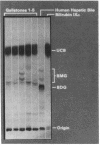
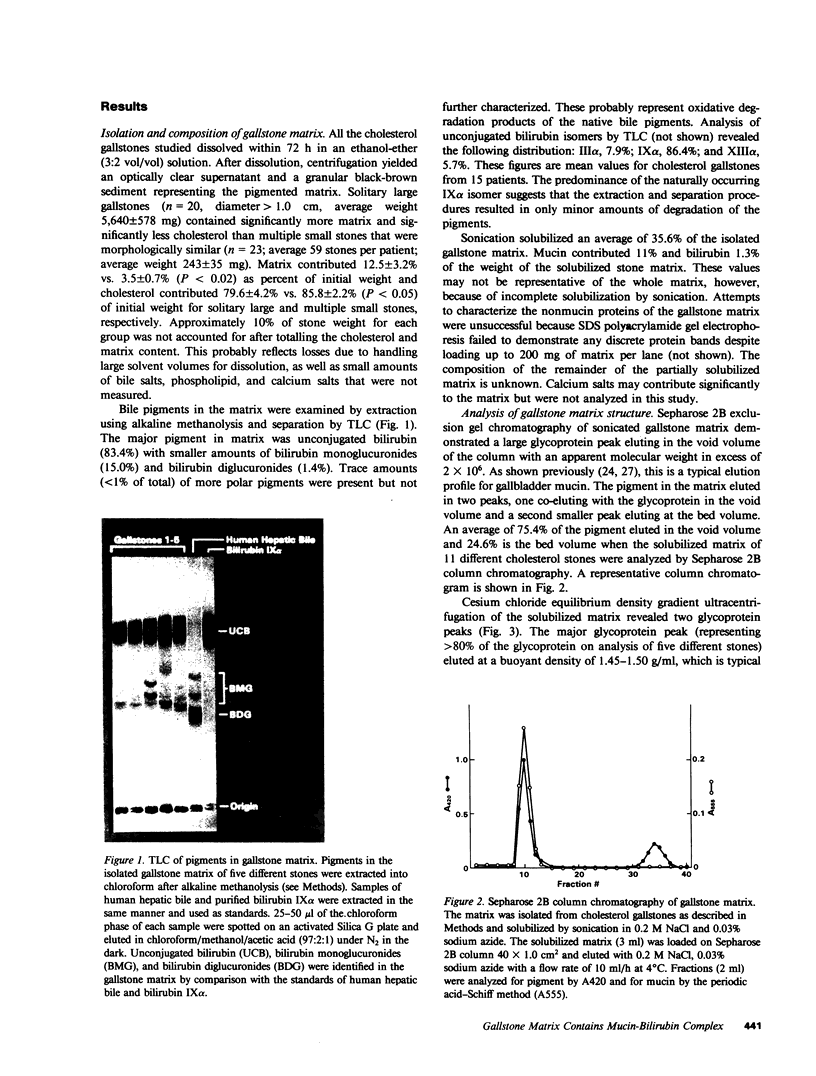
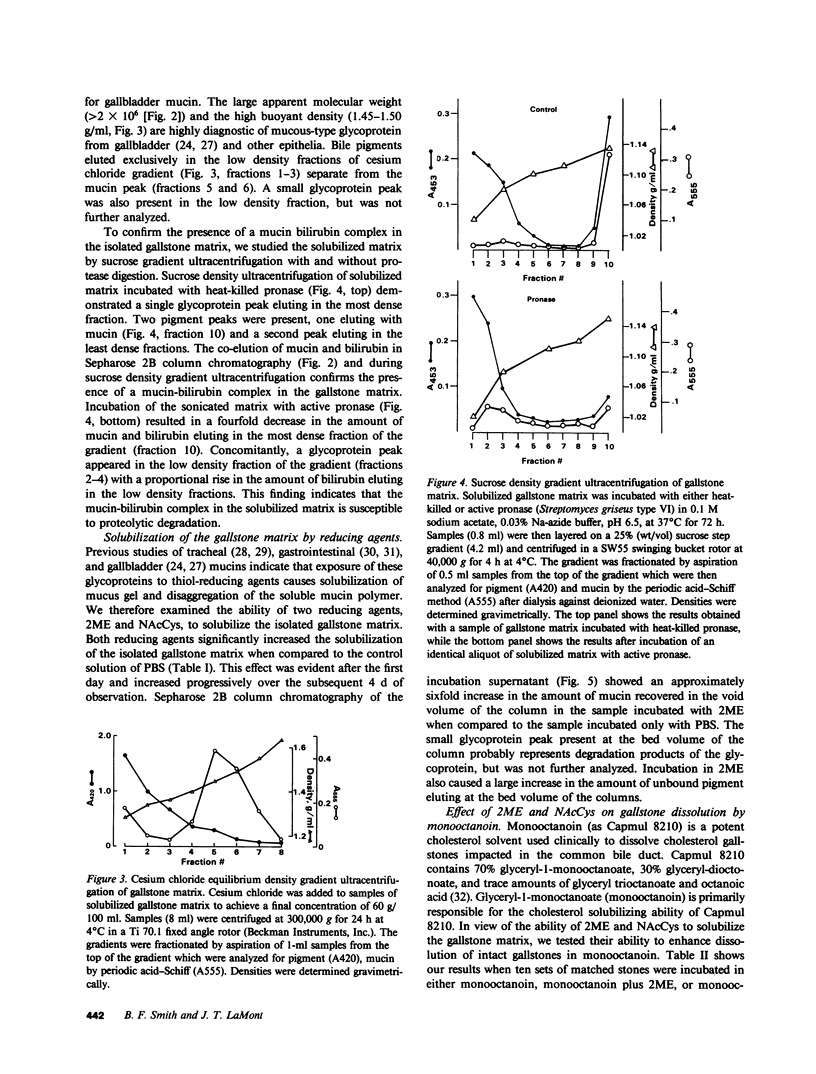
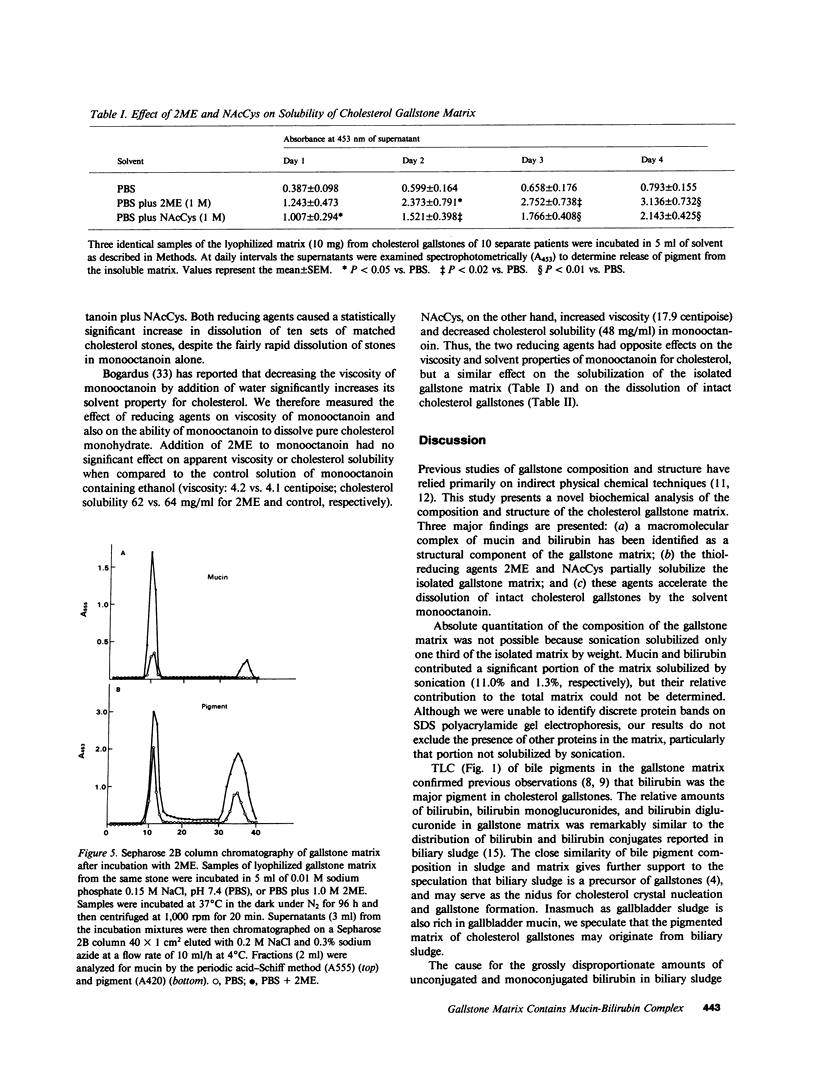
Abstract
The goals of this study were to isolate and characterize the nonlipid matrix of human cholesterol gallstones. The lipid portion of gallstones was dissolved in ethanol/ether, leaving an insoluble, granular, brown-black matrix that constituted 12.5% of solitary large stones and 3.5% of multiple small stones. The matrix was partially solubilized by sonication and studied by exclusion gel chromatography and density gradient ultracentrifugation. On Sepharose 2B column chromatography, bile pigment eluted with glycoprotein in the void volume, suggesting the presence of a high molecular weight complex (Mr greater than 2 X 10(6)). The identity of mucin in this complex was confirmed by its typical buoyant density during ultracentrifugation. The major bile pigments in the matrix were identified as bilirubin (84%) and bilirubin monoglucuronide (15%) by thin-layer chromatography. Because of their ability to solubilize mucin-type glycoproteins, we tested the ability of the reducing agents 2-mercaptoethanol (2ME) and N-acetylcysteine (NAcCys) to solubilize gallstone matrix. Both reducing agents caused a two- to threefold enhancement of matrix dissolution after 4 d compared to aqueous buffer alone (P less than 0.01). Sepharose 2B chromatography revealed that 2ME released a high molecular weight mucin-bilirubin complex as well as unbound pigment from the insoluble matrix. We also tested the effect of reducing agents on dissolution of matched cholesterol gallstones by monooctanoin, a cholesterol solvent. Both 2ME and NAcCys significantly accelerated gallstone dissolution in monooctanoin. Matched human cholesterol stones (n = 10) incubated for 4 d in monooctanoin plus either 2ME or NAcCys (1 M final concentration) weighed approximately half as much (P less than 0.01 for each) as stones incubated in monooctanoin alone. This study describes, for the first time, the isolation of a bilirubin-mucin complex in the insoluble matrix of human cholesterol gallstones. The ability of reducing agents to dissolve the matrix and thereby accelerate gallstone dissolution by monooctanoin in vitro may be relevant to gallstone dissolution in humans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen B., Bernhoft R., Blanckaert N., Svanvik J., Filly R., Gooding G., Way L. Sludge is calcium bilirubinate associated with bile stasis. Am J Surg. 1981 Jan;141(1):51–56. doi: 10.1016/0002-9610(81)90011-8. [DOI] [PubMed] [Google Scholar]
- BOGREN H., LARSSON K. ON THE PIGMENT IN BILIARY CALCULI. Scand J Clin Lab Invest. 1963;15:569–572. doi: 10.3109/00365516309051336. [DOI] [PubMed] [Google Scholar]
- Been J. M., Bills P. M., Lewis D. Microstructure of gallstones. Gastroenterology. 1979 Mar;76(3):548–555. [PubMed] [Google Scholar]
- Bills P. M., Lewis D. A structural study of gallstones. Gut. 1975 Aug;16(8):630–637. doi: 10.1136/gut.16.8.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogardus J. B. Unusual cholesterol solubility in water/glyceryl-1-monooctanoate solutions. J Pharm Sci. 1982 Mar;71(3):370–372. doi: 10.1002/jps.2600710331. [DOI] [PubMed] [Google Scholar]
- CARR J. J., DREKTER I. J. Simplified rapid technic for the extraction and determination of serum cholesterol without saponification. Clin Chem. 1956 Oct;2(5):353–368. [PubMed] [Google Scholar]
- Davis S. S., Scobie S., Inglis A. The effect of sulphydryl compounds and cross linking agents on the viscous and viscoelastic properties of mucus. Biorheology. 1975 Jun;12(3-4):225–232. doi: 10.3233/bir-1975-123-415. [DOI] [PubMed] [Google Scholar]
- Duvaldestin P., Mahu J. L., Metreau J. M., Arondel J., Preaux A. M., Berthelot P. Possible role of a defect in hepatic bilirubin glucuronidation in the initiation of cholesterol gallstones. Gut. 1980 Aug;21(8):650–655. doi: 10.1136/gut.21.8.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhoff P. A., Bhavanandan V. P., Davidson E. A. Purification, properties, and analysis of human asthmatic bronchial mucin. Biochemistry. 1979 May 29;18(11):2430–2436. doi: 10.1021/bi00578a044. [DOI] [PubMed] [Google Scholar]
- Fevery J., Blanckaert N., Leroy P., Michiels R., Heirwegh K. P. Analysis of bilirubins in biological fluids by extraction and thin-layer chromatography of the intact tetrapyrroles: application to bile of patients with Gilbert's syndrome, hemolysis, or cholelithiasis. Hepatology. 1983 Mar-Apr;3(2):177–183. doi: 10.1002/hep.1840030207. [DOI] [PubMed] [Google Scholar]
- Gordon E. R., Goresky C. A. A rapid and quantitative high performance liquid chromatographic method for assaying bilirubin and its conjugates in bile. Can J Biochem. 1982 Nov;60(11):1050–1057. doi: 10.1139/o82-135. [DOI] [PubMed] [Google Scholar]
- LaMont J. T., Ventola A. S., Trotman B. W., Soloway R. D. Mucin glycoprotein content of human pigment gallstones. Hepatology. 1983 May-Jun;3(3):377–382. doi: 10.1002/hep.1840030316. [DOI] [PubMed] [Google Scholar]
- Lee S. P., Carey M. C., LaMont J. T. Aspirin prevention of cholesterol gallstone formation in prairie dogs. Science. 1981 Mar 27;211(4489):1429–1431. doi: 10.1126/science.7466399. [DOI] [PubMed] [Google Scholar]
- Lee S. P. Hypersecretion of mucus glycoprotein by the gallbladder epithelium in experimental cholelithiasis. J Pathol. 1981 Jul;134(3):199–207. doi: 10.1002/path.1711340304. [DOI] [PubMed] [Google Scholar]
- Lee S. P., LaMont J. T., Carey M. C. Role of gallbladder mucus hypersecretion in the evolution of cholesterol gallstones. J Clin Invest. 1981 Jun;67(6):1712–1723. doi: 10.1172/JCI110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuschner U., Baumgärtel H. Gallstone dissolution in the biliary tract: in vitro investigations on inhibiting factors and special dissolution agents. Am J Gastroenterol. 1982 Apr;77(4):222–226. [PubMed] [Google Scholar]
- Levy P. F., Smith B. F., LaMont J. T. Human gallbladder mucin accelerates nucleation of cholesterol in artificial bile. Gastroenterology. 1984 Aug;87(2):270–275. [PubMed] [Google Scholar]
- MacLean-Fletcher S. D., Pollard T. D. Viscometric analysis of the gelation of Acanthamoeba extracts and purification of two gelation factors. J Cell Biol. 1980 May;85(2):414–428. doi: 10.1083/jcb.85.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki T., Matsushiro T., Suzuki N., Nakamura N. Role of sulfated glycoprotein in gallstone formation. Surg Gynecol Obstet. 1971 May;132(5):846–854. [PubMed] [Google Scholar]
- Maki T. Pathogenesis of calcium bilirubinate gallstone: role of E. coli, beta-glucuronidase and coagulation by inorganic ions, polyelectrolytes and agitation. Ann Surg. 1966 Jul;164(1):90–100. doi: 10.1097/00000658-196607000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Allen A. A colorimetric assay for glycoproteins based on the periodic acid/Schiff stain [proceedings]. Biochem Soc Trans. 1978;6(3):607–609. doi: 10.1042/bst0060607. [DOI] [PubMed] [Google Scholar]
- Mantle M., Allen A. Isolation and characterization of the native glycoprotein from pig small-intestinal mucus. Biochem J. 1981 Apr 1;195(1):267–275. doi: 10.1042/bj1950267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh A. F., Assisi F. Commercial bilirubin: A trinity of isomers. FEBS Lett. 1971 Nov 1;18(2):315–317. doi: 10.1016/0014-5793(71)80475-1. [DOI] [PubMed] [Google Scholar]
- McDonagh A. F., Assisi F. The ready isomerization of bilirubin IX- in aqueous solution. Biochem J. 1972 Sep;129(3):797–800. doi: 10.1042/bj1290797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing B., Bories C., Kunstlinger F., Bernier J. J. Does total parenteral nutrition induce gallbladder sludge formation and lithiasis? Gastroenterology. 1983 May;84(5 Pt 1):1012–1019. [PubMed] [Google Scholar]
- Park Y. H., Igimi H., Carey M. C. The "mirroring" of gallstones: description of a novel silvering method to determine the surface area of an irregular object. In vitro demonstration that multiple gallstones from the same gallbladder dissolve in unsaturated "bile" at the same rate. Gastroenterology. 1982 Nov;83(5):1071–1078. [PubMed] [Google Scholar]
- Pearson J. P., Kaura R., Taylor W., Allen A. The composition and polymeric structure of mucus glycoprotein from human gallbladder bile. Biochim Biophys Acta. 1982 Sep 7;706(2):221–228. doi: 10.1016/0167-4838(82)90490-3. [DOI] [PubMed] [Google Scholar]
- Phillips M. J., Funatsu K., Oda M., Edwards V., Mickle D. A. Dissolution of human cholesterol gallstones in vitro with ethanol and ether: a scanning and stereoscanning electron microscopic study. Lab Invest. 1978 Nov;39(5):497–504. [PubMed] [Google Scholar]
- Rose M. C., Lynn W. S., Kaufman B. Resolution of the major components of human lung mucosal gel and their capabilities for reaggregation and gel formation. Biochemistry. 1979 Sep 4;18(18):4030–4037. doi: 10.1021/bi00585a029. [DOI] [PubMed] [Google Scholar]
- Schoenfield L. J., Lachin J. M. Chenodiol (chenodeoxycholic acid) for dissolution of gallstones: the National Cooperative Gallstone Study. A controlled trial of efficacy and safety. Ann Intern Med. 1981 Sep;95(3):257–282. doi: 10.7326/0003-4819-95-3-257. [DOI] [PubMed] [Google Scholar]
- Smith B. F., LaMont J. T. Bovine gallbladder mucin binds bilirubin in vitro. Gastroenterology. 1983 Sep;85(3):707–712. [PubMed] [Google Scholar]
- Smith B. F., LaMont J. T. Hydrophobic binding properties of bovine gallbladder mucin. J Biol Chem. 1984 Oct 10;259(19):12170–12177. [PubMed] [Google Scholar]
- Starkey B. J., Snary D., Allen A. Characterization of gastric mucoproteins isolated by equilibrium density-gradient centrifugation in caesium chloride. Biochem J. 1974 Sep;141(3):633–639. doi: 10.1042/bj1410633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutor D. J., Wilkie L. I. Calcium in bile and calcium salts in gallstones. Clin Chim Acta. 1977 Aug 15;79(1):119–127. doi: 10.1016/0009-8981(77)90469-7. [DOI] [PubMed] [Google Scholar]
- Thistle J. L., Carlson G. L., Hofmann A. F., LaRusso N. F., MacCarty R. L., Flynn G. L., Higuchi W. I., Babayan V. K. Monooctanoin, a dissolution agent for retained cholesterol bile duct stones: physical properties and clinical application. Gastroenterology. 1980 May;78(5 Pt 1):1016–1022. [PubMed] [Google Scholar]
- Trotman B. W., Morris T. A., 3rd, Sanchez H. M., Soloway R. D., Ostrow J. D. Pigment versus cholesterol cholelithiasis: identification and quantification by infrared spectroscopy. Gastroenterology. 1977 Mar;72(3):495–498. [PubMed] [Google Scholar]
- Trotman B. W., Ostrow J. D., Soloway R. D. Pigment vs cholesterol cholelithiasis: comparison of stone and bile composition. Am J Dig Dis. 1974 Jul;19(7):585–590. doi: 10.1007/BF01073011. [DOI] [PubMed] [Google Scholar]
- WOMACK N. A., ZEPPA R., IRVIN G. L., 3rd The anatomy of gallstones. Ann Surg. 1963 May;157:670–686. doi: 10.1097/00000658-196305000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]