Abstract
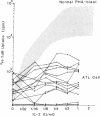
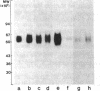
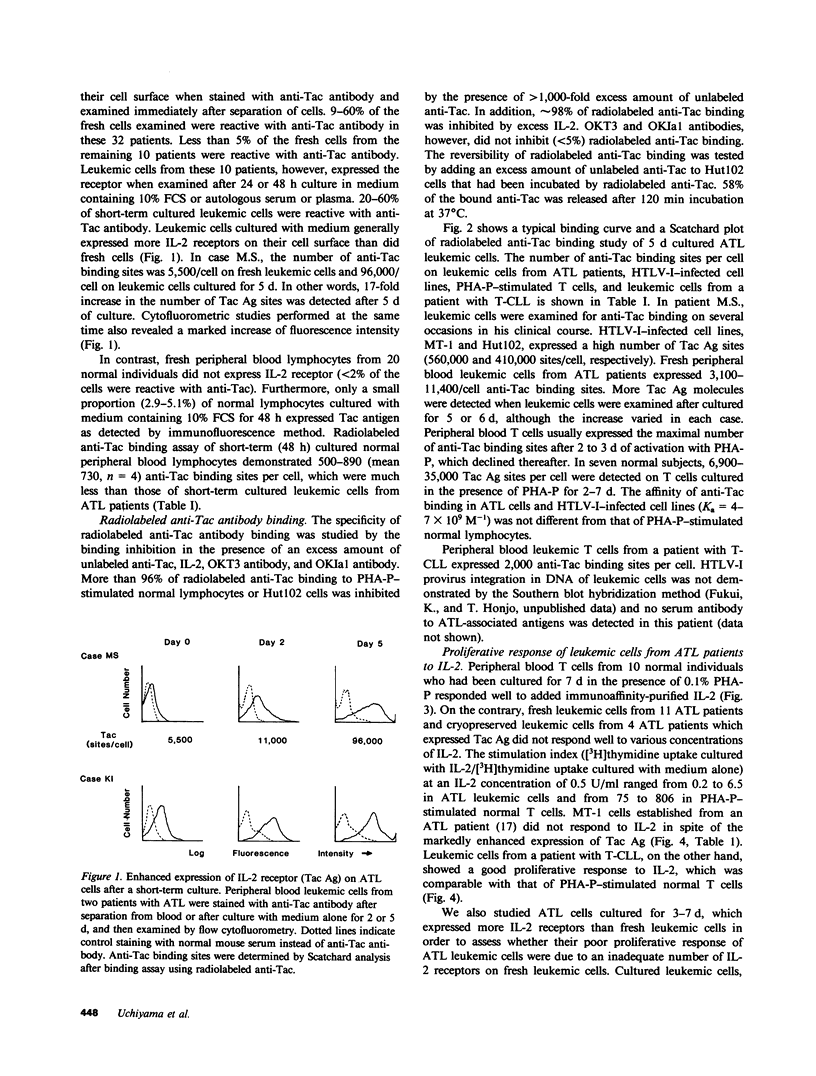
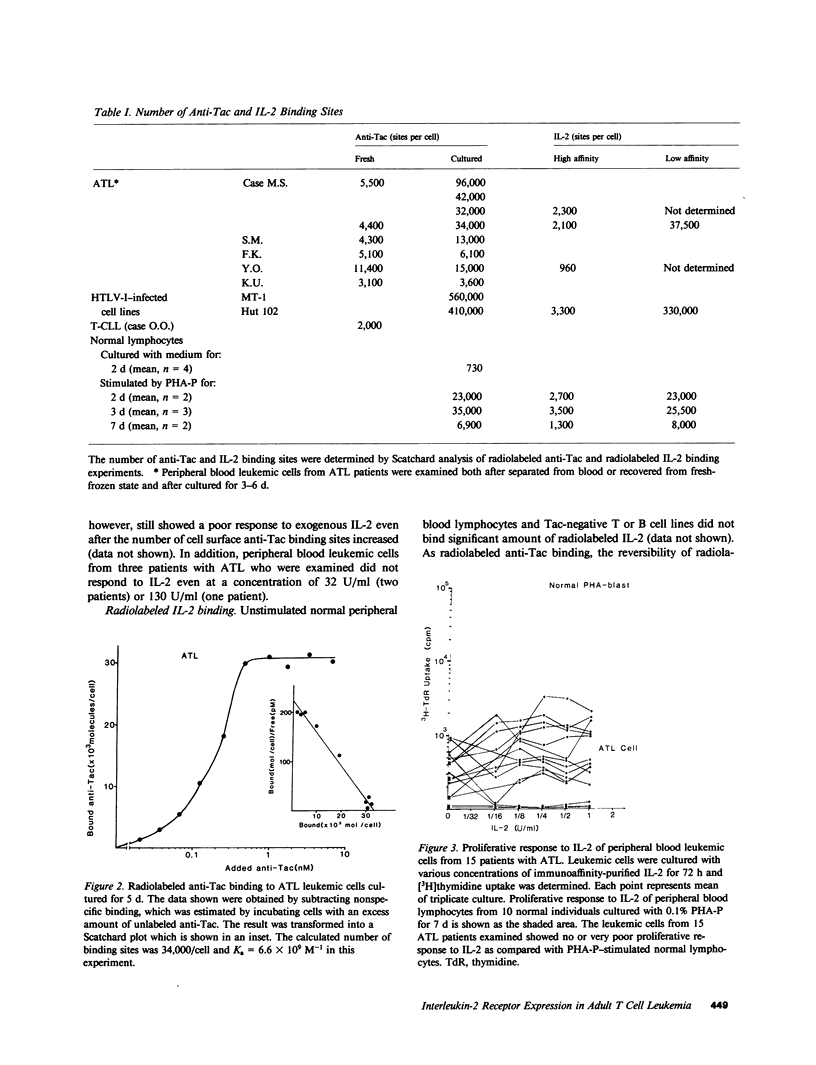
We studied the expression of the interleukin-2 (IL-2) receptor and the proliferative response to exogenous IL-2 of peripheral blood leukemic cells from patients with adult T cell leukemia (ATL) in order to see whether IL-2 receptor expressed on ATL cells is different from normal IL-2 receptor and whether it plays a role in the neoplastic growth in ATL. Peripheral blood leukemic cells from 42 patients with ATL examined expressed IL-2 receptors that were detected by anti-Tac monoclonal antibody when examined immediately after the separation of cells or after the culture for 24 or 48 h. The number of anti-Tac binding sites ranged from 3,100 to 11,400 in fresh cells and from 3,600 to 96,000/cell in short-term cultured leukemic cells, whereas phytohemagglutinin-P (PHA-P)-stimulated normal T cells exhibited 6,900-35,000 anti-Tac binding sites per cell. ATL-derived and human T cell leukemia/lymphoma virus, type I (HTLV-I)-infected cell lines such as MT-1 and Hut102 expressed a much higher number of anti-Tac binding sites. Leukemic cells from 15 patients with ATL examined showed no or very poor proliferative response to various concentrations of immunoaffinity-purified IL-2, although they expressed Tac antigen (Ag). Radiolabeled IL-2 binding experiments demonstrated that ATL leukemic cells could bind IL-2, and they expressed both high and low affinity IL-2 receptors, although the number of high affinity IL-2 receptor was much less than that of low affinity IL-2 receptor and that of anti-Tac binding sites. In contrast, leukemic T cells from a patient with T cell chronic lymphocytic leukemia (CLL), in whom HTLV-I infection was not demonstrated, responded as well as PHA-P-stimulated normal T cells, and their IL-2 receptors, unlike ATL cells, were modulated (down regulated) by anti-Tac antibody. No differences were noted between ATL cells and normal activated T cells in one-dimensional sodium dodecyl sulfate polyacrylamide gel electrophoresis analysis of the IL-2 receptor. Thus, leukemic cells in ATL spontaneously and continuously express IL-2 receptor, which appears to be abnormally regulated and unresponsive to IL-2. These results, taken together with those on normal IL-2 receptors on HTLV-I-negative T-CLL cells, suggest that abnormal expression of the IL-2 receptor in ATL is closely associated with HTLV-I infection and may play a role in the neoplastic growth of ATL cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K., Wong-Staal F., Gallo R. C. T-cell growth factor gene: lack of expression in human T-cell leukemia-lymphoma virus-infected cells. Science. 1984 Mar 9;223(4640):1086–1087. doi: 10.1126/science.6320374. [DOI] [PubMed] [Google Scholar]
- Barnes D. W. Epidermal growth factor inhibits growth of A431 human epidermoid carcinoma in serum-free cell culture. J Cell Biol. 1982 Apr;93(1):1–4. doi: 10.1083/jcb.93.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner W. A., Kalyanaraman V. S., Robert-Guroff M., Lister T. A., Galton D. A., Sarin P. S., Crawford M. H., Catovsky D., Greaves M., Gallo R. C. The human type-C retrovirus, HTLV, in Blacks from the Caribbean region, and relationship to adult T-cell leukemia/lymphoma. Int J Cancer. 1982 Sep 15;30(3):257–264. doi: 10.1002/ijc.2910300302. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Fabricant R. N., De Larco J. E., Todaro G. J. Nerve growth factor receptors on human melanoma cells in culture. Proc Natl Acad Sci U S A. 1977 Feb;74(2):565–569. doi: 10.1073/pnas.74.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. C., Wong-Staal F. Retroviruses as etiologic agents of some animal and human leukemias and lymphomas and as tools for elucidating the molecular mechanism of leukemogenesis. Blood. 1982 Sep;60(3):545–557. [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Bunn P. A., Russell E. K., Jaffe E. S., Schechter G. P., Guccion J. G. Mitogen requirements for the in vitro propagation of cutaneous T-cell lymphomas. Blood. 1980 Mar;55(3):409–417. [PubMed] [Google Scholar]
- Gill G. N., Lazar C. S. Increased phosphotyrosine content and inhibition of proliferation in EGF-treated A431 cells. Nature. 1981 Sep 24;293(5830):305–307. doi: 10.1038/293305a0. [DOI] [PubMed] [Google Scholar]
- Hahn B., Gallo R. C., Franchini G., Popovic M., Aoki T., Salahuddin S. Z., Markham P. D., Staal F. W. Clonal selection of human T-cell leukemia virus-infected cells in vivo and in vitro. Mol Biol Med. 1984 Feb;2(1):29–36. [PubMed] [Google Scholar]
- Hattori T., Uchiyama T., Toibana T., Takatsuki K., Uchino H. Surface phenotype of Japanese adult T-cell leukemia cells characterized by monoclonal antibodies. Blood. 1981 Sep;58(3):645–647. [PubMed] [Google Scholar]
- Hinuma Y., Komoda H., Chosa T., Kondo T., Kohakura M., Takenaka T., Kikuchi M., Ichimaru M., Yunoki K., Sato I. Antibodies to adult T-cell leukemia-virus-associated antigen (ATLA) in sera from patients with ATL and controls in Japan: a nation-wide sero-epidemiologic study. Int J Cancer. 1982 Jun 15;29(6):631–635. doi: 10.1002/ijc.2910290606. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino H., Esumi H., Miwa M., Shimoyama M., Minato K., Tobinai K., Hirose M., Watanabe S., Inada N., Kinoshita K. Establishment and characterization of 10 cell lines derived from patients with adult T-cell leukemia. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6061–6065. doi: 10.1073/pnas.80.19.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Crabtree G. R., Rudikoff S., Pumphrey J., Robb R. J., Krönke M., Svetlik P. B., Peffer N. J., Waldmann T. A. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Nikaido T., Shimizu A., Ishida N., Sabe H., Teshigawara K., Maeda M., Uchiyama T., Yodoi J., Honjo T. Molecular cloning of cDNA encoding human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):631–635. doi: 10.1038/311631a0. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Lange-Wantzin G., Sarin P. S., Mann D., Gallo R. C. Transformation of human umbilical cord blood T cells by human T-cell leukemia/lymphoma virus. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5402–5406. doi: 10.1073/pnas.80.17.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Guroff M., Nakao Y., Notake K., Ito Y., Sliski A., Gallo R. C. Natural antibodies to human retrovirus HTLV in a cluster of Japanese patients with adult T cell leukemia. Science. 1982 Feb 19;215(4535):975–978. doi: 10.1126/science.6760397. [DOI] [PubMed] [Google Scholar]
- Teshigawara K., Maeda M., Nishino K., Nikaido T., Uchiyama T., Tsudo M., Wano Y., Yodoi J. Adult T leukemia cells produce a lymphokine that augments interleukin 2 receptor expression. J Mol Cell Immunol. 1985;2(1):17–26. [PubMed] [Google Scholar]
- Tsudo M., Uchiyama T., Uchino H., Yodoi J. Failure of regulation of Tac antigen/TCGF receptor on adult T-cell leukemia cells by anti-Tac monoclonal antibody. Blood. 1983 May;61(5):1014–1016. [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Uchiyama T., Nelson D. L., Fleisher T. A., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. II. Expression of Tac antigen on activated cytotoxic killer T cells, suppressor cells, and on one of two types of helper T cells. J Immunol. 1981 Apr;126(4):1398–1403. [PubMed] [Google Scholar]
- Uchiyama T., Yodoi J., Sagawa K., Takatsuki K., Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977 Sep;50(3):481–492. [PubMed] [Google Scholar]
- Wano Y., Uchiyama T., Fukui K., Maeda M., Uchino H., Yodoi J. Characterization of human interleukin 2 receptor (Tac antigen) in normal and leukemic T cells: co-expression of normal and aberrant receptors on Hut-102 cells. J Immunol. 1984 Jun;132(6):3005–3010. [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Yodoi J., Takatsuki K., Masuda T. Letter: Two cases of T-cell chronic lymphocytic leukemia in Japan. N Engl J Med. 1974 Mar 7;290(10):572–573. doi: 10.1056/NEJM197403072901018. [DOI] [PubMed] [Google Scholar]
- Yodoi J., Uchiyama T., Maeda M. T-cell growth factor receptor in adult T-cell leukemia. Blood. 1983 Aug;62(2):509–511. [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]