should be set with the patient. Strong consensus
6. Informed consent: The patient‘s oral and/or written informed consent should be documented and should include considerations of driving ability and occupational considerations (possibly with the participation of the patient‘s family or guardian). Strong consensus
7. Titration and driving safety: Patients should be advised not to drive a car during the dose-finding phase or after dose changes. Strong consensus
Box 2. Key recommedations on treatment with opioid analgesics (clinical consensus points).
1. The differential indications of opioid analgesics:
The choice of drug should take account of the patient‘s accompanying conditions, contraindications for transdermal or oral administration, adverse effects, and the patient‘s preferences.
Strong consensus
2. Short- versus long-acting preparations: Extended-release and long-acting preparations are to be used. Consensus
3. Dosing: The drug should be taken in a fixed schedule that is timed according to the duration of its effect. Strong consensus
4. Switching between preparations: If clinical stability has been reached, a switch to another preparation with different pharmacokinetics and pharmacodynamics should only be carried out after discussion with the treating physician and with the patient‘s informed consent. Strong consensus
5. Initial dose: The initial dose should be low. Strong consensus
6. Optimal dose and treatment response: The optimal dose has been reached when the previously discussed goals of treatment have been attained and the adverse effects, if any, are mild and tolerable. Strong consensus
7. Maximum dose: The morphine equivalent dose should generally be no higher than 120 mg/d. Strong consensus
8. Long-term treatment: Treatment for longer than 3 months should be restricted to treatment responders. Strong consensus
9. The treatment of nausea: An antiemetic drug can be given as soon as opioid treatment is initiated. In 2–4 weeks, discontinuation of the antiemetic drug can be considered. Strong consensus
10. The treatment of constipation: Most patients should be given a laxative prophylactically. Many need laxatives for the duration of opioid treatment. Strong consensus
11. Pause in drug treatment: After 6 months of opioid treatment with a good response, a dose reduction or drug holiday should be discussed with the patient, to assess the indication for continued treatment and the response to the non-pharmacological treatments (e.g., multimodal therapy) that are being used in parallel. Strong consensus
12. Regular treatment monitoring: The physician giving opioids over the long term should determine at regular intervals whether the treatment goals are still being achieved and check for evidence of adverse effects (e.g., loss of libido, mental changes such as loss of interest or attention deficit, falls) or abuse of the prescribed drug. Strong consensus
The drug to be used should be chosen in consideration of the type of chronic pain syndrome from which the patient is suffering, any accompanying medical conditions, any contraindications, the patient‘s preferences, the good and bad effects of previous treatments, and the risk–benefit profile of alternative drugs and non-pharmacological treatments.
Patients with CNCP should not be given opioid analgesics as their sole treatment for this condition. Self-help resources and physical, physiotherapeutic, and/or psychotherapeutic techniques (including patient education) and/or lifestyle modification, should be used as complements to drug treatment for pain.
Emotional and functional disturbances of which pain is a major manifestation are contraindications to opioid treatment. Therefore, psychosocial screening is recommended; if evidence is found that the symptoms have a relevant psychosocial component, then the patient should be interviewed by a psychotherapist. The treating physician should be sure to inform the patient, before an opioid is taken, of the effects of the drug on driving ability, possible impaired performance and risks in the workplace, and any individually relevant risks, e.g., falls and confusion in the elderly or loss of libido in younger patients. Unjustified fears or unrealistic expectations should be addressed and corrected before opioid treatment is begun. The dose should start low and rise slowly; the patient should be told that adverse effects such as nausea and light-headedness, if they arise, may resolve spontaneously in 2–4 weeks even without any dose reduction. Opioid-induced constipation should be treated with adequate doses of laxatives (possibly prophylactically). A morphine equivalent dose above 120 mg per day is inadvisable.
The indications for terminating treatment with opioid analgesics are listed in eBox 2.
eBox 2. Key recommendations on the termination of treatment with an opioid analgesic (clinical consensus points).
Treatment with an opioid analgesic should be gradually discontinued in any of the following situations:
-
The individual goals of treatment are not met in the dose titration phase (up to 12 weeks), or adverse effects arise in this phase that are intolerable or not adequately treatable, in the view of either the patient or the physician.
Strong consensus
During further treatment, the individual goals of treatment are no longer being met, or adverse effects arise that are intolerable or not adequately treatable, in the view of either the patient or the physician. Strong consensus
The individual goals of treatment have been met by other medical means (e.g., surgery, radiotherapy, or treatment of an underlying illness) or by physical measures, physiotherapy, or psychotherapy. Strong consensus
The patient continues to abuse the prescribed opioid drug despite accompanying treatment by a specialist in drug addiction. Strong consensus
For patients with persistently severe pain and/or physical impairment despite the long-term use of opioids, opioid withdrawal can be considered as a therapeutic intervention within the framework of a multimodal treatment program. Recommendations for the treatment of special types of patients (the elderly, children, adolescents, pregnant women, and persons with mental disturbances, including substance abuse) are given in eBox 3.
eBox 3. Recommendations for special types of patients with CNCP (clinical consensus points).
-
The elderly:
The treatment should begin at a low dose.
Dose escalation should be slow.
Efficacy and tolerability should be checked at short intervals.
-
Children and adolescents:
Opioid analgesics should only be given in exceptional cases.
The treatment should be administered by specialized centers/pediatricians.
-
Pregnant women:
The termination of opioid therapy should be urgently advised if the patient is planning to beome pregnant.
If a patient taking opioid analgesics becomes pregnant, the termination of opioid treatment is advisable.
-
Patients with mental disorders:
The treatment should begin at a low dose.
Dose escalation should be slow.
Efficacy and tolerability should be checked at short intervals.
Concomitant treatment by a psychotherapist should be considered.
-
Patients with current substance dependence:
The treatment should be provided in close collaboration with physicians experienced in the care of patients with drug addiction.
Overview
Opioid analgesics are an important option for the drug treatment of chronic osteoarthritis pain, low back pain and neuropathic pain. Some, but not all, patients treated with opioid analgesics experience long-term relief (for at least 26 weeks) of pain and subjective physical impairment, without any major adverse effects. Opioid analgesics have significant risks (drug abuse, sexual dysfunction, elevated mortality); to minimize them, physicians must be aware of the contraindications and they must regularly reassess the efficacy and adverse effects of treatment. Pharmacotherapy should be combined with physical and physiotherapeutic measures and pain psychotherapy if indicated. Clearly, the short- and long-term opioid treatment of CNCP should not be expanded incautiously and unthinkingly; yet it must not be categorically rejected, either. A central aim of further research will be to assess the long-term efficacy and risks of opioid analgesics on the basis of pain registry data and routine data from health-insurance carriers.
Key Messages.
The long-term use (≥ 3 months) of opioid analgesics to treat chronic non-cancer pain is controversial in Germany, as in other countries, because of the discrepancy between clinical practice and the extant evidence base.
Opioid analgesics are an option for the short-term pharmacotherapy (4–12 weeks) of chronic pain due to osteoarthritis, diabetic polyneuropathy, and post-herpetic neuralgia, and of chronic low back pain.
Long-term opioid treatment (≥ 26 weeks) for these conditions is of benefit to about 25% of patients.
For other conditions, either short- or long-term treatment with opioid analgesics should be considered an individual therapeutic trial.
Opioid treatment for pain is contraindicated by primary headaches and by any functional or mental disorder of which pain is a leading manifestation.
To minimize the risks of opioid analgesic treatment (drug abuse, sexual dysfunction, increased mortality), physicians must be aware of its contraindications and must regularly reassess its efficacy and adverse effects.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
PD Dr. Häuser owns mutual stock funds that may contain pharmaceutical company stock. He has been paid for serving on an advisory board for Daiichi Sankyo. He has received lecture honoraria from the Abbott, Janssen-Cilag, MSD, Sharp & Dohme, and Pfizer companies.
Dr. Bock has received reimbursement of meeting participation fees from the Mundipharma and Grünenthal companies. He has received reimbursement of travel expenses and lecture honoraria from Mundipharma.
Prof. Petzke has served as a paid consultant for the Grünenthal, Epionics Spine, and Janssen-Cilag companies. He has received research support (third-party funding) and reimbursement of travel expenses from Janssen-Cilag.
Prof. Tölle has been paid for serving on advisory boards for Mundipharma, Janssen-Cilag, Grünenthal, and Ratiopharm. He has received support for travel expenses from Mundipharma and research support (third-party funding) from Pfizer.
Dr. Engeser and Dipl.-psych. Willweber-Strumpf state that they have no conflict of interest.
References
- 1.Häuser W, Wolfe F, Henningsen P, Schmutzer G, Brähler E, Hinz A. Untying chronic pain: prevalence and societal burden of chronic pain stages in the general population - a cross-sectional survey. BMC Public Health. 2014;14 doi: 10.1186/1471-2458-14-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.von Korff M, Kolodny A, Deyo RA, Chou R. Long-term opioid therapy reconsidered. Ann Intern Med. 2011;155:325–328. doi: 10.1059/0003-4819-155-5-201109060-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Werber A, Marschall U, L‘hoest H, Häuser W, Moradi M, Schiltenwolf M. Opioid therapy in the treatment of chronic pain conditions in Germany. Pain Physician. 2014 in press. [PubMed] [Google Scholar]
- 4.Schubert I, Ihle P, Sabatowski R. Increase in opiate prescription in Germany between 2000 and 2010: a study based on insurance data. Dtsch Arztebl Int. 2013;110(4):45–51. doi: 10.3238/arztebl.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinecke H, Sorgatz H. German Society for the Study of Pain (DGSS): S3 guideline LONTS. Long-term administration of opioids for non-tumor pain. Schmerz. 2009;23:440–447. doi: 10.1007/s00482-009-0839-9. [DOI] [PubMed] [Google Scholar]
- 6.Kissin I. Long-term opioid treatment of chronic nonmalignant pain: unproven efficacy and neglected safety? J Pain Res. 2013;6:513–529. doi: 10.2147/JPR.S47182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154:94–100. doi: 10.1016/j.pain.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaparro LE, Furlan AD, Deshpande A, Mailis-Gagnon A, Atlas S, Turk DC. Opioids compared to placebo or other treatments for chronic low-back pain. Cochrane Database Syst Rev. 2013;8 doi: 10.1002/14651858.CD004959.pub4. CD004959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNicol ED, Midbari A, Eisenberg E. Opioids for neuropathic pain. Cochrane Database Syst Rev. 2013;8 doi: 10.1002/14651858.CD006146.pub2. CD006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busse JW, Schandelmaier S, Kamaleldin M, et al. Opioids for chronic non-cancer pain: a protocol for a systematic review of randomized controlled trials. Syst Rev. 2013;2 doi: 10.1186/2046-4053-2-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brouwers M, Kho ME, Browman GP, et al. on behalf of the AGREE Next Steps Consortium: AGREE II: Advancing guideline development, reporting and evaluation in healthcare. J Clin Epidemol. 2010;63:1308–1311. doi: 10.1016/j.jclinepi.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38–47. doi: 10.7326/0003-4819-160-1-201401070-00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaefert R, Sommer C, Welsch P, Petzke F, Klose P, Häuser W. Opioids in chronic osteoarthritis pain - A systematic review and meta-analysis of efficacy and harms in randomized placebo-controlled studies of at least four weeks duration. Schmerz. 2014 doi: 10.1007/s00482-014-1451-1. dx.doi.org/10.1007/s00482-014-1451-1; epub ahead of print (last accessed on 2 September 2014) [DOI] [PubMed] [Google Scholar]
- 14.Sommer C, Welsch P, Petzke F, Schaefert R, Häuser W. Opioids in chronic neuropathic pain - a systematic review and meta-analysis of efficacy and harms in randomized placebo-controlled studies of at least four weeks duration. Schmerz. 2014 doi: 10.1007/s00482-014-1455-x. dx.doi.org/10.1007/s00482-014-1455-x; epub ahead of print (last accessed on 2 September 2014) [DOI] [PubMed] [Google Scholar]
- 15.Petzke F, Sommer C, Welsch P, Schaefert R, Klose P, Häuser W. Opioids in chronic low back pain - A systematic review and meta-analysis of efficacy and harms in randomized placebo-controlled studies of at least four weeks duration. Schmerz. 2014 doi: 10.1007/s00482-014-1449-8. dx.doi.org/10.1007/s00482-014-1449-8; epub ahead of print (last accessed on 3 September 2014) [DOI] [PubMed] [Google Scholar]
- 16.Welsch P, Sommer C, Schiltenwolf M, Häuser W. Opioids in chronic non-cancer pain: Are opioids superior to non-opioid analgesics? A systematic review and meta-analysis of efficacy and harms of randomized head to head comparisons of opioids versus non-opioid analgesics in studies of at least four weeks duration. Schmerz. 2014 doi: 10.1007/s00482-014-1436-0. DOI 10.1007/s00482-014-1423-5, last accessed on 3 September 2014) [DOI] [PubMed] [Google Scholar]
- 17.Lauche M, Klose P, Radbruch L, Welsch P, Häuser W. Opioids in chronic non-cancer pain: Are opioids different?- A systematic review and meta-analysis of efficacy and harms in randomized head to head comparisons of opioids in studies of at least four weeks duration. Schmerz. 2014 doi: 10.1007/s00482-014-1432-4. dx.doi.org/10.1007/s00482-014-1432-4; epub ahead of print (last accessed on 2 September 2014) [DOI] [PubMed] [Google Scholar]
- 18.Häuser W, Bernardy K, Maier C. Long-term opioid therapy in chronic non-cancer pain: A systematic review and meta-analysis of efficacy and harms in open-label extension trials with a study duration of at least 26 weeks duration. Schmerz. 2014 dx.doi.org/10.1007/s00482-014-1452-0; epub ahead of print (last accessed on 2 September 2014) [Google Scholar]
- 19.Arbeitsgemeinschaft wissenschaftlicher Fachgesellschaften AWMF. AWMF Regelwerk Leitlinien. www.awmf.org/leitlinien/awmf-regelwerk.html. (last accessed on 11 November 2013)
- 20.Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008;9:444–459. doi: 10.1111/j.1526-4637.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- 21.Maier C, Schaub C, Willweber-Strumpf A, Zenz M. Long-term efficiency of opioid medication in patients with chronic non-cancer-associated pain. Results of a survey 5 years after onset of medical treatment. Schmerz. 2005;19:410–417. doi: 10.1007/s00482-005-0432-9. [DOI] [PubMed] [Google Scholar]
- 22.Kipping K, Maier C, Bussemas H, Schwartzer A. Medication compliance in chronic pain. Pain Physician. 2014 in press. [PubMed] [Google Scholar]
- 23.Radbruch L, Glaeske G, Grond S, et al. Topical review on the abuse and misuse potential of tramadol and tilidine in Germany. Subst Abus. 2013;34:313–320. doi: 10.1080/08897077.2012.735216. [DOI] [PubMed] [Google Scholar]
- 24.Solomon DH, Rassen JA, Glynn RJ, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;13:1979–1986. doi: 10.1001/archinternmed.2010.450. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Setoguchi S, Cabral H, Jick S. Opioid use for noncancer pain and risk of fracture in adults: a nested case-control study using the general practice research database. Am J Epidemiol. 2013;178:559–569. doi: 10.1093/aje/kwt013. [DOI] [PubMed] [Google Scholar]
- e1.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- e2.Atkinson TJ, Schatman ME, Fudin J. The damage done by the war on opioids: the pendulum has swung too far. J Pain Res. 2014;12(7):265–268. doi: 10.2147/JPR.S65581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e3.Müller-Schwefe GHH. European survey of chronic pain patients: results for Germany. Curr Med Res Opin. 2011;27:2099–2106. doi: 10.1185/03007995.2011.621935. [DOI] [PubMed] [Google Scholar]
- e4.Müller-Schwefe GHH. Die Scheiterhaufen brennen wieder. Schmerztherapie. 2011;27:2–3. [Google Scholar]
- e5.Überall M. LONTS und die Macht der Zahlen. Schmerztherapie. 2010;26:8–11. [Google Scholar]
- e6.The Nordic Cochrane Centre, The Cochrane Collaboration. Copenhagen: Version 5.2; 2012. Review Manager (RevMan) (Computer program) [Google Scholar]
- e7.Cohen J. Statistical power analysis for the behavoral sciences. Hillsdale: Lawrence Erlbaum Associates. 1988 [Google Scholar]
- e8.Fayers PM, Hays RD. Don‘t middle your MIDs: regression to the mean shrinks estimates of minimally important differences. Qual Life Res. 2014;23:1–4. doi: 10.1007/s11136-013-0443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e9.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- e10.Oxford Centre for Evidence-based Medicine - Levels of Evidence (March 2009) www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ (last accessed on 18 March 2010)
- e11.Häuser W, Klose P, Welsch P, Petzke F, Nothacker M, Kopp I. Methodenreport der aktualisierten Leitlinie „Langzeitanwendung von Opioiden bei nicht tumorbedingten Schmerzen - LONTS”. Schmerz. 2014 doi: 10.1007/s00482-014-1462-y. DOI 10.1007/s00482-014-1462-y (last accessed on 4 September 2014) [DOI] [PubMed] [Google Scholar]
- e12.Härter M, Klesse C, Bermejo I, et al. Development of national guidelines for depression. Bundesgesundhbl Gesundheitsforsch Gesundheitsschutz. 2008;51:451–457. doi: 10.1007/s00103-008-0514-9. [DOI] [PubMed] [Google Scholar]
- e13.Hoffmann J. Methodische Basis für die Entwicklung der Konsensusempfehlungen. Z Gastroenterol. 2004;42:984–987. doi: 10.1055/s-2004-813496. [DOI] [PubMed] [Google Scholar]
- e14.Breivik H, Ljosaa TM, Stengaard-Pedersen K. A 6-months, randomised, placebo-controlled evaluation of efficacy and tolerability of a low-dose 7-day buprenorphine transdermal patch in osteoarthritis patients naive to potent opioids. Scand J Pain. 2010;1:122–141. doi: 10.1016/j.sjpain.2010.05.035. [DOI] [PubMed] [Google Scholar]
- e15.Allan L, Richarz U, Simpson K, Slappendel R. Transdermal fentanyl versus sustained release oral morphine in strong-opioid naïve patients with chronic low back pain. Spine. 2005;30:2484–2490. doi: 10.1097/01.brs.0000186860.23078.a8. [DOI] [PubMed] [Google Scholar]
- e16.Wild JE, Grond S, Kuperwasser B, et al. Long-term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Pract. 2010;10:416–427. doi: 10.1111/j.1533-2500.2010.00397.x. [DOI] [PubMed] [Google Scholar]
- e17.Brennan MJ. The effect of opioid therapy on endocrine function. Am J Med. 2013;126:12–18. doi: 10.1016/j.amjmed.2012.12.001. [DOI] [PubMed] [Google Scholar]
- e18.De Maddalena C, Bellini M, Berra M, Meriggiola MC, Aloisi AM. Opioid-induced hypogonadism: why and how to treat it. Pain Physician. 2012;15:111–118. [PubMed] [Google Scholar]
- e19.Häuser W, Bock F, Engeser P, et al. Empfehlungen der aktualisierten Leitlinie „Langzeitanwendung von Opioiden bei nicht tumorbedingten Schmerzen - LONTS”. Schmerz. 2014 doi: 10.1007/s00482-014-1463-x. DOI 10.1007/s00482-014-1462-y (last accessed on 2 September 2014) [DOI] [PubMed] [Google Scholar]