Abstract
Purpose.
Canonical Wnt signaling has emerged as a critical regulator of aqueous outflow facility and intraocular pressure (IOP). In this study, we examine the role of canonical Wnt signaling on extracellular matrix (ECM) expression in the trabecular meshwork (TM) and explore the molecular mechanisms involved.
Methods.
β-catenin localization in human TM tissue was examined using immunofluorescent staining. Primary human TM cells were incubated with lithium chloride (LiCl) and the effect on active β-catenin expression was assessed by immunoblot. Adenovirus expressing a dominant-negative TCF4 mutant that lacks a β-catenin binding domain was used. Changes in the levels of the microRNA-29 (miR-29) family and ECM proteins were determined by real-time quantitative PCR and immunoblot analysis, respectively.
Results.
β-catenin was expressed throughout the TM, with localization primarily to the plasma membrane. Incubation of TM cells with lithium chloride increased the expression of active β-catenin. Lithium chloride treatment upregulated miR-29b expression, and suppressed the levels of various ECM proteins under both basal and TGF-β2 stimulatory conditions. Infection of TM cells with a dominant-negative TCF4 mutant induced ECM levels without a significant change in the expression of the miR-29 family.
Conclusions.
Collectively, our data identify the canonical Wnt signaling pathway as an important modulator of ECM expression in the TM and provide a mechanistic framework for its regulation of outflow facility and IOP.
Keywords: Wnt signaling, β-catenin, TCF4, miR-29, extracellular matrix, trabecular meshwork, glaucoma, TGF-β2, SPARC
Collectively, our data identify the canonical Wnt signaling pathway as an important modulator of extracellular matrix expression in human trabecular meshwork and provide a mechanistic framework for its regulation of outflow facility and intraocular pressure.
Introduction
Glaucoma is a leading cause of irreversible blindness.1,2 Elevated intraocular pressure (IOP) in patients with primary open-angle glaucoma (POAG) is caused by impaired aqueous humor drainage and leads to a progressive optic nerve damage that results in visual field loss.3,4 Lowering IOP is currently the only rigorously proven treatment for POAG.5,6 Thus, further insight into the molecular mechanisms governing IOP regulation is critical for the identification and development of novel therapeutic agents.
The majority (80%–90%) of aqueous humor outflow occurs through the trabecular meshwork (TM), with the remaining 10%–20% going through the ciliary body face.7 Regulation of aqueous outflow is thought to take place at the juxtacanalicular (JCT) region, an amorphous layer comprised of TM and inner wall Schlemm's canal (SC) endothelial cells and their surrounding extracellular matrix (ECM).8 The extracellular matrix in this region undergoes continuous turnover, with alterations in the equilibrium between ECM synthesis and breakdown shown to influence outflow facility.9–14
Matricellular proteins are nonstructural secreted glycoproteins that mediate cellular control over their surrounding ECM. The matricellular family is comprised of SPARC (secreted protein acidic and rich in cysteine), thrombospondins (TSP)-1 and -2, tenascins-C and –X, hevin, and osteopontin.15 Matricellular proteins have been associated with processes, including tissue fibrosis and aberrant tissue remodeling, that perturb ECM homeostasis and may have a role in IOP regulation and glaucoma pathogenesis. We have previously identified SPARC, TSP-1, and TSP-2 as important modulators of IOP, aqueous outflow facility, and segmental outflow in mice.16–18 We have further shown that SPARC overexpression elevates IOP in perfused cadaveric human anterior segments, with a corresponding qualitative increase seen in the JCT ECM.19
Canonical Wnt signaling is a critical regulator of outflow facility and IOP. Secreted frizzled-related protein 1 (sFRP-1) decreases outflow facility and increases IOP by antagonizing Wnt signaling.20 Levels of sFRP-1 are elevated in glaucomatous TM cells.20 The effects of sFRP-1 on outflow facility and IOP are mediated by inhibition of the canonical Wnt/β-catenin signaling pathway, with no changes observed in actin cytoskeletal organization (noncanonical pathway). The importance of canonical Wnt signaling in the regulation of IOP has been further demonstrated using a specific Wnt/β-catenin pathway inhibitor, Dickkopf-related protein 1 (DKK1).21 The molecular mechanisms underlying the effect of canonical Wnt signaling on outflow facility and IOP remain largely unknown.
In nonocular tissues, activation of canonical Wnt signaling regulates matricellular protein production.22,23 Additionally, treatment with lithium chloride (LiCl), which stabilizes and activates β-catenin, has been demonstrated to upregulate miR-29 family expression in nonocular cells.22,24 MicroRNAs are small (~22 nucleotides), single-stranded noncoding RNAs that modulate the posttranscriptional expression of genes.25 We and others have identified the miR-29 family as an important regulator of matricellular and ECM synthesis in the TM.26–28 We hypothesized that activation of Wnt/β-catenin signaling in the TM may suppress ECM expression via the induction of the miR-29 family.
Materials and Methods
Trabecular Meshwork Cell Culture
Primary human trabecular meshwork (TM) cells were isolated and cultured in accordance with the tenets of the Declaration of Helsinki as previously described.29 Independent human TM cell strains were generated from donors ranging in age from 12 to 75 years and no known history of ocular disease. Cell cultures, unless otherwise stated, were maintained in Dulbecco's Modified Eagle's Medium (Invitrogen-Gibco, Grand Island, NY, USA) supplemented with 20% fetal bovine serum (FBS), 1% L-glutamine (2 mM), and 0.1% gentamicin (10 μg/mL) at 37°C in 10% CO2 atmosphere. Trabecular meshwork cells from passage 4 to 5 were used for all experiments.
Lithium Chloride and SB216763 Experiments
Primary human TM cells were plated and grown to 100% confluency. Cells were then washed with PBS and incubated for 19 hours in serum-free media. Cells were subsequently cultured for 24 hours in serum-free media containing either 20 mM lithium chloride (Sigma-Aldrich Corp., St. Louis, MO, USA) or sodium chloride (NaCl) control. For experiments with SB216763, cells were cultured for 24 hours in serum-free media containing either 10 μM SB216763 or DMSO vehicle (EMD Millipore Corp., Milford, MA, USA).
For stimulatory experiments with TGF-β2, confluent TM cells were washed with PBS and incubated for 19 hours in serum-free media. Cells were then cultured for 1 hour in serum-free media containing either 20 mM LiCl or NaCl control. Trabecular meshwork cells were subsequently incubated for 24 hours with 2.5 ng/mL activated, recombinant human TGF-β2 (R&D Systems, Minneapolis, MN, USA) or 4 mM HCl solution containing 0.1% human serum albumin as the vehicle.
For experiments assessing activated β-catenin levels, 100% confluent TM cells were washed with PBS and incubated for 19 hours in serum-free media. Cells were then cultured for 4 hours in serum-free media containing either 20 mM LiCl or NaCl control and lysed for immunoblot analysis.
Adenovirus-Mediated Infection
Primary human TM cells at 95% to 100% confluency were infected in 2% FBS media containing either Ad-TCF4 (HUGO gene symbol: TCF7L2) dominant negative virus (multiplicity of infection [MOI] = 25; Vector Biolabs, Philadelphia, PA, USA) or Ad-null control virus (MOI = 25), both of which use human adenovirus type 5 vectors with E1/E3 deletion. After 18 hours, an equal volume of 10% FBS media was added to each well and cells were incubated for an additional 24 hours. Cells were then washed with PBS and incubated for 24 hours in serum-free media.
MicroRNA Isolation and Real-Time TaqMan PCR
Following the completion of the respective experiments, cells were lysed and microRNA isolated using an miRNA isolation kit (PureLink; Invitrogen). We reverse transcribed RNA using a microRNA reverse transcription kit (TaqMan; Applied Biosystems, Foster City, CA, USA). Quantitative real-time PCR was performed, as instructed by the manufacturer, using microRNA assays for mature miR-29a/b/c and internal control RNU6B (Applied Biosystems). Levels of the miR-29 family were normalized to internal control RNU6B using the formula 2−ΔCt. Relative fold changes in miR expression between control and treatment groups were calculated using the 2−ΔΔCt method.
Immunoblot Analysis
For detection of ECM, conditioned media from TM cell cultures was harvested and centrifuged at 5000 rpm for 10 minutes at 4°C. The supernatant was concentrated (Amicon Ultra-4 Filter Unit, 10 kDa; Millipore Corp.), and protein content quantified (DC Protein Assay; Bio-Rad, Hercules, CA, USA). For detection of active β-catenin levels, TM cells were lysed on ice with cold ×1 RIPA buffer containing 0.5% aprotinin, 0.1% EDTA, 1% EGTA, 0.5% phenylmethanesulfonyl fluoride, and 0.01% leupeptin. Samples were subsequently centrifuged at 18,000g for 15 minutes at 4°C and supernatant protein content isolated and quantified. For all experiments, equal amounts of protein were treated with ×6 reducing buffer and boiled for 5 minutes. Electrophoreses in 10% SDS-polyacrylamide gels was performed on all samples, and proteins were transferred to nitrocellulose membranes (0.45-μm pore size; Invitrogen). Membranes were blocked for 1 hour at room temperature in a 1:1 mixture of 1xTBS-T (20 mM Tris-HCl [pH 7.6], 137 mM NaCl, 0.1% Tween-20) and blocking buffer (Rockland, Inc., Gilbertsville, PA, USA), then incubated overnight at 4°C with the indicated primary antibody at 1:1000 for active β-catenin (Millipore Corp.); 1:10,000 for SPARC (Haematologic Technologies Inc., Essex Junction, VT, USA); 1:1000 for thrombospondin-1 (R&D Systems); 1:1000 for collagen I (Rockland, Inc.); 1:1000 for collagen IV (Rockland, Inc.); and 1:200 for laminin (Sigma-Aldrich Corp.). After incubation with the primary antibody, the membranes were washed three times with 1xTBS-T and incubated at room temperature for 1 hour with dye-conjugated affinity purified 680 anti-mouse or 800 anti-rabbit IgG antibodies (IRDye; 1:10,000 dilution; Rockland, Inc.). Membranes were then washed three times with 1xTBS-T, scanned, and integrated band intensities calculated using an infrared imaging system (Odyssey; LI-COR, Inc., Lincoln, NE, USA).
Immunofluorescence
Human donor eyes (aged 21, 65, and 70 years) were immersion-fixed in 10% neutral buffered formalin, dehydrated in sequential ethanol solutions (75%, 85%, 95%, 100%), then embedded in paraffin. Sections (6 μm) were mounted on poly-L-lysine–coated glass slides and baked for 2 hours at 60°C. Slides were then deparaffinized in xylene, sequentially rehydrated in ethanol solutions, and washed three times for 10 minutes in phosphate-buffered saline containing 0.1% Tween-20 (PBS-T). After 1 hour of incubation in 10% goat serum, tissues were permeabilized for 5 minutes in 0.2% Triton ×-100 in 1× PBS and washed three times with PBS-T. Prepared sections were incubated overnight at 4°C with either primary mouse β-catenin monoclonal antibody diluted 1:200 in PBS (#2677 Cell Signaling, Inc., Danvers, MA, USA) or in PBS alone. Slides were washed three times in PBS-T and then incubated in 1:200 goat anti-mouse AlexFluor 594 secondary immunoglobulin G (IgG; Invitrogen), followed by three additional washes with PBS-T. Nuclei were stained using DAPI antifade reagent (SlowFade Gold; Invitrogen). Labeled tissues were imaged and analyzed by fluorescent light microscopy (Zeiss Observer 3.1; Carl Zeiss MicroImaging GmbH, Göttingen, Germany).
Statistics
Statistical analyses were performed using statistical software (GraphPad Prism 6; GraphPad Software, Inc., La Jolla, CA, USA). Comparisons between two groups were made using a two-tailed Student's t-test. Comparisons between multiple groups were made using one-way ANOVA followed by Tukey's HSD post hoc test. Differences were considered significant at P < 0.05.
Results
Immunolocalization of β-catenin in Human Trabecular Meshwork
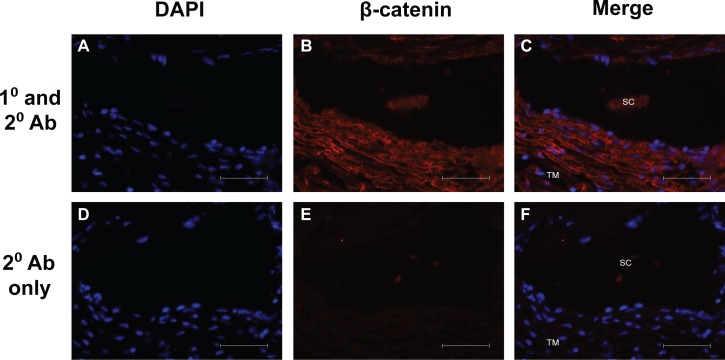
To determine the localization of β-catenin in the human trabecular meshwork, immunofluorescent staining of β-catenin in normal human TM tissue was performed. β-catenin was expressed throughout the TM, and it appears to localize to the plasma membrane (Fig. 1).
Figure 1.
Immunolocalization of β-catenin in human trabecular meshwork from a 21-year-old donor. Representative images demonstrate β-catenin expression throughout the TM. β-catenin appears to localize to the plasma membrane. Scale bars: 50 μm.
Lithium Chloride Induces Nuclear Translocation of β-Catenin
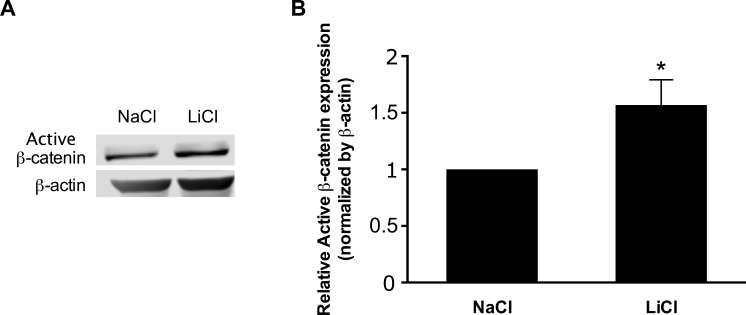
Lithium chloride (LiCl) treatment mimics activation of canonical Wnt signaling by inhibiting glycogen synthase kinase 3β (GSK3β), which results in the stabilization and nuclear translocation of β-catenin.22 To confirm the activation of β-catenin by lithium chloride, primary human TM cells were incubated with LiCl or NaCl control and protein levels of active β-catenin were assessed. Treatment of TM cells with LiCl increased the expression of active β-catenin compared with control cells (Fig. 2).
Figure 2.
Lithium chloride increases expression of active β-catenin protein in primary human TM cells. (A) Representative immunoblot and (B) densitometric analysis of active β-catenin protein expression from cell lysates of primary human TM cells incubated for 4 hours with either LiCl or NaCl vehicle. Data are expressed as the mean ± SEM (*P < 0.05 versus corresponding control group; n = 4, where “n” refers to the number of independent experiments performed using “n” different human TM cell strains).
Lithium Chloride Suppresses ECM Protein Levels
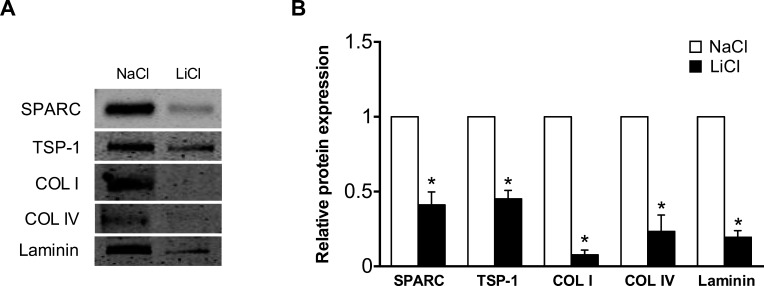
Recent studies have identified the canonical Wnt signaling pathway as an important regulator of outflow facility and IOP.20,21 Since alterations in ECM levels influence aqueous outflow, we examined the effect of Wnt signaling activation on ECM expression. Incubation of TM cells with LiCl resulted in a significant reduction in the levels of various matricellular and ECM proteins, including SPARC, TSP-1, collagen I, collagen IV, and laminin (Fig. 3). Similarly, treatment of cells with SB216763, a selective inhibitor of GSK3 that has previously been shown to activate β-catenin signaling in TM cells, suppressed expression of TSP-1, collagen I, collagen IV, and laminin (Supplementary Fig. S1).30
Figure 3.
Lithium chloride treatment suppresses ECM protein levels. (A) Representative immunoblot and (B) densitometric analysis of ECM proteins from conditioned media of primary human TM cells incubated for 24 hours with either LiCl or NaCl control. Data are expressed as the mean ± SEM (*P < 0.001 versus corresponding control group; n = 4, where “n” refers to the number of independent experiments performed using “n” different human TM cell strains).
MiR-29b Expression Is Upregulated by Lithium Chloride
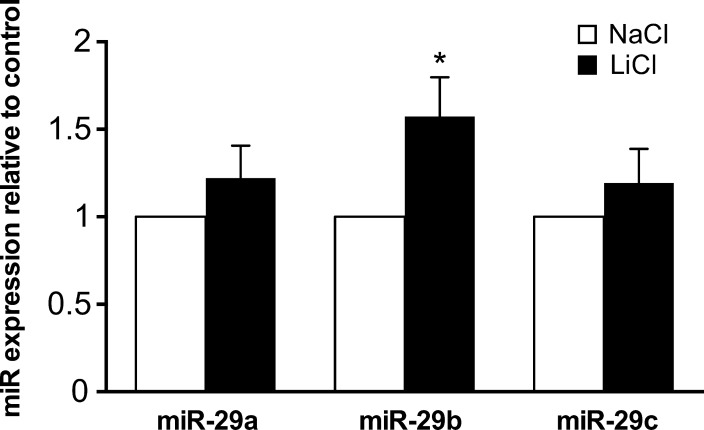
The human microRNA-29 family consists of three paralogs: miR-29a, miR-29b, and miR-29c.31 To examine whether canonical Wnt signaling influences miR-29 family expression in the TM, primary human TM cells were incubated for 24 hours with lithium chloride. Lithium chloride stimulation resulted in a significant upregulation of miR-29b levels, with no significant change seen in miR-29a or miR-29c expression (Fig. 4).
Figure 4.
Lithium chloride upregulates expression of miR-29b. The levels of mature miR-29a, miR-29b, and miR-29c were quantified by real-time PCR in primary human TM cells cultured for 24 hours with either LiCl or NaCl control. Data are expressed as the mean ± SEM (*P < 0.05 versus corresponding control group; n = 9, where “n” refers to the number of independent experiments performed using “n” different human TM cell strains).
Lithium Chloride Suppresses ECM Protein Levels Under TGF-β2 Stimulatory Conditions
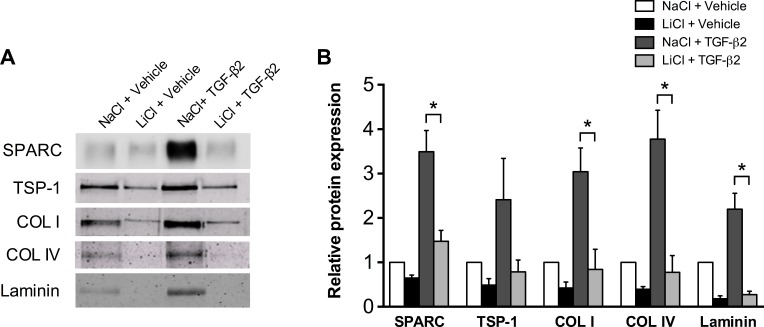
Cytokine levels of TGF-β2 are significantly increased in the aqueous humor of patients with POAG compared with age-matched controls.32,33 Multiple studies have demonstrated an important role for TGF-β2 in the deposition of extracellular matrix in the TM.34–36 We next investigated if activation of canonical Wnt signaling could suppress TGF-β2 mediated induction of ECM proteins. Pretreatment of TM cells with lithium chloride inhibited the upregulation of various ECM proteins by TGF-β2, including SPARC, collagen I, collagen IV, and laminin (Fig. 5).
Figure 5.
Lithium chloride suppresses ECM protein levels under TGF-β2 stimulatory conditions. (A) Representative immunoblot and (B) densitometric analysis of ECM proteins from conditioned media of primary human TM cells pretreated for 1 hour with LiCl or NaCl control followed by 24-hour stimulation with 2.5 ng/mL activated, recombinant human TGF-β2 or HCl vehicle. Data are expressed as the mean ± SEM (*P < 0.05; ANOVA, Tukey's HSD post hoc test; n = 6, where “n” refers to the number of independent experiments performed using “n” different human TM cell strains).
Dominant-Negative TCF4 Upregulates ECM Protein Levels
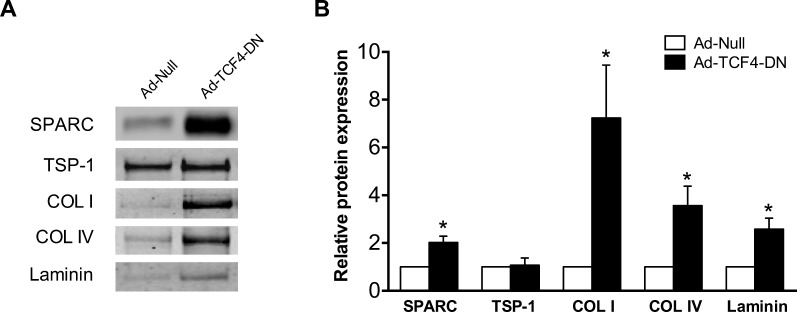
T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors function as important mediators of Wnt/β-catenin signaling and gene expression.37 The family of TCF consists of four genes in mammals: LEF1, TCF3, TCF4, and TCF7.38 To begin to assess the role of this family in the regulation of ECM levels, TM cells were infected with control adenovirus or virus expressing a dominant-negative TCF4 mutant (TCF4-DN) that lacks a β-catenin binding domain. Dominant-negative TCF4 mutant resulted in a significant upregulation of multiple ECM proteins, including SPARC, collagen I, collagen IV, and laminin. Levels of TSP1 were not significantly changed (Fig. 6).
Figure 6.
Dominant-negative TCF4 increases ECM protein levels. (A) Representative immunoblot and (B) densitometric analysis of ECM proteins from conditioned media of TM cells infected with TCF4-DN or null-control virus. Data are expressed as the mean ± SEM. *P < 0.05 versus corresponding control group; n = 4, where “n” refers to the number of independent experiments performed using “n” different human TM cell strains).
MiR-29 Family Expression Is Not Affected by Dominant-Negative TCF4
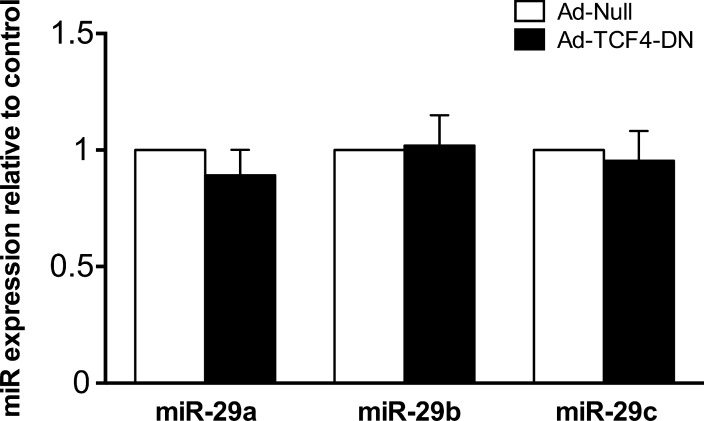
To further investigate the signaling pathway mediating the regulation of ECM protein levels by TCF4, we assessed the effect of dominant-negative TCF4 on miR-29 family expression. Two binding sites of TCF/LEF are located within the proximal promoter of miR-29b1/a and have been shown to regulate promoter activity.24,31 The levels of mature miR-29 were not significantly affected by TCF4-DN when compared with cells expressing control adenovirus (Fig. 7).
Figure 7.
Dominant-negative TCF4 does not affect miR-29 family expression. Primary human TM cells were infected with either TCF4-DN or null-control virus. Cells were lysed and relative levels of mature miR-29 family expression were measured by quantitative real-time PCR. Data are expressed as the mean ± SEM (n = 7, where “n” refers to the number of independent experiments performed using “n” different human TM cell strains).
Discussion
The Wnt/β-catenin signaling pathway is a critical mediator of aqueous outflow and IOP.20,21 Taken together, the data presented here identify the canonical Wnt signaling pathway as an important regulator of ECM expression in the TM and provide mechanistic insight into its effects on outflow facility and IOP (see working model; Fig. 8).
Figure 8.
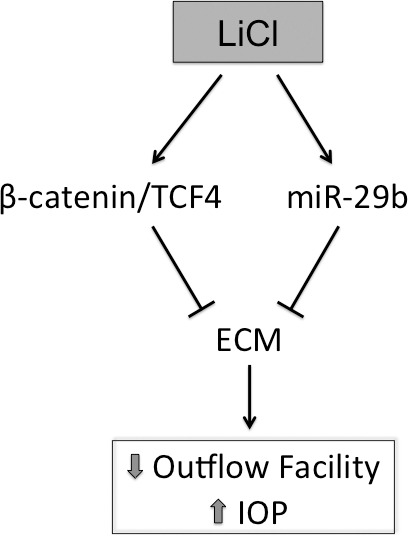
Working model for the suppression of ECM levels by lithium chloride.
Immunofluorescent analysis of β-catenin expression in normal human TM tissue revealed expression throughout the TM, and β-catenin appears to localize to the cellular membrane. These results are consistent with those previously reported by Stamer and colleagues,39 who demonstrated β-catenin localization primarily at the cellular membrane in the TM and inner wall of Schlemm's canal in perfused, enucleated human eyes. Wang et al.20 have previously reported that 50% to 60% of β-catenin in human TM cells is associated with the plasma membrane. The localization of β-catenin to the cellular membrane suggests that under basal conditions, β-catenin's major function may involve cadherin-based cellular adhesion and cell-cell communication. While dysregulated expression of the Wnt antagonist sFRP-1 has been reported in glaucomatous TM cells,20 alterations in β-catenin expression and/or localization in POAG remain unknown.
Using the GSK3β inhibitor, lithium chloride, we activated β-catenin expression in primary human TM cells. Analysis of various matricellular and ECM proteins from TM-conditioned media revealed significant reductions in their levels under both basal and TGF-β2 conditions, suggesting that activation of canonical Wnt signaling may function as an important regulator of ECM expression. Suppression of ECM proteins was also seen using the selective GSK3 inhibitor SB216763. Of note, SPARC did not show a significant change compared with control-treated cells. Further, the mean decrease in ECM proteins with SB216763 treatment was less than that observed in LiCl-treated cells, suggesting that LiCl may also regulate ECM expression through additional pathways independent of GSK3. Prior work has demonstrated that Wnt pathway ligands do not affect actin cytoskeletal organization in the TM, suggesting that regulation of ECM levels may serve as an important mechanism for the observed changes seen in outflow facility and IOP with Wnt signaling modulation.21
Recent studies by our laboratory and others have identified the miR-29 family as an important regulatory node for ECM synthesis.26–28 Trabecular meshwork cells incubated with LiCl displayed a significant induction in miR-29b expression, suggesting that suppression of ECM protein levels by LiCl may be mediated in part via the upregulation of miR-29b. Together, modulation of miR-29b levels by various stimuli including oxidative stress,28 TGF-β2,26,27 and lithium chloride suggests a central role for miR-29b in the coordinated regulation of ECM synthesis in the TM. Luna et al.40 have demonstrated successful anterior chamber microRNA delivery in rat eyes. Further investigation into the effects of miR-29b on ECM synthesis and IOP using in vivo models is necessary.
The T-cell factor family of transcription factors, consisting of LEF1, TCF3, TCF4, and TCF7, function as important mediators of Wnt/β-catenin signaling. Infection of TM cells with a dominant-negative TCF4 mutant, which lacks a β-catenin binding domain, resulted in a significant upregulation of various matricellular and ECM proteins, further supporting the importance of canonical Wnt signaling in the regulation of ECM homeostasis. Trabecular meshwork cells infected with Ad-TCF4-DN did not demonstrate any significant changes in miR-29 family expression at 66 hours. While it is possible that miR-29 may not be necessary for TCF4-mediated changes in ECM levels, early time point changes in miR-29 expression cannot be excluded and are difficult to assess given the constraints of adenoviral tissue culture experimentation. Prior work in human TM cells identified expression of TCF3, TCF4, and TCF7 transcripts.20,41 Functional redundancy has been reported between TCF/LEFs, however, significant heterogeneity via alternative promoters and alternative splicing has also enabled unique gene targeting and may help further explain observed differences in the modulation of miR-29 as well as the matricellular protein TSP-1, which may be regulated by other TCF/LEFs, independently of TCF4.37 Characterization of the specific TCF/LEF isoforms present in glaucomatous TM cells is necessary, as changes in TCF/LEF isoform expression have been associated with disease development.37
We have demonstrated that SPARC overexpression upregulates ECM levels in the juxtacanicular region of perfused human anterior segments, with a corresponding increase seen in IOP.19 Interestingly, previous reports in nonocular tissues have shown that SPARC can induce canonical Wnt signaling via the activation of integrin-linked kinase, which inhibits GSK3β and allows for stabilization and nuclear translocation of β-catenin signaling.42,43 As we have demonstrated, canonical Wnt signaling suppresses matricellular and ECM protein levels secreted by TM cells. Therefore, the activation of canonical Wnt signaling by SPARC could serve as a feedback loop to limit ECM levels in the TM. Further investigation is necessary to determine whether such regulatory feedback is present in TM cells.
Collectively, the results presented here suggest that the canonical Wnt signaling pathway may function as a critical regulator of ECM levels in the TM, and lend further insight into the complex molecular mechanisms mediating its effect on outflow facility and IOP. The dysregulation of canonical Wnt signaling in glaucomatous TM cells, and altered cellular localization of β-catenin by TGF-β2, suggest that modulation of this pathway may serve as an important therapeutic avenue for POAG.20,44 Interestingly, statins (HMG-CoA reductase inhibitors) have been shown to activate canonical Wnt signaling via the inhibition of GSK3β.45–47 Statin treatment increases aqueous outflow facility in porcine anterior segments48 and has been associated with a significant reduction in the risk of open-angle glaucoma in specific patient populations.49–51 Systematic screening of statins and other FDA-approved drugs for activation of canonical Wnt signaling is warranted and may further accelerate the identification of novel compounds for the treatment of glaucoma.
Acknowledgments
Supported by Fight for Sight Summer Student Fellowship (GV); Howard Hughes Medical Institute Research Training Fellowship for Medical Students (AC); National Eye Institute EY 019654-01 (DJR); and EY 014104 (MEEI Vision-Core Grant).
Disclosure: G. Villarreal Jr, None; A. Chatterjee, None; S.S. Oh, None; D.-J. Oh, None; M.H. Kang, None; D.J. Rhee, Alcon (F, C), Aquesys (F, C), Merck (F, C), Aerie (C), Allergan (C), Johnson & Johnson (C), Santen (C)
References
- 1. Allingham RR, Liu Y, Rhee DJ. The genetics of primary open-angle glaucoma: A review. Exp Eye Res. 2009; 88: 837–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009; 360: 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Larsson LI, Rettig ES, Brubaker RF. Aqueous flow in open-angle glaucoma. Arch Ophthalmol. 1995; 113: 283–286. [DOI] [PubMed] [Google Scholar]
- 4. Nemesure B, Honkanen R, Hennis A, Wu SY, Leske MC. Barbados Eye Studies Group. Incident open-angle glaucoma and intraocular pressure. Ophthalmology. 2007; 114: 1810–1815. [DOI] [PubMed] [Google Scholar]
- 5. The advanced glaucoma intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS investigators. Am J Ophthalmol. 2000; 130: 429–440. [DOI] [PubMed] [Google Scholar]
- 6. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: Results from the early manifest glaucoma trial. Arch Ophthalmol. 2002; 120: 1268–1279. [DOI] [PubMed] [Google Scholar]
- 7. Pederson JE, Gaasterland DE, MacLellan HM. Uveoscleral aqueous outflow in the rhesus monkey: importance of uveal reabsorption. Invest Ophthalmol Vis Sci. 1977; 16: 1008–1007. [PubMed] [Google Scholar]
- 8. Bill A. Editorial: the drainage of aqueous humor. Invest Ophthalmol. 1975; 14: 1–3. [PubMed] [Google Scholar]
- 9. Knepper PA, Farbman AI, Telser AG. Exogenous hyaluronidases and degradation of hyaluronic acid in the rabbit eye. Invest Ophthalmol Vis Sci. 1984; 25: 286–293. [PubMed] [Google Scholar]
- 10. Barany EH, Scotchbrook S. Influence of testicular hyaluronidase on the resistance to flow through the angle of the anterior chamber. Acta Physiol Scand. 1954; 30: 240–248. [DOI] [PubMed] [Google Scholar]
- 11. Bradley JM, Vranka J, Colvis CM, et al. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998; 39: 2649–2658. [PubMed] [Google Scholar]
- 12. Keller KE, Aga M, Bradley JM, Kelley MJ, Acott TS. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2009; 88: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fuchshofer R, Tamm ER. Modulation of extracellular matrix turnover in the trabecular meshwork. Exp Eye Res. 2009; 88: 683–688. [DOI] [PubMed] [Google Scholar]
- 14. Liton PB, Gonzalez P, Epstein DL. The role of proteolytic cellular systems in trabecular meshwork homeostasis. Exp Eye Res. 2009; 88: 724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rhee DJ, Haddadin RI, Kang MH, Oh DJ. Matricellular proteins in the trabecular meshwork. Exp Eye Res. 2009; 88: 694–703. [DOI] [PubMed] [Google Scholar]
- 16. Haddadin RI, Oh DJ, Kang MH, et al. SPARC-null mice exhibit lower intraocular pressures. Invest Ophthalmol Vis Sci. 2009; 50: 3771–3777. [DOI] [PubMed] [Google Scholar]
- 17. Haddadin RI, Oh DJ, Kang MH, et al. Thrombospondin-1 (TSP1)-null and TSP2-null mice exhibit lower intraocular pressures. Invest Ophthalmol Vis Sci. 2012; 53: 6708–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Swaminathan SS, Oh DJ, Kang MH, et al. Secreted protein acidic and rich in cysteine (SPARC)-null mice exhibit more uniform outflow. Invest Ophthalmol Vis Sci. 2013; 54: 2035–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oh DJ, Kang MH, Ooi YH, Choi KR, Sage EH, Rhee DJ. Overexpression of SPARC in human trabecular meshwork increases intraocular pressure and alters extracellular matrix. Invest Ophthalmol Vis Sci. 2013; 54: 3309–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang WH, McNatt LG, Pang IH, et al. Increased expression of the Wnt antagonist sFRP-1 in glaucoma elevates intraocular pressure. J Clin Invest. 2008; 118: 1056–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mao W, Millar JC, Wang WH, et al. Existence of the canonical Wnt signaling pathway in the human trabecular meshwork. Invest Ophthalmol Vis Sci. 2012; 53: 7043–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kapinas K, Kessler CB, Delany AM. MiR-29 suppression of osteonectin in osteoblasts: Regulation during differentiation and by canonical Wnt signaling. J Cell Biochem. 2009; 108: 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Scheller EL, Chang J, Wang CY. Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J Dent Res. 2008; 87: 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kapinas K, Kessler C, Ricks T, Gronowicz G, Delany AM. MiR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop. J Biol Chem. 2010; 285: 25221–25231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: MicroRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009; 11: 228–234. [DOI] [PubMed] [Google Scholar]
- 26. Villarreal G Jr, Oh DJ, Kang MH, Rhee DJ. Coordinated regulation of extracellular matrix synthesis by the microRNA-29 family in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2011; 52: 3391–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luna C, Li G, Qiu J, Epstein DL, Gonzalez P. Cross-talk between miR-29 and transforming growth factor-betas in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011; 52: 3567–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luna C, Li G, Qiu J, Epstein DL, Gonzalez P. Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Mol Vis. 2009; 15: 2488–2497. [PMC free article] [PubMed] [Google Scholar]
- 29. Oh DJ, Martin JL, Williams AJ, Russell P, Birk DE, Rhee DJ. Effect of latanoprost on the expression of matrix metalloproteinases and their tissue inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006; 47: 3887–3895. [DOI] [PubMed] [Google Scholar]
- 30. Shen X, Ying H, Yue BY. Wnt activation by wild type and mutant myocilin in cultured human trabecular meshwork cells. PLoS One. 2012; 7: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kriegel AJ, Liu Y, Fang Y, Ding X, Liang M. The miR-29 family: genomics, cell biology, and relevance to renal and cardiovascular injury. Physiol Genomics. 2012; 44: 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res. 1994; 59: 723–727. [DOI] [PubMed] [Google Scholar]
- 33. Fuchshofer R, Tamm ER. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012; 347: 279–290. [DOI] [PubMed] [Google Scholar]
- 34. Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006; 47: 226–234. [DOI] [PubMed] [Google Scholar]
- 35. Gottanka J, Chan D, Eichhorn M, Lutjen-Drecoll E, Ethier CR. Effects of TGF-beta2 in perfused human eyes. Invest Ophthalmol Vis Sci. 2004; 45: 153–158. [DOI] [PubMed] [Google Scholar]
- 36. Kang MH, Oh DJ, Kang JH, Rhee DJ. Regulation of SPARC by transforming growth factor beta-2 in human trabecular meshwork. Invest Ophthalmol Vis Sci. 2013; 54: 2523–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cadigan KM, Waterman ML. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb Perspect Biol. 2012; 4: 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009; 17: 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stamer WD, Read AT, Sumida GM, Ethier CR. Sphingosine-1-phosphate effects on the inner wall of Schlemm's canal and outflow facility in perfused human eyes. Exp Eye Res. 2009; 89: 980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Luna C, Li G, Huang J, et al. Regulation of trabecular meshwork cell contraction and intraocular pressure by miR-200c. PLoS One. 2012; 7: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shyam R, Shen X, Yue BY, Wentz-Hunter KK. Wnt gene expression in human trabecular meshwork cells. Mol Vis. 2010; 16: 122–129. [PMC free article] [PubMed] [Google Scholar]
- 42. Nie J, Sage EH. SPARC functions as an inhibitor of adipogenesis. J Cell Commun Signal. 2009; 3: 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nie J, Sage EH. SPARC inhibits adipogenesis by its enhancement of beta-catenin signaling. J Biol Chem. 2009; 284: 1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wecker T, Han H, Borner J, Grehn F, Schlunck G. Effects of TGF-beta2 on cadherins and beta-catenin in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2013; 54: 6456–6462. [DOI] [PubMed] [Google Scholar]
- 45. Salins P, Shawesh S, He Y, et al. Lovastatin protects human neurons against Abeta-induced toxicity and causes activation of beta-catenin-TCF/LEF signaling. Neurosci Lett. 2007; 412: 211–216. [DOI] [PubMed] [Google Scholar]
- 46. Bergmann MW, Rechner C, Freund C, Baurand A, El Jamali A, Dietz R. Statins inhibit reoxygenation-induced cardiomyocyte apoptosis: Role for glycogen synthase kinase 3beta and transcription factor beta-catenin. J Mol Cell Cardiol. 2004; 37: 681–690. [DOI] [PubMed] [Google Scholar]
- 47. Lin CL, Cheng H, Tung CW, et al. Simvastatin reverses high glucose-induced apoptosis of mesangial cells via modulation of Wnt signaling pathway. Am J Nephrol. 2008; 28: 290–297. [DOI] [PubMed] [Google Scholar]
- 48. Song J, Deng PF, Stinnett SS, Epstein DL, Rao PV. Effects of cholesterol-lowering statins on the aqueous humor outflow pathway. Invest Ophthalmol Vis Sci. 2005; 46: 2424–2432. [DOI] [PubMed] [Google Scholar]
- 49. Stein JD, Newman-Casey PA, Talwar N, Nan B, Richards JE, Musch DC. The relationship between statin use and open-angle glaucoma. Ophthalmology. 2012; 119: 2074–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marcus MW, Muskens RP, Ramdas WD, et al. Cholesterol-lowering drugs and incident open-angle glaucoma: A population-based cohort study. PLoS One. 2012; 7: . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McGwin G Jr, McNeal S, Owsley C, Girkin C, Epstein D, Lee PP. Statins and other cholesterol-lowering medications and the presence of glaucoma. Arch Ophthalmol. 2004; 122: 822–826. [DOI] [PubMed] [Google Scholar]