Abstract
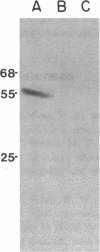
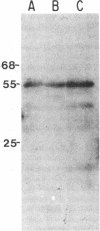
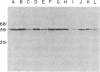
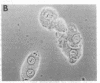
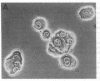
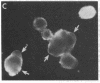
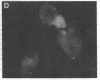
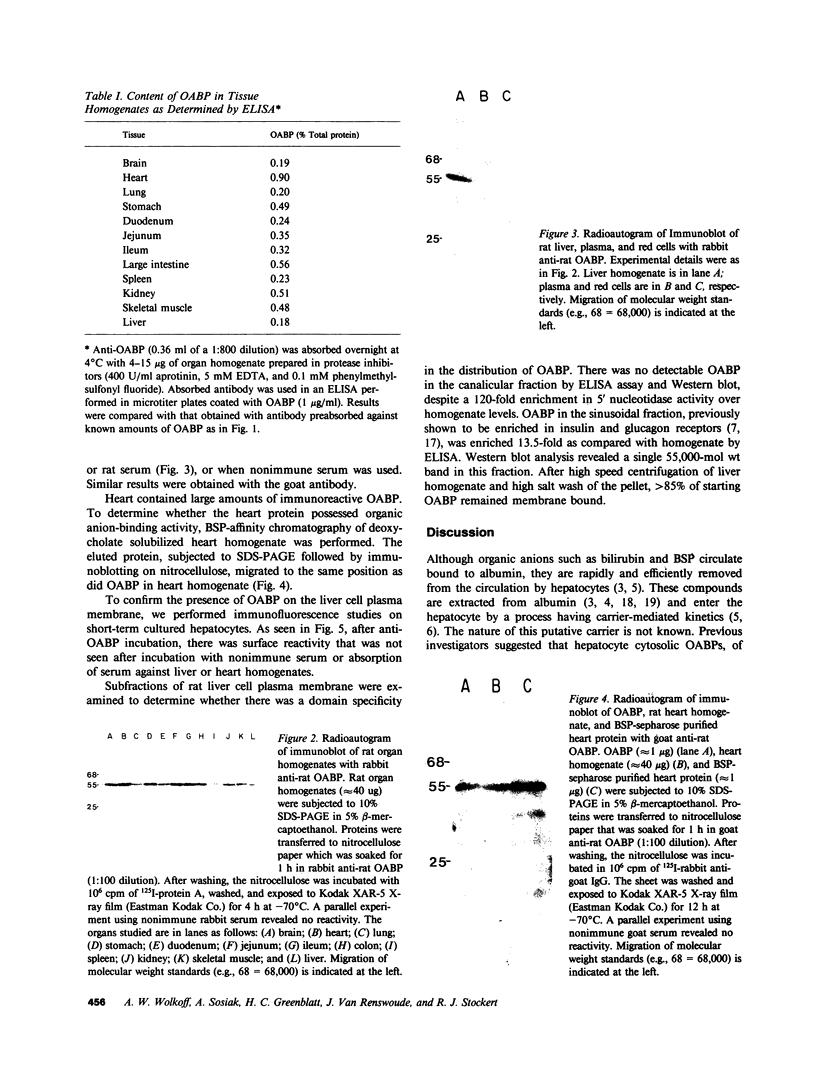
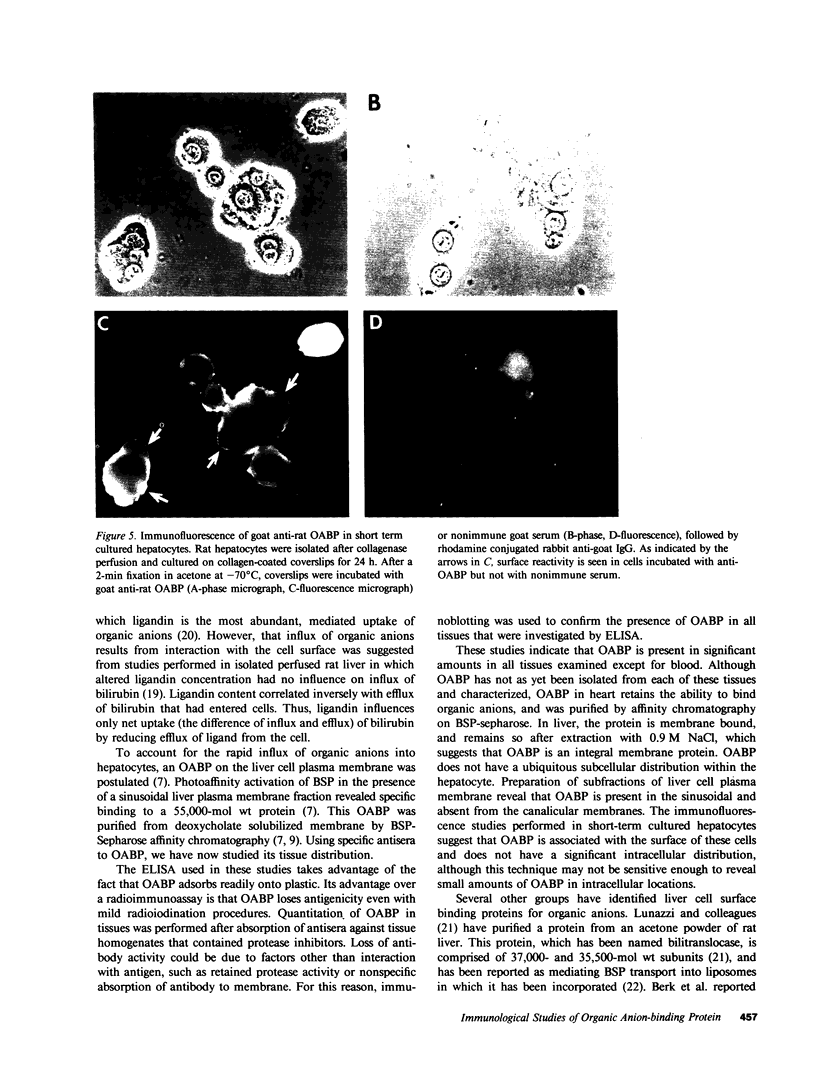
The mechanism of organic anion uptake by hepatocytes has kinetics that suggest facilitated diffusion, and carrier-mediated membrane transport has been postulated. In previous studies, we purified a 55,000-mol wt organic anion-binding protein (OABP) by affinity chromatography on sulfobromophthalein (BSP)-Sepharose of deoxycholate solubilized liver cell plasma membrane preparations. Using specific goat and rabbit antibodies to OABP, we have now investigated the distribution of this protein in liver fractions and other tissues by an enzyme-linked immunosorbent assay and by the immunoblot (Western blot) procedure. These studies indicated that OABP is present in significant amounts in all tissues examined except for blood. Although OABP has not as yet been isolated from each of these tissues and characterized, OABP in heart retained the ability to bind organic anions, and was purified by affinity chromatography on BSP-sepharose. In liver, OABP was membrane bound and remained so after extraction with 0.9 M NaCl, which suggests that it is an intrinsic membrane protein. OABP did not have a ubiquitous subcellular distribution within the hepatocyte. Preparation of subfractions of liver cell plasma membrane revealed that OABP is present in the sinusoidal and absent from the canalicular membrane. Immunofluorescence studies performed in short-term cultured hepatocytes suggest that OABP is associated with the surface of these cells and does not have a significant intracellular distribution.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker K. J., Bradley S. E. Binding of sulfobromophthalein (BSP) sodium by plasma albumin. Its role in hepatic BSP extraction. J Clin Invest. 1966 Feb;45(2):281–287. doi: 10.1172/JCI105341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Evans W. H., Bergeron J. J., Geschwind I. I. Distribution of insulin receptor sites among liver plasma membrane subfractions. FEBS Lett. 1973 Aug 15;34(2):259–262. doi: 10.1016/0014-5793(73)80807-5. [DOI] [PubMed] [Google Scholar]
- Fleischner G., Robbins J., Arias I. M. Immunological studies of Y protein. A major cytoplasmic organic anion-binding protein in rat liver. J Clin Invest. 1972 Mar;51(3):677–684. doi: 10.1172/JCI106856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORESKY C. A. A linear method for determining liver sinusoidal and extravascular volumes. Am J Physiol. 1963 Apr;204:626–640. doi: 10.1152/ajplegacy.1963.204.4.626. [DOI] [PubMed] [Google Scholar]
- GORESKY C. A. THE HEPATIC UPTAKE AND EXCRETION OF SULFOBROMOPHTHALEIN AND BILIRUBIN. Can Med Assoc J. 1965 Apr 17;92:851–857. [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R. D., Stroupe S. D. Kinetics and mechanism of bilirubin binding to human serum albumin. J Biol Chem. 1978 Jun 25;253(12):4370–4377. [PubMed] [Google Scholar]
- Kramer W., Burckhardt G., Wilson F. A., Kurz G. Bile salt-binding polypeptides in brush-border membrane vesicles from rat small intestine revealed by photoaffinity labeling. J Biol Chem. 1983 Mar 25;258(6):3623–3627. [PubMed] [Google Scholar]
- Lunazzi G., Tiribelli C., Gazzin B., Sottocasa G. Further studies on bilitranslocase, a plasma membrane protein involved in hepatic organic anion uptake. Biochim Biophys Acta. 1982 Feb 23;685(2):117–122. doi: 10.1016/0005-2736(82)90087-6. [DOI] [PubMed] [Google Scholar]
- Notermans S., Hagenaars A. M., Kozaki S. The enzyme-linked immunosorbent assay (ELISA) for the detection and determination of Clostridium botulinum toxins A, B, and E. Methods Enzymol. 1982;84:223–238. doi: 10.1016/0076-6879(82)84020-2. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Paumgartner G., Reichen J. Kinetics of hepatic uptake of unconjugated bilirubin. Clin Sci Mol Med. 1976 Aug;51(2):169–176. doi: 10.1042/cs0510169. [DOI] [PubMed] [Google Scholar]
- Petzinger E., Joppen C., Frimmer M. Common properties of hepatocellular uptake of cholate, iodipamide and antamanide, as distinct from the uptake of bromosulfophthalein. Naunyn Schmiedebergs Arch Pharmacol. 1983 Mar;322(2):174–179. doi: 10.1007/BF00512393. [DOI] [PubMed] [Google Scholar]
- Reichen J., Berk P. D. Isolation of an organic anion binding protein from rat liver plasma membrane fractions by affinity chromatography. Biochem Biophys Res Commun. 1979 Nov 28;91(2):484–489. doi: 10.1016/0006-291x(79)91547-x. [DOI] [PubMed] [Google Scholar]
- Scharschmidt B. F., Waggoner J. G., Berk P. D. Hepatic organic anion uptake in the rat. J Clin Invest. 1975 Nov;56(5):1280–1292. doi: 10.1172/JCI108204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Baldini G., Sandri G., Lunazzi G., Tiribelli C. Reconstitution in vitro of sulfobromophthalein transport by bilitranslocase. Biochim Biophys Acta. 1982 Feb 23;685(2):123–128. doi: 10.1016/0005-2736(82)90088-8. [DOI] [PubMed] [Google Scholar]
- Stollman Y. R., Gärtner U., Theilmann L., Ohmi N., Wolkoff A. W. Hepatic bilirubin uptake in the isolated perfused rat liver is not facilitated by albumin binding. J Clin Invest. 1983 Aug;72(2):718–723. doi: 10.1172/JCI111021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel W., Gerber M. A., Glezerov V., Thung S. N., Kochwa S., Berk P. D. Physicochemical and immunohistological studies of a sulfobromophthalein- and bilirubin-binding protein from rat liver plasma membranes. J Clin Invest. 1983 Jun;71(6):1796–1805. doi: 10.1172/JCI110935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilmann L., Stollman Y. R., Arias I. M., Wolkoff A. W. Does Z-protein have a role in transport of bilirubin and bromosulfophthalein by isolated perfused rat liver? Hepatology. 1984 Sep-Oct;4(5):923–926. doi: 10.1002/hep.1840040523. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland T., Nassal M., Kramer W., Fricker G., Bickel U., Kurz G. Identity of hepatic membrane transport systems for bile salts, phalloidin, and antamanide by photoaffinity labeling. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5232–5236. doi: 10.1073/pnas.81.16.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Functional polarity of the rat hepatocyte surface membrane. Isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-Canalicular and contiguous surfaces of the hepatocyte. Biochem J. 1975 Feb;146(2):375–388. doi: 10.1042/bj1460375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse E., Knook D. L. The investigation of sinusoidal cells: a new approach to the study of liver function. Prog Liver Dis. 1979;6:153–171. [PubMed] [Google Scholar]
- Wolkoff A. W., Bhargava M. M., Chung C., Gatmaitan Z. Purification of ligandin by affinity chromatography on sulfobromophthalein-agarose gel. Proc Soc Exp Biol Med. 1979 Feb;160(2):150–153. doi: 10.3181/00379727-160-40408. [DOI] [PubMed] [Google Scholar]
- Wolkoff A. W., Chung C. T. Identification, purification, and partial characterization of an organic anion binding protein from rat liver cell plasma membrane. J Clin Invest. 1980 May;65(5):1152–1161. doi: 10.1172/JCI109770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkoff A. W., Goresky C. A., Sellin J., Gatmaitan Z., Arias I. M. Role of ligandin in transfer of bilirubin from plasma into liver. Am J Physiol. 1979 Jun;236(6):E638–E648. doi: 10.1152/ajpendo.1979.236.6.E638. [DOI] [PubMed] [Google Scholar]
- Wolkoff A. W., Klausner R. D., Ashwell G., Harford J. Intracellular segregation of asialoglycoproteins and their receptor: a prelysosomal event subsequent to dissociation of the ligand-receptor complex. J Cell Biol. 1984 Feb;98(2):375–381. doi: 10.1083/jcb.98.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler K., Frimmer M., Fasold H. Further characterization of membrane proteins involved in the transport of organic anions in hepatocytes. Comparison of two different affinity labels: 4,4'-diisothiocyano-1,2-diphenylethane-2,2'-disulfonic acid and brominated taurodehydrocholic acid. Biochim Biophys Acta. 1984 Jan 11;769(1):117–129. doi: 10.1016/0005-2736(84)90015-4. [DOI] [PubMed] [Google Scholar]
- von Dippe P., Levy D. Characterization of the bile acid transport system in normal and transformed hepatocytes. Photoaffinity labeling of the taurocholate carrier protein. J Biol Chem. 1983 Jul 25;258(14):8896–8901. [PubMed] [Google Scholar]