Introduction
We live or die by blood loss. Platelets, the anuclear cells designed to stop bleeding, have their limits. In the past two decades, a range of intravenously administered products have been pursued to augment platelets and stop internal bleeding. Traumatic injury, thrombocytopenia, intraoperative bleeding, hemophilia, and other diseases that result in excessive bleeding could potentially be mitigated by this kind of product. Traumatic injury, for example, is a leading cause of death for young people worldwide, and many of those deaths are a result of excessive hemorrhage (surpassed only by CNS injury).1,2 Hemorrhage as a result of these traumatic injuries accounts for more than a third of prehospital mortality for civilians and is the leading cause of preventable deaths for soldiers.2–4 We know that time is absolutely critical for halting hemorrhage and saving lives: more than 80% of battlefield casualties and one-third of civilian trauma casualties occur before the patient ever reaches a hospital.2,3 This means that first responders have to be able to provide the care that can halt hemorrhage and keep patients alive until they can reach a hospital.
The current methods for first responders to treat patients are limited. In cases of injury to the extremities, tourniquets are a simple and affordable way to halt bleeding.5 Pressure and the use of appropriate dressings are also helpful when the injury is external.5,6 Some of the available dressings include the popular QuikClot, which has been shown in a pig liver injury model to be significantly better at reducing blood loss and improving survival when compared with gauze.7 One notable issue found with the first generation of this design was that is was highly exothermic, and was associated with tissue damage.7 Because of this, the mineral zeolite was replaced with kaolin, which is the currently available version of the product. Chitosan is another treatment that has been considered for use as a hemostatic agent, although the duration of efficacy in halting bleeding has been questioned.8 Treatments that act directly on the clotting cascade have also been considered. Holcomb et al. showed that a fibrin sealant foam, which can be introduced into the body cavity, can reduce bleeding and bond with injured surfaces in a rat model.9 This type of technology does not rely on the ability to place direct pressure, but does still require access to the injury site. In addition, the fibrin is expensive and difficult to store, making it much less practical for field use.8 While there is no ideal dressing for hemostasis, there are many options currently being investigated that have significant ability to halt bleeding.
A gap remains, however, in the available treatments to halt internal bleeding. For internal injuries, termed noncompressible, neither dressings nor pressure and tourniquets are adequate solutions. This has led to study of the potential for an injectable suspension of particles to aid hemostasis. Such particles must act on the clotting cascade to halt bleeding without activating excessive clotting, which could result in stroke or infarction. In addition, these particles would ideally be affordable, easily stored, and simple for field use. Several groups have investigated use of particles that interact with platelets or the clotting cascade to help aid hemostasis.
Cell-derived hemostatic agents
The clotting cascades, and primarily, platelets, were the first place that researchers looked for methods to halt non-compressible bleeding. Platelets are anuclear cells, derived from budding off from their precursor megakaryocytes, which circulate in the bloodstream in a quiescent state until injury occurs. They are typically 2–3 microns and are bi-discoid in their quiescent state. They contain alpha and dense granules, which contain pro-coagulation signals and clotting factors. The contents of these granules can be quickly secreted through a transport system called the open canalicular system.26
Primary hemostasis involves the adhesion, activation, and aggregation of platelets to form the platelet plug.27 When the blood vessel is injured, the damaged endothelial lining exposes the underlying layer subendothelium matrix28, which includes collagen29, fibronectin30, and laminin.31 Platelets can bind directly to this matrix through the glycoprotein GPIa/IIa receptor.26 However, these interactions are most effective in low shear binding.31 Under high shear, platelets bind mostly to von Willibrand Factor (vWF), which is itself bound to subendothelial matrix.32 This association is brief however, as platelet GPIb-V-IX/vWF binding has a very high dissociation rate. This leads to the characteristic “rolling” of platelets along a damaged endothelium, slowing its progress enough to allow for additional integrin binding to extracellular matrix proteins (e.g. GPVI-collagen or GPIa/IIa-fibrin) to finally arrest its motion.26
Platelets can become activated by a number of different mechanisms, including adenosine diphosphate (ADP), thromboxane, thrombin, and cyclooxygenase.26 Upon activation, they change their conformation from a bi-discoid shape to a stellate morphology. Rapid polymerization of actin filament in the cytoskeleton causes both the gross shape change as well as specific conformational change of the surface receptor glycoprogtein IIb/IIIa (GPIIb/IIIa, integrin α2bβ3) and release of platelet alpha and dense granules.26
The change in the GPIIb/IIIa conformation exposes binding domains for both fibrinogen and vWF. Interestingly, fibrinogen has multiple binding domains, including the common RGD (arg-gly-asp) motif at each end, as well as a dodecapeptide-H12, which allows for it to act as a platelet-platelet bridging molecule.33–35 Upon activation, and in concert with fibrinogen, and other extracellular matrix (ECM) proteins, platelets rapidly aggregate, adhere to the injury surface, and begin to spread across the surface, such that they become indistinguishable from one another under scanning electron microscopy.26
Thromboerythrocytes
One of the first groups that considered this potential application for injectable particles was Coller et al. They looked at the use of what they called ‘thromboerythrocytes.’10 These particles use a peptide containing the sequence arginine-glycine-aspartic acid to bind to activated platelets, which display the GPIIbIIIa receptor. This sequence was bound through a heterobifunctional crosslinking agent to the surface amino groups on erythrocytes. In prior work, they had shown how important the length of the crosslinker is for establishing interaction between the activated platelets and the RGD linked particles36 They were then able to make thromboerythrocytes that interacted selectively in vitro with platelets activated by the presence of ADP. This work established the importance of the RGD sequence in development of particles capable of interacting with activated platelets.
Synthocytes
The next group that looked at an intravenously injectable particle to improve clotting was Levi et al. They developed particles made of albumin coated with fibrinogen (a blood protein that contains the RGD sequence).11 These particles were approximately 3.5–4.5 microns in size, larger than many of current particle formulations. They showed a reduction in bleed time in an in vivo ear injury model in thrombocytopenic rabbit. In this model they pre-injected the rabbits with the particles prior to injury. They did show with this model that the particles significantly reduce the bleed times of the animals, demonstrating that particles using the RGD sequence to bind activated platelets can, indeed, have an effect on bleeding outcomes.11 However, the size of these particles is problematic because particles of that size can be filtered in the capillary beds of the lungs.37
Thrombosomes
Another approach to the generation of particles that can perform this function is the use of modified platelets, which can both initiate and strengthen clotting. This is the method that Fitzpatrick et al. have looked at. They have shown that they can collect and freeze-dry platelets stabilized with trehalose in a form that is still similar to native platelets.12 The majority of the particles are in the 1–5 micron range (the size range of native platelets). In vitro studies show that the particles display GPIIbIIIa and can still perform the functions necessary for hemostasis. In vivo studies show that the particles stay in circulation after injection for the same amount of time as a normal platelet transfusion and no adverse effects of injection were apparent. In vivo testing with the ear bleed model in thrombocytopenic rabbits showed that the particles also significantly reduced blood loss.12,13 Safety testing of these particles was also done in a non-human primate model (rhesus macaque) without any adverse events reported. However, biologically derived particles may also lead to potential for adverse immune responses and disease transfer.38
Drugs Interacting with the Clotting Cascade
Tranexamic Acid
Tranexamic acid is a strong antagonist of plasminogen activation, and therefore acts as an antifibrinolytic. There is convincing evidence that its administration after trauma greatly improves outcomes, reducing (all cause) mortality by 23.9%-17.4%, in one study of 896 wounded soldiers at a military hospital in Afghanistan.14,15 However, it has the potential drawbacks of increasing risk of diffuse intravascular coagulation, increased risk when administered 3–8 hours after trauma, and increased risk when co-administered with blood products. The concurrent use of a permissive hypotension approach may also increase tranexamic acid’s risks since this approach leads to a decreased glomerular filtration rate, and hence a delayed clearance rate. This may lead to a change in the drug’s efficacy and safety.14 Nevertheless, it has tremendous potential, and may become incorporated in standard damage control resuscitation.39
Recombinant Factor VIIa (rFVIIa)
The administration of recombinant factor VIIa intravenously to reduce bleeding after acute trauma has been a topic of debate.5,19,20,40 Several studies have shown that perioperative administration of rFVIIa reduces the volume of blood transfusion. However, it is unclear whether the benefit is large enough to have any associated effect on mortality after hemorrhagic trauma.16–18 Its potential use in the prehospital phase is further diminished due to its high cost, potential for adverse effects, and necessity to be stored at 2–8 degrees C.18,20
Synthetic intravenous hemostats
The use of synthetic methods to generate particles has become more popular in recent years because they offer improved control over the final product and concerns about outcomes with large particles. Early studies of particle biodistribution showed that smaller particles (in the range of a micron or less) are taken up by the reticuloendothelial system and remain in the liver. Larger particles, greater than 5 microns in diameter, are mechanically filtered by the capillary beds of the lungs within minutes of administration.37 Particles of larger size were shown by Ilium et al. to cause immediate toxicity and resulted in death of rabbits injected with the particles within four minutes of injection.37 This filtering by the capillary beds is concerning and makes it doubtful that microparticles of this size would ever be usable in humans. This means that the ability to control size and make particles in the nano size range is necessary for developing a successful particle. This is of particular concern in the cases of cell-derived hemostatic particles. In all cases, these particles were greater than one micron in size.10,11,13 In that size range, the particles have the potential to cause serious side effects if they become trapped. Synthetic particles tend to be smaller in size, which is more likely to safely navigate small capillaries.21,24 This has led to more study of synthetic particle formulations in recent years.
Liposomes
Liposomes are a popular type of synthetic nanoparticle used primarily for drug delivery. Okamura et al. have developed hemostatic liposomes coated with an alternative peptide: the dodecapeptide HHLGGAKQAGDV (H12) which was shown in vitro to suppress platelet aggregation, suggesting interaction with activated platelets. The liposomes with this peptide are much smaller than the comparable particles derived from cells: they are typically around 260nm in diameter (+/− 60 nm). In addition, liposomes allow encapsulation of drugs, in this case ADP within area enclosed by the lipid bilayer. The use of ADP there may enhance platelet aggregation by activation of additional platelets.22,23 These particles are entirely synthetic, but still offer a significant improvement in bleeding time in both thrombocytopenic rat and thrombocytopenic rabbit models when compared with saline as a treatment.22
PLGA nanoparticles
Bertram et al. have also shown that synthetic particles can be a functional alternative to particles derived from blood cells and proteins. These particles are made with a biodegradable polymer core (poly(lactic-co-glycolic acid) with polyethylene glycol as the spacer used to attach the RGD peptide. They have been shown to be effective in a rat femoral artery injury model at significantly reducing bleeding time.24 These particles were also later shown to effectively reduce lethality in a rat liver injury model by Shoffstall et al.25 The trend toward synthetically based particles offers several advantages. Size can be more easily controlled with synthetic particles and tend to be smaller, which may be an advantage. In addition, both the liposomes and the particles with a polymer core can be drug loaded to offer additional treatment at the injury site if desired.
Injury and Bleeding Models
The choice of model in these examinations is important for understanding how treatments may improve outcomes. In vivo models of hemostasis are generally designed to assess the impact of a treatment (or knockout of a specific pathway) on clotting time, blood loss, degree of shock, and/or survival.41 These models consist of controlled hemorrhage, uncontrolled hemorrhage, specific organ systems (e.g. CNS), or polytrauma.41 There are a variety of in vivo models that have been tested in uncontrolled hemorrhagic trauma, where the efficacy of a therapy such as synthetic platelets could be tested, including in rodents, large mammals, and non-human primates (Table 2).
Table 2.
Advantages and disadvantages of various species in bleeding hemorrhagic trauma models.
| Species | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| Mouse | Economy, wide availability of genetically-mutated strains, and the associated host of immune/biochemical assays | High platelet count, limited correlation to human physiology, and an insensitivity to many coagulation defects, small size makes some interventions technically challenging | 42–47 |
| Rat | Similar to mouse, surgical procedures are technically easier to perform on the rat, and they are still as widely available and relatively cheap like mice. The coagulation cascade and associated mechanism appear to be relatively well-conserved: including platelet physiology, hepatic blood flow, and blood pressure regulation | High platelet count and a relatively lower clotting time compared to humans, small size still presents some technical challenges | 22–24,45–60 |
| Rabbit | Platelet counts, physiology and in vitro clotting parameters are extremely well-correlated to humans, making them a viable candidate for an in vivo model of hemorrhage, and experimental therapies. | More expensive and fewer on-shelf bio-assays than rats or mice, insensitive to thrombin receptor peptides that activate human platelets. | 12,45–47,59–61 |
| Pig | The cardiovascular system is well-correlated with human parameters and the comparable size allows for devices to be used in both clinical and research environment without modification. Wound-healing process appears to be similar to the human due to similarities between porcine and human skin. | Expensive, need technically-trained staff, sensitivity to complement activation related pseudoallergy (CARPA). | 41,45–47,62–65 |
| Primate | Best animal analog of humans available. | Controversial, very expensive, highly regulated. These studies are generally performed prior to initiating a clinical trial to test for safety/efficacy. | 13,46,47 |
Rodents
Tail, Ear Bleeding Time
Murine hemorrhagic trauma models are generally limited to tail bleeding times in the literature.42–44,48,50 The advantages of using mice include their economy and wide availability, and availability of genetically-mutated strains, and the associated host of immune/biochemical assays.41 However, the disadvantages are a limited correlation to human physiology, and an insensitivity to many coagulation defects.42 The latter may possibly be due to the low-flow allowing vasospasm and other compensatory mechanisms to allow for normal bleeding times.42 Rat models that have been investigated include the same bleeding time models as the mouse.51–55 However, these studies are more often performed to look at the physiology and response to experimental treatments.41 Surgical procedures are technically easier to perform on the rat, blood volume is significantly greater, and they are still as widely available and relatively inexpensive, like mice.41
Lethal Liver Injury
There have also been a multitude of trauma models developed in the rat. One of the most widely published is a model of uncontrolled hemorrhage from a liver resection with or without fluid resuscitation.9,56–58 The main outcomes studied in this model have included blood loss, survival, and blood metabolic outcomes (oxygenation) depending on the resuscitation paradigm.57 The advantages include having survival as an experimental outcome and requiring only very simple surgery and measurement techniques.
The disadvantages of this model include a large variability (depending strongly on rat body mass and liver resection).9 Rats also tend to have a higher platelet count and a relatively lower clotting time compared to humans.45 However, while there are moderate differences in hemodynamics and physiology from humans and larger animals, the coagulation cascade and associated mechanism appear to be relatively well-conserved: including platelet physiology, hepatic blood flow, and blood pressure regulation.46,47,49,59
Rabbit
Thrombocytopenia
Normal rabbit platelet counts, physiology and in vitro clotting parameters are extremely well-correlated to humans,45,47 making them a viable candidate for an in vivo model of hemorrhage, and experimental therapies. There are well-established protocols for inducing thrombocytopenia either chemically (busulfan)66, radiation-therapy, or repeat blood draws/transfusion, and have been tested in conjunction with other synthetic platelet treatments.11,21,22,61
Blood Loss and Bleeding Times
The majority of studies that have used the rabbit in a model of hemostasis have looked at bleeding times, or blood loss from surgical incisions.11,67–71 The limitations of bleeding times in both human and animal models is widely recognized.48,72 While it may produce a repeatable model, conclusions drawn must be limited to scope of isolated vascular injury, whereas the majority of clinical traumas are much more complex.42,48
Lethal Liver Injury
Recently, Nishikawa et al. have published a paper investigating the use of synthetic platelets to increase survival in a model of a liver trauma in thrombocytopenic rabbits.61 This appears to be the first lethal liver trauma model developed in the rabbit, and appears to induce a repeatable injury.
Pig
The pig is the standard model for uncontrolled hemorrhagic trauma, when investigating the physiological impact of a potential therapy.62–65,73–76 The cardiovascular system is well-correlated with human parameters and the comparable size allows for devices to be used in both clinical and research environment without modification.41 Furthermore, the wound-healing process appears to be similar to the human due to similarities between porcine and human skin.41 However, a porcine model is more expensive due to the equipment and need to for a technically-trained staff.41 Furthermore, there is an increasing body of evidence that suggests pigs may be especially sensitive to complement activation and related pseudoallergy (CARPA).77–79 Briefly, a pseudoallergy is observed after intravenous infusions of certain nanoparticle formulations. Symptoms include severe hypotension, cardiopulmonary dysfunction, and, if severe enough, death. Interestingly, other species are less susceptible to symptoms. However, it has been observed directly in both swine and dogs.79,80
Primate
Non-human primates are the best animal analog of humans available. However, this makes their use in medical research controversial and, due to the administrative overhead, expensive. Thus, the use of primates in trauma is not well suited for establishing efficacy in experimental therapies, except where the anatomical/physiological differences in other animals precludes their use. The majority of non-human primate studies (in hemorrhagic trauma) are for preclinical studies, where the main goal is testing safety.
Several hemorrhagic trauma models have been established.12,81–83 Recently, of particular interest, Thrombosomes (a lyophilized hemostatic agent derived from platelets), underwent a safety study in rhesus macaques, in the presence of a hemorrhagic liver trauma.12
Challenges for Nanoparticle Formulations
There remain, however, many challenges that need to be addressed before particles such as those discussed above can be translated for use by first responders. While micro- and nanoparticles appear to offer many advantages for targeting and drug delivery, there are key issues of stability and evasion of the immune system that must be addressed.
Blood Circulation Time
The adsorption of proteins is also a significant concern for any surface or particle that will contact blood because it is believed to cause rapid uptake of particles by the RES system. It is well understood that when the surface of a biomaterial is put into the body, it is immediately covered with a protein corona.85,86 Understanding the degree to which nanoparticles will be taken up by phagocytosis and maximizing evasion of the RES system are considered necessary for the success of nanoparticle treatments. In addition, the formation of the protein corona alters the interaction of nanoparticles with their environments, which may effect the ability of nanoparticles targeted to activated platelets to interact with their receptors.85,86 PEGylation of nanoparticles is the most typical method used to attempt to overcome protein adsorption. It has been shown that for polymeric nanoparticles, PEG content between 2 and 5 weight %, with a PEG molecular weight of 5000 grams/mole is optimal for prevention of protein adsorption.87 Even with the use of PEG to prevent protein adsorption, there will still be protein adsorption, and the result is that these types of nanoparticles will have a relatively short circulation time.
Storage and Resuspension (stability)
Particle stability in storage is another concern for successfully translating particles to a clinical setting. The need for refrigeration significantly hurts the chances that a technology will be successful in a military setting or useful for first responders. Alternative treatments, such as blood transfusions and rVIIa require refrigeration, which is a significant impediment to use in the field. Some of the proposed treatment can be stored at ambient temperatures, such as the thrombosomes.13 Storage of liposomes has proven challenging. They are most easily stored in suspension, which is stable for several months when refrigerated.88 They can be stored longer by freeze drying, but will still suffer degradation, and keep best when maintained at cold temperatures, although they do have a glass transition in the range of up to 40 °C, beyond which no particle structure would be expected to be maintained.89,90
Polymers offer a potentially more stable solution. Polymeric nanoparticles are most often freeze-dried, which is more stable than storage in aqueous solution.88 While PLGA, used by Bertam and Shoffstall et al., has a relatively low glass transition temperature of around 40 °C, PLA, another polymer used frequently for nanoparticle formulation, is an alternative polymer that has a higher glass transition of 50 °C, which could offer a solution to temperature degradation. It is also crystalline, which offers additional stability, and will form stereocomplexes when both L- and D- lactide polymers are present.91,92
Nanoparticles are stored in suspension, frozen, or as a lyophilized product, depending on their stability in each phase. For biodegradable polymer nanoparticles, they are generally lyophilized for long-term storage. However, due to crystallization during processing, aggregates are formed, which then require sonication in order to recover pre-lyophilization size distributions.93 This has led to the development and use of cryo- and lyo-protectants. Some of these involve sugars such as trehalose and sucrose, polymers PVA, poly(vinyl pyrrolidone) (PVP), and even matrices, such as gelatin.88 Some concerns exist as to the concentrations of these excipients required in order to stabilize nanoparticles, for example a 1:1 w/w ratio in the case of trehalose, which could constitute a substantial dose of sugar depending on the application.88 While trehalose has been shown to be safe at oral doses up to 50g, its metabolism with i.v. delivery may require further investigation in regards to safety and toxicity.94 The use of surfactants such as Tween 80 (polysorbate 80), and other excipients are used to minimize the energy required to resuspend nanoparticles after lyophliiziation, however toxicity is always a potential concern.95
Complement activation
Complement activation related pseudoallergy (CARPA) is elicited readily in pigs during administration of certain liposomes and several polymeric-based nanoparticles.77 The hallmark symptoms of CARPA appear within 1–3 minutes following particle administration and include cardiopulmonary distress (including increase in heart rate, hypotension, decreased cardiac output, decreased pulmonary pressures, and decreased blood gas levels), and a characteristic flushing of the skin (erythema) upon reperfusion of the tissues.77–79 Surprisingly, unless symptoms are so severe they lead to mortality, these issues spontaneously resolve within minutes. Regardless, the consequences of a severe CARPA episode during administration of intravenous hemostats could be catastrophic, and the mitigation by pharmaceutical prophylaxis is likely contraindicated. Therefore, the development of robust models to ensure a minimized risk for CARPA during nanoparticle administration, and elucidating its mechanisms so that patient-exclusion criteria, based on heighted risk factors, will be paramount to the clinical translation of intravenous hemostatic technologies.
Complement activation is classically induced by traumas, invading bacteria, and viruses; experimentally, it is induced with endotoxin lipopolysaccharide (LPS). Pigs, compared to humans, are especially sensitive to CARPA, endotoxemia and sepsis, which make them good models for those pathologies.96 Administration of Doxil (a liposome-based chemotherapeutic) causes mild-to-moderate hypersensitivity reactions in 0–25% of humans97, sometimes even subclinically, yet causes moderate-to-lethal reactions in swine.98 Interestingly, drastic response to nanoparticles in mice and rat models are not readily observed, making them very poor models for detecting CARPA. It is most likely, as has been previously reported, that the pig species simply has a heightened immune response to pathogens, potentially due to their selective breeding in the meat industry, where susceptibility to disease has been strongly selected against.96
While these reactions are thought to be pseudoallergies, in at least one study, repeat administrations (of a novel pegylated micelle) increased the severity of reactions, measured by histamine release and complement activation, consistent with antibody-mediated reactions.99,100 Since the precise mechanism has not yet been elucidated, methods to design around this adverse reaction are also somewhat poorly understood. Three main strategies have been employed to date: prophylaxis, tachyphylaxis, and tuning surface chemistry.
Mitigation of response
Prophylaxis
Szebeni et al.78,77 describes the nonspecific complement activation to infusions of nanoparticle drug carrier systems and discuss that the reaction can be prevented with preconditioning with low doses of the nanoparticle carrier or pharmacologically with indomethacin (a potent complement inhibitor).
Diphenhydramine, phenylephrine, epinephrine and steroids may also be used in conjunction to reverse the anaphylaxis induced by CARPA.79 Unfortunately for the application of intravenous hemostatic agents to be administered during trauma, co-administration with additional pharmaceuticals should be avoided if possible.
Tachyphylaxis
One potential method for reducing the onset of CARPA is to infuse the nanoparticles slowly (or with multiple small doses) (tachyphylaxis)78 This appeared to prevent the onset of CARPA and reduce the severity of any symptoms. It relies on a desensitization mechanism. However, since the present therapy will rely on rapid administration after hemorrhagic injury, tachyphylaxis does not appear to be a viable option.
Tuning particle charge
Currently, the most viable option for prevention of CARPA appears to be tuning the surface chemistry of the nanoparticles to have a neutral zeta potential.98 While the symptoms appear to be exaggerated in pigs, there does appear to be a large human population of individuals that experience CARPA symptoms, when administered the novel liposomal chemotherapeutic Doxil.97 In one particular study, a post-hoc analysis demonstrated that 45% of patients receiving Doxil showed symptoms of CARPA (grade 2 and 3), where grade 2 is symptomatic but does not require intervention, and grade 3 is a severe reaction requiring anti-allergic medication and cessation of the infusion. The responding population received infusions at a mean rate of 0.51 mg/min, with a mean total 151.8 mg, compared to those that showed no symptoms, receiving a mean infusion rate of 0.23 mg/min, total 70.1 mg.97 However, it is important to note that even in patients not showing symptoms, complement activation (SC5b-9 elevation) is present, which may aggravate other pathways during trauma.97 While the infusion rate of 0.2 mg/min (or tachyphylaxis) may be an acceptable method to mitigate the induction of CARPA for chemotherapy, this is not an acceptable practice for intravenous hemostat applications, where a large bolus is attempting to be infused as quickly as possible to prevent blood loss.
Conclusion
Bleeding resulting from traumatic injury remains a leading cause of death for young people. There are few treatments that first responders can offer for non-compressible bleeding, but nanoparticles are being investigated as a way to fill this gap in treatment. These treatments typically use peptide sequences to bind to activated platelets and crosslink them to stabilize the platelet plug. Research in this area has evolved from biologically based microparticles towards synthetic nanoparticles. Reduction in size and use of appropriate synthetic materials can improve circulation time and reduce the risks associated with larger particles. However, there are still concerns about protein adsorption, immune response, and stability of this type of particle that must be overcome before these particles can be translated for clinical use.
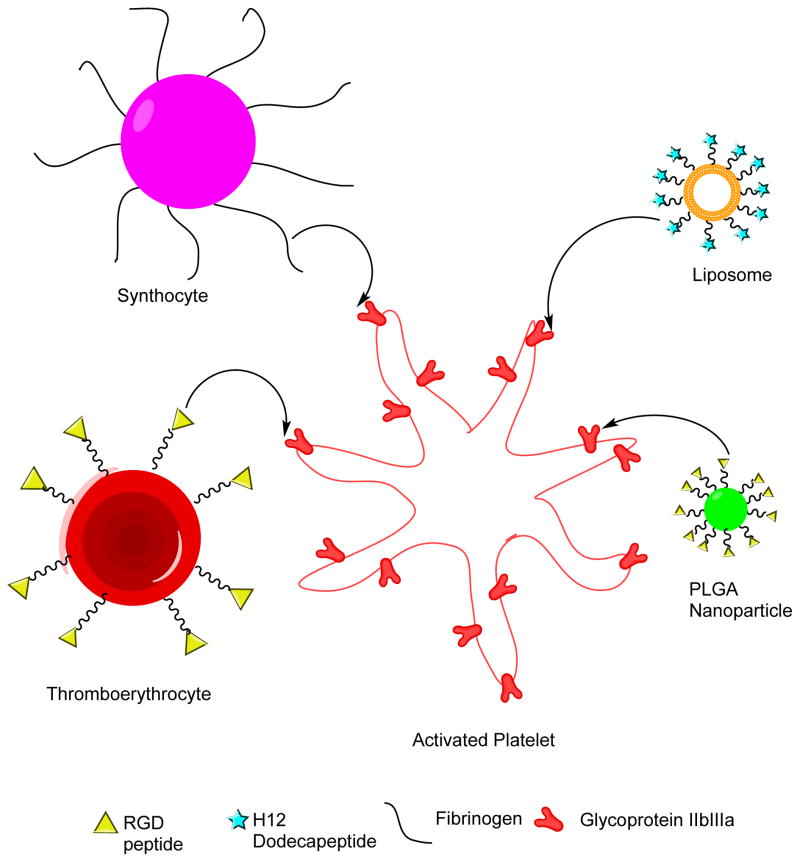
Figure 1.
Summary of Intravenous Hemostat Designs including thromboerythrocytes (Arginine-Glycine-Aspartic Acid (RGD) decorated red blood cell fragements), synthocytes (fibrinogen coated albumin particles), liposomes coated with H12 and PLGA-PEG nanoparticles decorated with RGD.
Table 1.
Summary of experimental substitutes, their efficacy, and references.
| Cell derived substitutes | Results | References: |
|---|---|---|
| Thromboerythrocytes | In vitro demonstration the interaction of activated platelets with RGD linked to red blood cell fragment | 10 |
| Synthocytes | Reduction in bleeding time of ear bleed and surgical incision bleed in thrombocytopenic rabbits | 11 |
| Thrombosomes | Retention of normal expression of GPIIbIIIa and normal aggregation. Reduction in blood loss in ear bleed model of thrombocytopenic rabbits. | 12,13 |
| Drugs: | ||
| Tranexamic Acid | Improves outcomes in battlefield mortality, however there is evidence of risk of diffuse intravascular coagulation. | 14,15 |
| Recombinant Factor VIIa | Reduces bleeding after acute trauma to a small degree, but has potential for adverse effects and is difficult to store appropriately | 16–20 |
| Synthetic substitutes: | ||
| Liposomes | Reduced bleeding time in thrombocytopenic rat and rabbit bleeding models | 21–23 |
| PLGA Nanoparticles | Reduction in bleed time in rat femoral artery injury. Increased survival in rat liver injury model. | 24,25 |
Table 3.
Common hemorrhagic injury models with advantages, disadvantages and example references.
| Model | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| Bleeding time (tail, ear, isolated vessel) *Survival time, rather than bleed time is main outcome |
Simple, easy to perform and measure. Blood loss as well as bleeding time and survival time are common outomes | Limited correlation to survival, and insensitive to certain types of coagulation defects | Mouse 42–44 Rat 22–24,48,50–55 Rabbit 11 Pig 84 Human 72 |
| Liver trauma | Simple, easy to perform, blood loss and survival as main outcomes, potentially better correlation to functional outcomes | May require larger number of animals depending on impact of treatment | Rat 9,56–58 Rabbit 61 Pig 64 Primate 13 |
| Polytrauma | Clinically relevant | Technically difficult, requires trained staff | Pig 41,62,63,65 |
References
- 1.Krug EG, Sharma GK, Lozano R. Am J Public Health. 2000;90:523–526. doi: 10.2105/ajph.90.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kauvar DS, Lefering R, Wade CE. J Trauma Inj Infect Crit Care. 2006;60:S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 3.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, Butler FK, Kotwal RS, Holcomb JB, Wade C, Champion H, Lawnick M, Moores L, Blackbourne LH. J Trauma Acute Care Surg. 2012;73:S431–S437. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- 4.Champion HR, Bellamy RF, Roberts CP, Leppaniemi A. J Trauma. 2003;54:S13–19. doi: 10.1097/01.TA.0000057151.02906.27. [DOI] [PubMed] [Google Scholar]
- 5.Clifford CC. Mil Med. 2004;169:8–10. 4. doi: 10.7205/milmed.169.12s.8. [DOI] [PubMed] [Google Scholar]
- 6.Butler FK. J Trauma. 2010;69(Suppl 1):S10–13. doi: 10.1097/TA.0b013e3181e4220c. [DOI] [PubMed] [Google Scholar]
- 7.Pusateri AE, Delgado AV, Dick EJ, Martinez RS, Holcomb JB, Ryan KL. J Trauma. 2004;57:555–62. doi: 10.1097/01.ta.0000136155.97758.cd. discussion 562. [DOI] [PubMed] [Google Scholar]
- 8.Kheirabadi BS, Acheson EM, Deguzman R, Sondeen JL, Ryan KL, Delgado A, Dick EJ, Jr, Holcomb JB. J Trauma. 2005;59:25–34. doi: 10.1097/01.ta.0000171458.72037.ee. discussion 34–35. [DOI] [PubMed] [Google Scholar]
- 9.Holcomb JB, McClain JM, Pusateri AE, Beall D, Macaitis JM, Harris RA, MacPhee MJ, Hess JR. J Trauma. 2000;49:246–250. doi: 10.1097/00005373-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Coller BS, Springer KT, Beer JH, Mohandas N, Scudder LE, Norton KJ, West SM. J Clin Invest. 1992;89:546–555. doi: 10.1172/JCI115619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levi M, Friederich PW, Middleton S, de Groot PG, Wu YP, Harris R, Biemond BJ, Heijnen HFG, Levin J, ten Cate JW. Nat Med. 1999;5:107–111. doi: 10.1038/4795. [DOI] [PubMed] [Google Scholar]
- 12.Fitzpatrick GM, Cliff R, Tandon N. Transfusion (Paris) 2013;53:100S–106S. doi: 10.1111/trf.12043. [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick G, Vibhudatta A, Agashe H, Dee J. Vox Sang. 2010;99:261–261. [Google Scholar]
- 14.Rappold JF, Pusateri AE. Transfusion (Paris) 2013;53:96S–99S. doi: 10.1111/trf.12042. [DOI] [PubMed] [Google Scholar]
- 15.Pusateri AE, Weiskopf RB, Bebarta V, Butler F, Cestero RF, Chaudry IH, Deal V, Dorlac WC, Gerhardt RT, Given MB, Hansen DR, Hoots WK, Klein HG, Macdonald VW, Mattox KL, Michael RA, Mogford J, Montcalm-Smith EA, Niemeyer DM, Prusaczyk WK, Rappold JF, Rassmussen T, Rentas F, Ross J, Thompson C, Tucker LD, TUDH RR D. S. Committee. Shock. 2013;39:121–126. doi: 10.1097/SHK.0b013e318280409a. [DOI] [PubMed] [Google Scholar]
- 16.Boffard KD, Riou B, Warren B, Choong PI, Rizoli S, Rossaint R, Axelsen M, Kluger Y. The Journal of trauma. 2005;59:8–15. doi: 10.1097/01.ta.0000171453.37949.b7. discussion 15–8. [DOI] [PubMed] [Google Scholar]
- 17.Duchesne JC, Mathew KA, Marr AB, Pinsky MR, Barbeau JM, Mcswain NE. Am Surg. 2008;74:1159–1165. [PubMed] [Google Scholar]
- 18.Morse BC, Dente CJ, Hodgman EI, Shaz BH, Nicholas JM, Wyrzykowski AD, Salomone JP, Vercruysse GA, Rozycki GS, Feliciano DV. Am Surg. 2011;77:1043–1049. [PubMed] [Google Scholar]
- 19.Stein DM, Dutton RP. Curr Opin Crit Care. 2004:10. doi: 10.1097/01.ccx.0000144770.96342.65. [DOI] [PubMed] [Google Scholar]
- 20.Martinowitz U, Zaarur M, Yaron BL, Blumenfeld A, Martonovits G. Mil Med. 2004;169:16–18. 4. doi: 10.7205/milmed.169.12s.16. [DOI] [PubMed] [Google Scholar]
- 21.Okamura Y, Eto K, Maruyama H, Handa M, Ikeda Y, Takeoka S. Nanomed. 2010;6:391–6. doi: 10.1016/j.nano.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Okamura Y, Takeoka S, Teramura Y, Maruyama H, Tsuchida E, Handa M, Ikeda Y. Transfusion (Paris) 2005;45:1221–1228. doi: 10.1111/j.1537-2995.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- 23.Okamura Y, Takeoka S, Eto K, Maekawa I, Fujie T, Maruyama H, Ikeda Y, Handa M. J Thromb Haemost. 2009;7:470–477. doi: 10.1111/j.1538-7836.2008.03269.x. [DOI] [PubMed] [Google Scholar]
- 24.Bertram JP, Williams CA, Robinson R, Segal SS, Flynn NT, Lavik EB. Sci Transl Med. 2009;1:11ra22. doi: 10.1126/scitranslmed.3000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shoffstall AJ, Atkins KT, Groynom RE, Varley ME, Everhart LM, Lashof-Sullivan MM, Martyn-Dow B, Butler RS, Ustin JS, Lavik EB. Biomacromolecules. 2012;13:3850–3857. doi: 10.1021/bm3013023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michelson AD. Platelets. 2. Academic Press/Elsevier; Amsterdam; Boston: 2007. [Google Scholar]
- 27.Guyton AC, Hall JE. Textbook of medical physiology. 11. Elsevier Saunders; Philadelphia: 2006. [Google Scholar]
- 28.Nievelstein PF, de Groot PG. Haemostasis. 1988;18:342–59. doi: 10.1159/000215816. [DOI] [PubMed] [Google Scholar]
- 29.Nievelstein PF, D’Alessio PA, Sixma JJ. Arteriosclerosis. 1988;8:200–6. doi: 10.1161/01.atv.8.2.200. [DOI] [PubMed] [Google Scholar]
- 30.Nievelstein PF, Sixma JJ. Blood. 1988;72:82–8. [PubMed] [Google Scholar]
- 31.Hindriks G, Ijsseldijk MJ, Sonnenberg A, Sixma JJ, de Groot PG. Blood. 1992;79:928–35. [PubMed] [Google Scholar]
- 32.Alevriadou BR, Moake JL, Turner NA, Ruggeri ZM, Folie BJ, Phillips MD, Schreiber AB, Hrinda ME, McIntire LV. Blood. 1993;81:1263–76. [PubMed] [Google Scholar]
- 33.Andrieux A, Hudry-Clergeon G, Ryckewaert JJ, Chapel A, Ginsberg MH, Plow EF, Marguerie G. The Journal of biological chemistry. 1989;264:9258–65. [PubMed] [Google Scholar]
- 34.Lam SC, Plow EF, Smith MA, Andrieux A, Ryckwaert JJ, Marguerie G, Ginsberg MH. The Journal of biological chemistry. 1987;262:947–50. [PubMed] [Google Scholar]
- 35.Weisel JW, Nagaswami C, Vilaire G, Bennett JS. The Journal of biological chemistry. 1992;267:16637–43. [PubMed] [Google Scholar]
- 36.Beer JH, Springer KT, Coller BS. Blood. 1992;79:117–128. [PubMed] [Google Scholar]
- 37.Ilium L, Davis SS, Wilson CG, Thomas NW, Frier M, Hardy JG. Int J Pharm. 1982;12:135–146. [Google Scholar]
- 38.Blajchman Ma. Vox Sang. 1998;74:315–319. doi: 10.1111/j.1423-0410.1998.tb05437.x. [DOI] [PubMed] [Google Scholar]
- 39.Morrison JJ, Rasmussen TE. Recent Adv Future Dir Trauma Care. 2012;92:843–858. [Google Scholar]
- 40.Patanwala AE. Am J Health Syst Pharm. 2008;65:1616–1623. doi: 10.2146/ajhp080008. [DOI] [PubMed] [Google Scholar]
- 41.Frink M, Andruszkow H, Zeckey C, Krettek C, Hildebrand F. J Biomed Biotechnol. 2011;2011:1–15. doi: 10.1155/2011/797383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greene TK, Schiviz A, Hoellriegl W, Poncz M, Muchitsch EM. Journal of thrombosis and haemostasis_: JTH. 2010;8:2820–2. doi: 10.1111/j.1538-7836.2010.04084.x. [DOI] [PubMed] [Google Scholar]
- 43.Broze GJ, Yin ZF, Lasky N. Thrombosis and haemostasis. 2001;85:747–8. [PubMed] [Google Scholar]
- 44.Modery-Pawlowski CL, Tian LL, Ravikumar M, Wong TL, Gupta AS. Biomaterials. 2013 doi: 10.1016/j.biomaterials.2012.12.045. [DOI] [PubMed] [Google Scholar]
- 45.Siller-Matula JM, Plasenzotti R, Spiel A, Quehenberger P, Jilma B. Thrombosis and haemostasis. 2008;100:397–404. [PubMed] [Google Scholar]
- 46.Boxenbaum H. Journal of pharmacokinetics and biopharmaceutics. 1980;8:165–76. doi: 10.1007/BF01065191. [DOI] [PubMed] [Google Scholar]
- 47.Rao GHR. Handbook of platelet physiology and pharmacology. Kluwer Academic; Boston: 1999. [Google Scholar]
- 48.Dejana E, Quintana A, Callioni A, de Gaetano G. Thrombosis research. 1979;15:199–207. doi: 10.1016/0049-3848(79)90065-3. [DOI] [PubMed] [Google Scholar]
- 49.Langleben D, Moroz LA. Proc Soc Exp Biol Med. 1991;196:270–2. doi: 10.3181/00379727-196-43187. [DOI] [PubMed] [Google Scholar]
- 50.De Clerck F, Goossens J, Reneman R. Thrombosis research. 1976;8:179–93. doi: 10.1016/0049-3848(76)90261-9. [DOI] [PubMed] [Google Scholar]
- 51.Johansen PB, Henriksen L, Andresen PR, Lauritzen B, Jensen KL, Juhl TN, Tranholm M. Thrombosis and haemostasis. 2008;99:956–62. doi: 10.1160/TH07-12-0738. [DOI] [PubMed] [Google Scholar]
- 52.Hui YH, Huang NH, Ebbert L, Bina H, Chiang A, Maples C, Pritt M, Kern T, Patel N. Journal of pharmacological and toxicological methods. 2007;56:256–64. doi: 10.1016/j.vascn.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Buczko W, Gambino MC, De Gaetano G. European journal of pharmacology. 1984;103:261–8. doi: 10.1016/0014-2999(84)90486-2. [DOI] [PubMed] [Google Scholar]
- 54.Shek PN, Howe SA. Journal of immunological methods. 1982;53:255–60. doi: 10.1016/0022-1759(82)90148-x. [DOI] [PubMed] [Google Scholar]
- 55.Butler KD, Maguire ED, Smith JR, Turnbull AA, Wallis RB, White AM. Thrombosis and haemostasis. 1982;47:46–9. [PubMed] [Google Scholar]
- 56.Matsuoka T, Hildreth J, Wisner DH. J Trauma. 1995;39:674–680. doi: 10.1097/00005373-199510000-00010. [DOI] [PubMed] [Google Scholar]
- 57.Matsuoka T, Wisner DH. The Journal of trauma. 1996;41:439–45. doi: 10.1097/00005373-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 58.Ryan KL, Cortez DS, Dick EJ, Jr, Pusateri AE. Resuscitation. 2006;70:133–144. doi: 10.1016/j.resuscitation.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 59.Szczepanska-Sadowska E, Noszczyk B, Lon S, Stepniakowski K, Budzikowski A, Paczwa P. Ann N Acad Sci. 1993;689:677–9. doi: 10.1111/j.1749-6632.1993.tb55626.x. [DOI] [PubMed] [Google Scholar]
- 60.Kinlough-Rathbone RL, Rand ML, Packham MA. Blood. 1993;82:103–106. [PubMed] [Google Scholar]
- 61.Nishikawa K, Hagisawa K, Kinoshita M, Shono S, Katsuno S, Doi M, Yanagawa R, Suzuki H, Iwaya K, Saitoh D, Sakamoto T, Seki S, Takeoka S, Handa M. Journal of thrombosis and haemostasis_: JTH. 2012;10:2137–48. doi: 10.1111/j.1538-7836.2012.04889.x. [DOI] [PubMed] [Google Scholar]
- 62.Alam HB, Bice LM, Butt MU, Cho SD, Dubick MA, Duggan M, Englehart MS, Holcomb JB, Morris MS, Prince MD, Schreiber MA, Shults C, Sondeen JL, Tabbara M, Tieu BH, Underwood SA. The Journal of trauma. 2009;67:856–64. doi: 10.1097/TA.0b013e3181b5ae75. [DOI] [PubMed] [Google Scholar]
- 63.Alam HB, Hamwi KB, Duggan M, Fikry K, Lu J, Fukudome EY, Chong W, Bramos A, Kim K, Velmahos G. The Journal of trauma. 2011;70:636–45. doi: 10.1097/TA.0b013e31820d0dcc. [DOI] [PubMed] [Google Scholar]
- 64.Gurney J, Philbin N, Rice J, Arnaud F, Dong F, Wulster-Radcliffe M, Pearce LB, Kaplan L, McCarron R, Freilich D. The Journal of trauma. 2004;57:726–38. doi: 10.1097/01.ta.0000147520.84792.b4. [DOI] [PubMed] [Google Scholar]
- 65.Velmahos GC, Spaniolas K, Duggan M, Alam HB, Tabbara M, de Moya M, Vosburgh K. The Journal of trauma. 2007;63:285–8. doi: 10.1097/TA.0b013e3180d0a6ea. discussion 288–90. [DOI] [PubMed] [Google Scholar]
- 66.Markwardt F, Nowak G, Glusa E, Hoffman A. Thrombosis research. 1979;15:79–88. doi: 10.1016/0049-3848(79)90054-9. [DOI] [PubMed] [Google Scholar]
- 67.Blajchman MA, Lee DH. Transfusion medicine reviews. 1997;11:95–105. doi: 10.1053/tm.1997.0110095. [DOI] [PubMed] [Google Scholar]
- 68.Blajchman MA, Senyi AF, Hirsh J, Genton E, George JN. The Journal of clinical investigation. 1981;68:1289–94. doi: 10.1172/JCI110375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blajchman MA, Bordin JO, Bardossy L, Heddle NM. British journal of haematology. 1994;86:347–50. doi: 10.1111/j.1365-2141.1994.tb04737.x. [DOI] [PubMed] [Google Scholar]
- 70.Blajchman MA, Senyi AF, Hirsh J, Genton E, George JN. The Journal of clinical investigation. 1981;68:1289–94. doi: 10.1172/JCI110375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buchanan MR, Blajchman MA, Dejana E, Mustard JF, Senyi AF, Hirsh J. Prostaglandins and medicine. 1979;3:333–42. doi: 10.1016/0161-4630(79)90026-0. [DOI] [PubMed] [Google Scholar]
- 72.Lucas ON. J Can Dent Assoc Tor. 1966;32:599–602. [PubMed] [Google Scholar]
- 73.Dong F, Hall CH, Golech SA, Philbin NB, Rice JP, Gurney J, Arnaud FG, Hammett M, Ma X, Flournoy WS, Hong J, Kaplan LJ, Pearce LB, McGwin G, Ahlers S, McCarron R, Freilich D. Shock. 2006;25:50–5. doi: 10.1097/01.shk.0000187982.56030.94. [DOI] [PubMed] [Google Scholar]
- 74.Philbin N, Rice J, Gurney J, McGwin G, Arnaud F, Dong F, Johnson T, Flournoy WS, Ahlers S, Pearce LB, McCarron R, Freilich D. Resuscitation. 2005;66:367–78. doi: 10.1016/j.resuscitation.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 75.Sava J, Velmahos GC, Karaiskakis M, Kirkman P, Toutouzas K, Sarkisyan G, Chan L, Demetriades D. The Journal of trauma. 2003;54:590–4. doi: 10.1097/01.TA.0000056162.86054.00. [DOI] [PubMed] [Google Scholar]
- 76.Jaskille A, Schechner A, Park K, Williams M, Wang D, Sava J. The Journal of trauma. 2005;59:1305–8. doi: 10.1097/01.ta.0000198374.16218.ca. discussion 1308. [DOI] [PubMed] [Google Scholar]
- 77.Szebeni J, Bedőcs P, Csukás D, Rosivall L, Bünger R, Urbanics R. Nanotoxicity Bench Bedside. 2012;64:1706–1716. doi: 10.1016/j.addr.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 78.Szebeni J, Bedocs P, Urbanics R, Bunger R, Rosivall L, Toth M, Barenholz Y. J Controlled Release. 2012;160:382–387. doi: 10.1016/j.jconrel.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 79.Johnson RA, Simmons KT, Fast JP, Schroeder CA, Pearce RA, Albrecht RM, Mecozzi S. J Pharm Sci. 2011;100:2685–2692. doi: 10.1002/jps.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Szebeni J, Bedocs P, Csukas D, Rosivall L, Bunger R, Urbanics R. Advanced drug delivery reviews. 2012;64:1706–16. doi: 10.1016/j.addr.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 81.Deitch EA, Feketeova E, Adams JM, Forsythe RM, Xu DZ, Itagaki K, Redl H. Shock. 2006;25:460–3. doi: 10.1097/01.shk.0000209551.88215.1e. [DOI] [PubMed] [Google Scholar]
- 82.Deitch EA, Forsythe R, Anjaria D, Livingston DH, Lu Q, Xu DZ, Redl H. Shock. 2004;22:221–8. doi: 10.1097/01.shk.0000133592.55400.83. [DOI] [PubMed] [Google Scholar]
- 83.Miller DL, Rayner AA, Girton M, Doppman JL. Investigative radiology. 1985;20:68–72. doi: 10.1097/00004424-198501000-00018. [DOI] [PubMed] [Google Scholar]
- 84.Clay JG, Grayson JK, Zierold D. Mil Med. 2010;175:280–284. doi: 10.7205/milmed-d-09-00185. [DOI] [PubMed] [Google Scholar]
- 85.Lynch I, Dawson KA. Nano Today. 2008;3:40–47. [Google Scholar]
- 86.Mahmoudi M, Lynch I, Ejtehadi MR, Monopoli MP, Bombelli FB, Laurent S. Chem Rev. 2011;111:5610–5637. doi: 10.1021/cr100440g. [DOI] [PubMed] [Google Scholar]
- 87.Gref R, Lück M, Quellec P, Marchand M, Dellacherie E, Harnisch S, Blunk T, Müller R. Colloids Surf B Biointerfaces. 2000;18:301–313. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 88.Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Adv Drug Deliv Rev. 2006;58:1688–1713. doi: 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 89.Demetzos C. J Liposome Res. 2008;18:159–173. doi: 10.1080/08982100802310261. [DOI] [PubMed] [Google Scholar]
- 90.Crommelin DJA, van Bommel EMG. Pharm Res. 1984;1:159–163. doi: 10.1023/A:1016344523988. [DOI] [PubMed] [Google Scholar]
- 91.Shin EJ, Jones AE, Waymouth RM. Macromolecules. 2012;45:595–598. [Google Scholar]
- 92.Tsuji H. Macromol Biosci. 2005;5:569–597. doi: 10.1002/mabi.200500062. [DOI] [PubMed] [Google Scholar]
- 93.D’Addio SM, Kafka C, Akbulut M, Beattie P, Saad W, Herrera M, Kennedy MT, Prud’homme RK. Molecular pharmaceutics. 2010;7:557–64. doi: 10.1021/mp900260q. [DOI] [PubMed] [Google Scholar]
- 94.Richards AB, Krakowka S, Dexter LB, Schmid H, Wolterbeek AP, Waalkens-Berendsen DH, Shigoyuki A, Kurimoto M. Food and chemical toxicology_: an international journal published for the British Industrial Biological Research Association. 2002;40:871–98. doi: 10.1016/s0278-6915(02)00011-x. [DOI] [PubMed] [Google Scholar]
- 95.Varma RK, Kaushal R, Junnarkar AY, Thomas GP, Naidu MU, Singh PP, Tripathi RM, Shridhar DR. Arzneimittel-Forschung. 1985;35:804–8. [PubMed] [Google Scholar]
- 96.Fairbairn L, Kapetanovic R, Sester D, Hume D. J Leukoc Biol. 2011:89. doi: 10.1189/jlb.1110607. [DOI] [PubMed] [Google Scholar]
- 97.Chanan-Khan A, Szebeni J, Savay S, Liebes L, Rafique NM, Alving CR, Muggia FM. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2003;14:1430–1437. doi: 10.1093/annonc/mdg374. [DOI] [PubMed] [Google Scholar]
- 98.Szebeni J, Bedőcs P, Rozsnyay Z, Weiszhár Z, Urbanics R, Rosivall L, Cohen R, Garbuzenko O, Báthori G, Tóth M, Bünger R, Barenholz Y. Nanomedicine Nanotechnol Biol Med. 2012;8:176–184. doi: 10.1016/j.nano.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 99.Marjan J, Xie Z, Devine DV. Biochim Biophys Acta BBA - Biomembr. 1994;1192:35–44. doi: 10.1016/0005-2736(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 100.Simmons K. PhD Thesis. The University of Wisconson; Madison: 2012. [Google Scholar]