Abstract
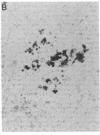
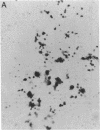
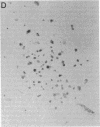
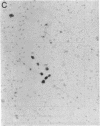
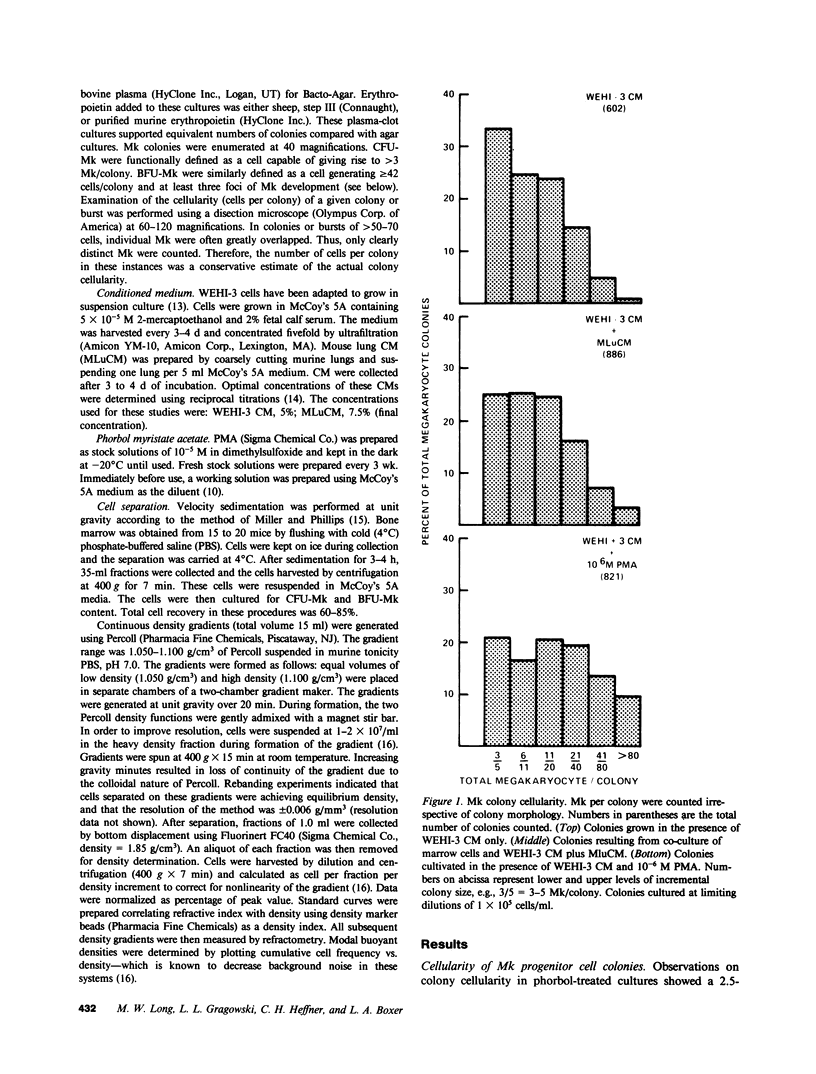
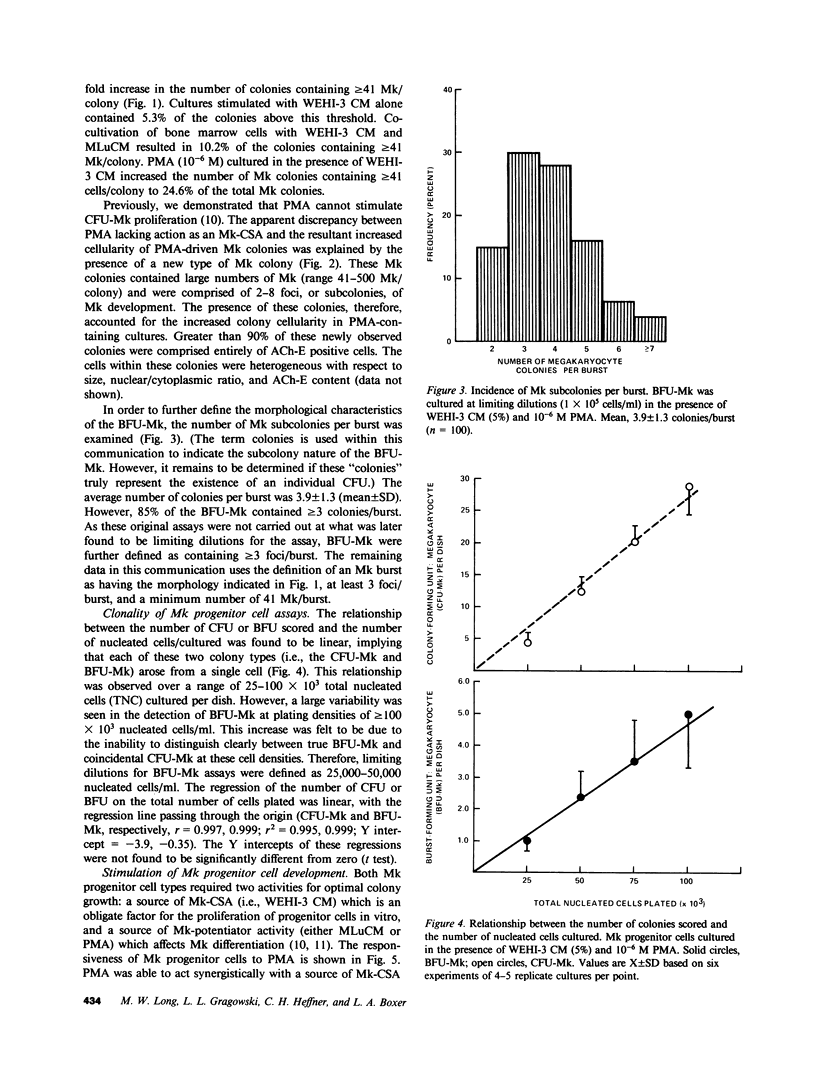
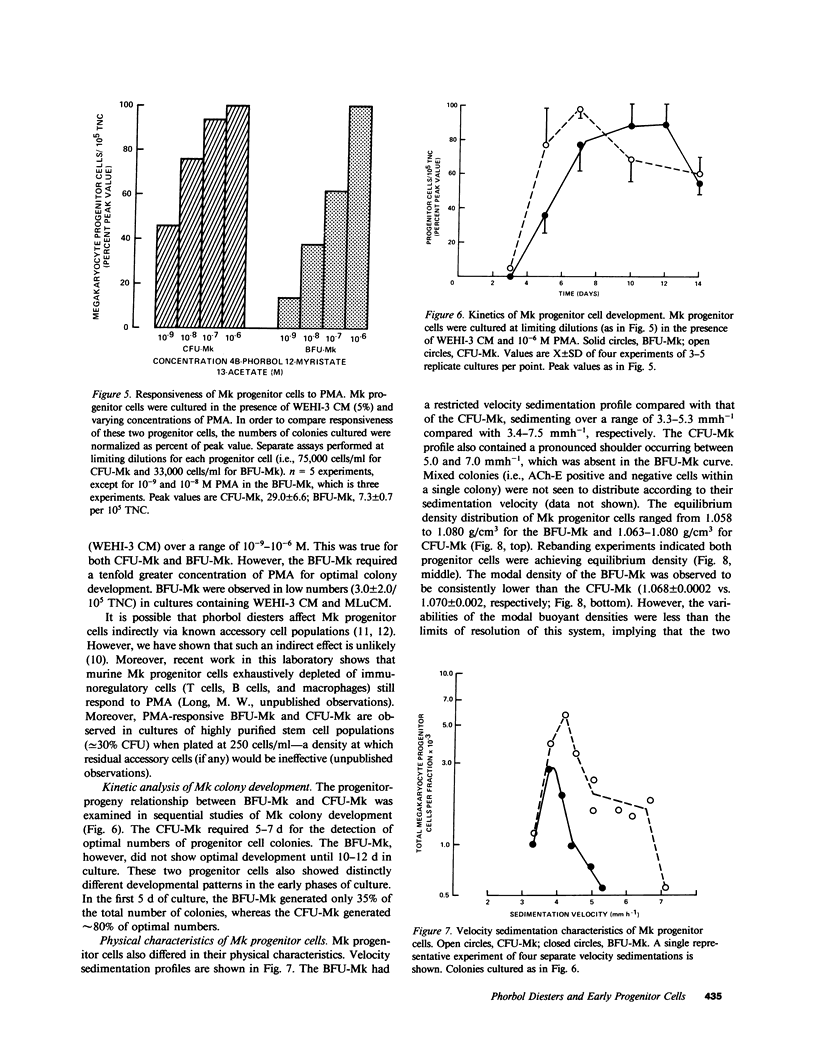
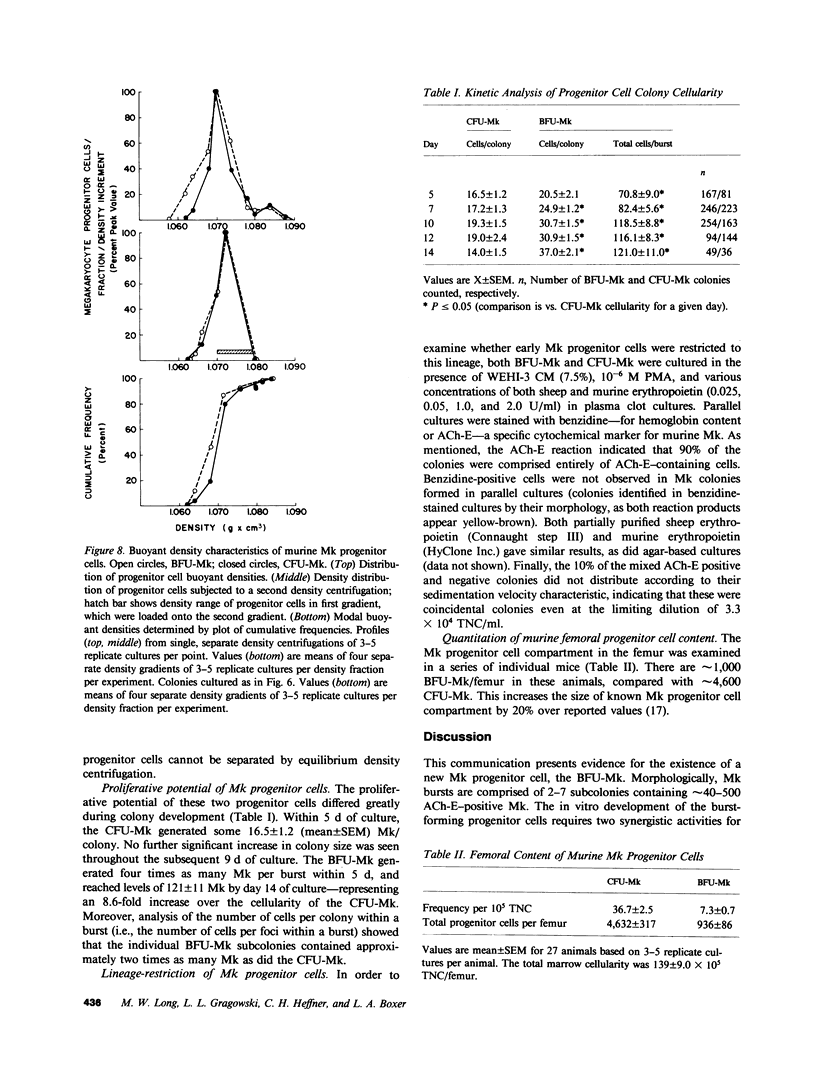
When murine (C57BL/6) bone marrow cells are cultivated with WEHI-3 conditioned media, a source of megakaryocyte-colony-stimulating activity (Mk-CSA), and phorbol myristate acetate (PMA), a previously undetected population of megakaryocyte (Mk) progenitor cells is observed. These new Mk colonies are reminiscent of erythroid bursts, in that they contain large numbers (40-500) of Mk and multiple foci (2-7) of development. These burst-forming units, Mk (BFU-Mk), are defined as having greater than or equal to 42 cells/colony and, at least, three foci of Mk development (colonies grown in soft agar cultures, all studies done at limiting dilutions; colonies detected by acetylcholinesterase [ACh-E] staining). CFU-Mk and BFU-Mk require two activities for optimal growth: Mk-CSA and PMA. However, the BFU-Mk require a tenfold greater concentration of PMA for optimal development (10(-6) vs. 10(-7) M). BFU-Mk detection is linear (over a range of 25-100 X 10(3) cells/ml), with the regression line passing through the origin. Bone marrow frequencies of these two progenitor cells are CFU-Mk, 36.7 +/- 2.5, and BFU-Mk, 7.3 +/- 0.7 per 10(5) total nucleated cells (mean +/- SEM; n = 28). The BFU-Mk have a restricted velocity sedimentation range (3.3-4.5 mmh-1 vs. 3.3-6.8 mmh-1 for CFU-Mk). Modal buoyant densities are 1.068 +/- 0.0002 and 1.070 +/- 0.002 for BFU-Mk and CFU-Mk, respectively. Thus, these cells are found among the smallest and less dense of the Mk progenitors, and are not clumps or clusters of CFU-Mk. Kinetic analysis indicates that CFU-Mk require 5-7 d for optimal growth, whereas BFU-Mk require 10-12 d. Examination of the proliferative potential (cells per colony) shows 19.3 +/- 1.5 cells per colony (n = 246 colonies) for day 10 CFU-Mk, vs. 118 +/- 6.0 for day 10 BFU-Mk (n = 163). Analysis of the cellularity/subcolony within each burst indicates 37.0 +/- 2.1 (n = 146) Mk/colony and 3.9 +/- 0.1 subcolonies/burst (n = 100). Finally, greater than 90% of the BFU-Mk contain only ACh-E positive cells, indicating that these are not mixed colonies. These results indicate that the BFU-Mk, compared with the CFU-Mk, require an increased amount of stimulation in order to differentiate, show delayed in vitro development, and have a higher proliferative potential. These data are consistent with the hypothesis that these cells are early progenitor cells in the Mk lineage that antedate the CFU-Mk.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines P., Bol S., Rosendaal M. Characterization of a developmentally early macrophage progenitor found in normal mouse marrow. Br J Haematol. 1981 May;48(1):147–153. doi: 10.1111/j.1365-2141.1981.00147.x. [DOI] [PubMed] [Google Scholar]
- Bol S., Visser J., van den Engh G. The physical separation of three subpopulations of granulocyte/macrophage progenitor cells from mouse bone marrow. Exp Hematol. 1979 Nov;7(10):541–553. [PubMed] [Google Scholar]
- Bradley T. R., Hodgson G. S. Detection of primitive macrophage progenitor cells in mouse bone marrow. Blood. 1979 Dec;54(6):1446–1450. [PubMed] [Google Scholar]
- Fibach E., Gambari R., Shaw P. A., Maniatis G., Reuben R. C., Sassa S., Rifkind R. A., Marks P. A. Tumor promoter-mediated inhibition of cell differentiation: suppression of the expression of erythroid functions in murine erythroleukemia cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1906–1910. doi: 10.1073/pnas.76.4.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscove N. N., Sieber F. Erythroid progenitors in mouse bone marrow detected by macroscopic colony formation in culture. Exp Hematol. 1975 Jan;3(1):32–43. [PubMed] [Google Scholar]
- Iscove N. N., Sieber F., Winterhalter K. H. Erythroid colony formation in cultures of mouse and human bone marrow: analysis of the requirement for erythropoietin by gel filtration and affinity chromatography on agarose-concanavalin A. J Cell Physiol. 1974 Apr;83(2):309–320. doi: 10.1002/jcp.1040830218. [DOI] [PubMed] [Google Scholar]
- Jackson C. W. Cholinesterase as a possible marker for early cells of the megakaryocytic series. Blood. 1973 Sep;42(3):413–421. [PubMed] [Google Scholar]
- Levin J., Levin F. C., Penington D. G., Metcalf D. Measurement of ploidy distribution in megakaryocyte colonies obtained from culture: with studies of the effects of thrombocytopenia. Blood. 1981 Feb;57(2):287–297. [PubMed] [Google Scholar]
- Long M. W., Smolen J. E., Szczepanski P., Boxer L. A. Role of phorbol diesters in in vitro murine megakaryocyte colony formation. J Clin Invest. 1984 Nov;74(5):1686–1692. doi: 10.1172/JCI111585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M. W., Williams N. Differences in the regulation of megakaryocytopoiesis in the murine bone marrow and spleen. Leuk Res. 1982;6(5):721–728. doi: 10.1016/0145-2126(82)90089-3. [DOI] [PubMed] [Google Scholar]
- Long M. W., Williams N. Immature Megakaryocytes in the Mouse: Morphology and quantitation by acetylcholinesterase staining. Blood. 1981 Nov;58(5):1032–1039. [PubMed] [Google Scholar]
- MacVittie T. J., McCarthy K. F. The detection of in vitro monocyte-macrophage colony-forming cells in mouse thymus and lymph nodes. J Cell Physiol. 1977 Aug;92(2):203–207. doi: 10.1002/jcp.1040920208. [DOI] [PubMed] [Google Scholar]
- Metcalf D., MacDonald H. R. Heterogeneity of in vitro colony- and cluster-forming cells in the mouse marrow: segregation by velocity sedimentation. J Cell Physiol. 1975 Jun;85(3):643–654. doi: 10.1002/jcp.1040850317. [DOI] [PubMed] [Google Scholar]
- Metcalf D., MacDonald H. R., Odartchenko N., Sordat B. Growth of mouse megakaryocyte colonies in vitro. Proc Natl Acad Sci U S A. 1975 May;72(5):1744–1748. doi: 10.1073/pnas.72.5.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., MacDonald H. R., Odartchenko N., Sordat B. Growth of mouse megakaryocyte colonies in vitro. Proc Natl Acad Sci U S A. 1975 May;72(5):1744–1748. doi: 10.1073/pnas.72.5.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Nakeff A., Dicke K. A., Noord van M. J. Megakaryocytes in agar cultures of mouse bone marrow. Ser Haematol. 1975;8(1):4–21. [PubMed] [Google Scholar]
- Ralph P., Moore M. A., Nilsson K. Lysozyme synthesis by established human and murine histiocytic lymphoma cell lines. J Exp Med. 1976 Jun 1;143(6):1528–1533. doi: 10.1084/jem.143.6.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendaal M., Hodgson G. S., Bradley T. R. Organization of haemopoietic stem cells: the generation-age hypothesis. Cell Tissue Kinet. 1979 Jan;12(1):17–29. doi: 10.1111/j.1365-2184.1979.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Sieber F., Stuart R. K., Spivak J. L. Tumor-promoting phorbol esters stimulate myelopoiesis and suppress erythropoiesis in cultures of mouse bone marrow cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4402–4406. doi: 10.1073/pnas.78.7.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thean L. E., Hodgson G. S., Bertoncello I., Radley J. M. Characterization of megakaryocyte spleen colony-forming units by response to 5-fluorouracil and by unit gravity sedimentation. Blood. 1983 Oct;62(4):896–901. [PubMed] [Google Scholar]
- Wagemaker G., Peters M. F., Bol S. J. Induction of erythropoietin responsiveness in vitro by a distinct population of bone marrow cells. Cell Tissue Kinet. 1979 Sep;12(5):521–537. doi: 10.1111/j.1365-2184.1979.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Williams N., Eger R. R., Jackson H. M., Nelson D. J. Two-factor requirement for murine megakaryocyte colony formation. J Cell Physiol. 1982 Jan;110(1):101–104. doi: 10.1002/jcp.1041100116. [DOI] [PubMed] [Google Scholar]
- Williams N., Jackson H. Kinetic analysis of megakaryocyte numbers and ploidy levels in developing colonies from mouse bone marrow cells. Cell Tissue Kinet. 1982 Sep;15(5):483–494. doi: 10.1111/j.1365-2184.1982.tb01571.x. [DOI] [PubMed] [Google Scholar]
- Williams N., Jackson H., Meyers P. Isolation of pluripotent hemopoietic stem cells and clonable precursor cells of erythrocytes, granulocytes, macrophages and megakaryocytes from mouse bone marrow. Exp Hematol. 1979 Nov;7(10):524–534. [PubMed] [Google Scholar]
- Williams N., Jackson H. Regulation of proliferation of murine megakaryocyte progenitor cells by cell cycle. Blood. 1978 Jul;52(1):163–170. [PubMed] [Google Scholar]