Abstract
Functional microbial amyloids are ubiquitous in nature and some contribute to the pathogenesis of infectious diseases. Three pathogenic microbial amyloids are compared and their contribution to the disease process explained. The recent demonstration and visualization of fungal amyloid in human invasive candidiasis is discussed. Moreover, the binding of host serum amyloid P component to Candida functional amyloid in invasive human disease is presented in light of its possible role of masking fungi from the host defenses.
Keywords: Amyloid, Functional amyloid, Fungal amyloid, Serum amyloid P component, Candidiasis, Candida albicans, Candida Als proteins, merozoite surface protein 2, curli, microbial adherence
Amyloid is a term ordinarily used to describe protein formed from spontaneously self-propagating, insoluble, β-sheet rich fibrils. These fibrils are resistant to enzymatic digestion, have characteristic patterns observed through electron microscopy, and special tinctorial properties such as staining with the dyes, Congo red and thioflavin-T. Although amyloid fibers have been associated with disease, it is now apparent the presence of amyloid is not always pathological. Amyloidoses are diseases in which amyloid deposits accumulate extracellularly and disrupt the structure and function of tissues and organs. The most common is AL amyloidosis caused by monoclonal immunoglobulin light chain deposition in the glomeruli of kidneys of patients with various plasma cell dyscrasias. One amyloidosis, AA, occurs in response to chronic inflammatory disorders including such infectious diseases as tuberculosis and chronic bacterial osteomyelitis. Examples of pathological amyloid deposits, but not considered amyloidoses, include Aβ-amyloid found in the plaques of Alzheimer's disease and prions found in the spongiform encephalopathies. These disease states are often characterized by protein misfolding that exposes amyloid-forming properties.
The structure of amyloid assemblies can lead to functional properties. Amyloid's tensile strength and resistance to degradation are advantageous in nanotechnology where it is used to manufacture tissue scaffolding, nanowires and nanotubes, (1). Natural amyloids include fibrils in skin cell melanosomes that impart a characteristic ultrastructure to the organelle, as seen on electron microscopy and are necessary for proper assembly and deposition of melanin (2).
Microbes elaborate amyloids that are used to fasten the microorganisms to a substratum. They are ubiquitous in nature and are important components of microbial biofilms (3, 4). Since microbial amyloids perform a beneficial function for the microorganism they are referred to as “functional amyloids” (5). These fibrils serve to attach a microbe to a substratum and thus, secure a survival advantage for the microorganism. Some functional amyloids attach microbes to inanimate surfaces, others to host cells, still others attach microbes to one another and some amyloids serve to stabilize the biofilm during infection (6). We will briefly discuss three microbial functional amyloid proteins presumed to be integral to the pathogenesis of disease in humans. These are, the curli protein of Escherichia coli, merozoite surface protein 2 (MSPII) of Plasmodium falciparum merozoites and the Als cell surface adhesins of Candida albicans.
Three Pathogenic Amyloid adhesins of microbes
Curli
Pathogenic Escherichia coli strains responsible for acute diarrheal diseases express multiple adhesins or attachment proteins including fimbriae which are long, hair-like appendages (microns in length) and short, amyloid fibrils known as curli. Fimbriae are resilient fibrils composed of repeating units of amino acids and the length of the assembled fiber provides for long range interactions with substrata. They can bend and resist torsion and stretch to 5 times their normal length. Fimbriae are important in colonization of a surface, e.g., the intestinal wall (Table 1). Curli proteins are highly hydrophobic, attached to the cell membrane and contribute not only to adherence to tissue, but also attachment of bacterium to other bacteria, i.e., cell-to-cell aggregation which is critical for biofilm formation. Curli are found throughout Enterobacteriaciae and in some species such as E.coli and Salmonella spp., are important in the pathogenesis of disease. In murine models, enterohemorrhagic E. coli express curli in order to insure cell-to-cell aggregation and adherence to intestinal cells. Curli are the product of several proteins. One subunit attaches to the outer cell membrane and then nucleates another protein on top of itself, a process that continues one after another to form a fibril (3). It is interesting to note that some secreted proteins of E. coli form amyloid ropes (> cm in length) that demonstrate characteristics of amyloid, i.e., the ability to self-propagate (7).
Table 1.
Characteristics of three microbial cell surface amyloids that mediate adherence of the microbe to human tissue and cells. These amyloids are all expressed under physiological conditions. GPI: glycosylphosphatidyl inositol anchor.
| Characteristic | E. coli curli | P. falciparum MSPII | C. albicans Als proteins |
|---|---|---|---|
| Mol. Weight | polymers of ∼15kDa protein | 30 kDA | heavily glycosylated, >200 kD |
| GPI cell surface anchor? | no | yes | yes |
| Location of amyloid | entire structure is attached to the outer cell membrane | N-terminus of the protein | a threonine-rich repeat region adjacent to N-terminal immunoglobulin region |
| Agglutination or Aggregation of microbes? | yes | not known | yes |
| Amyloid fibers demonstrated? | yes | yes | yes |
| Attachment targets | host proteins, other E. coli | possibly glycophorin | peptides/proteins of other C. albicans or host tissue |
| Vaccine candidate | antibodies to curli can be detected in patients with disease (Bian et al. 2000) | yes, one of several surface proteins being included in potential vaccines | yes (Edwards 2012) |
Merozoite surface protein 2 (MSP2)
The most lethal form of malaria is caused by Plasmodium falciparum, which stands out from other less virulent species of malaria by reason of its ability to parasitize a high percentage of red blood cells. The stage of the parasite that attaches and enters the red blood cell is the merozoite. It is released initially from the liver, enters red blood cells and ultimately ruptures the cell. The parasites attach and enter the red blood cell in order to obtain their food source, hemoglobin. The initial contact of a recently released merozoite and red blood cell is thought to occur by random collision. On the surface coat of P. falciparum merozoites are numerous proteins that extend out from the parasite surface like small knobs (8). There are number of merozoite surface proteins believed to be important in the adherence of the parasite to the red blood cell (several of these proteins are being incorporated into vaccines). MSP2 is one of these surface proteins and is unique in that it contains a functional amyloid in the N-terminus and is likely important in attachment of the merozoite to the red blood cell (9) (Table 1). The amyloid fibrils of MSP2 are resistant to proteinase, stain with Congo red and are formed under physiologic conditions, all characteristics of amyloid fibers (10). After attachment of the merozoite to the red blood cell, the parasite reorients on the cell surface and, using different adhesins, penetrates the cell. Once it is intracellular the merozoite is free to digest hemoglobin.
Als proteins of Candida albicans
There are 8 different Als proteins in C. albicans located on the cell surface of yeasts and hyphae of this opportunistic pathogen. Although curli and MSP2 proteins appear to be required for pathogenesis of disease in the human, the intended purpose of Als proteins in C. albicans is likely to serve a social function, i.e., aggregating fungal cells to another as this is the preferred phenotype in nature. Candida albicans is considered a commensal of human mucous surfaces. However, if host defenses are compromised the Als proteins can mediate adherence of the fungus to peptides and proteins the fungus does not ordinarily encounter, e.g., human endothelium or urothelial cells. The best studied of the Als proteins, Als5p when attached to the surface of non-pathogenic Saccharyomyces cerevisiae (baker's yeast) has been used to model the functions of the different regions of the Als proteins. The protein is connected to the cell wall by a GPI anchor where a C-terminal Stalk region raises the protein above the cell surface (Figure 1). The Stalk is followed by multiple 36-amino acid Tandem Repeats (the number of repeats varies in each protein), which are instrumental in cell-to-cell aggregation. A Threonine-Rich domain forms a connection between the Tandem Repeats, and the N-terminal Immunoglobulin region binds to various peptides exposed on host cells and fungal cells (Figure 1). The Threonine-Rich domain is formed of β-sheets that form amyloid fibrils. In addition to amyloid formation this region contributes to the adherence process by stabilizing adherence. It is characteristic of C. albicans adherence and aggregation that it is very resistant to physical and chemical force, for example, 8M urea and formamide are required to undue adherence (as they are to break apart amyloid). We have termed the Als proteins the “perfect adhesive”: binding to a wide variety of protein targets with almost unbreakable adhesion even in the face of shear forces (11). These cell surface proteins likely contribute to the phenotypic characteristics of the fungus (Table 2).
Figure 1.
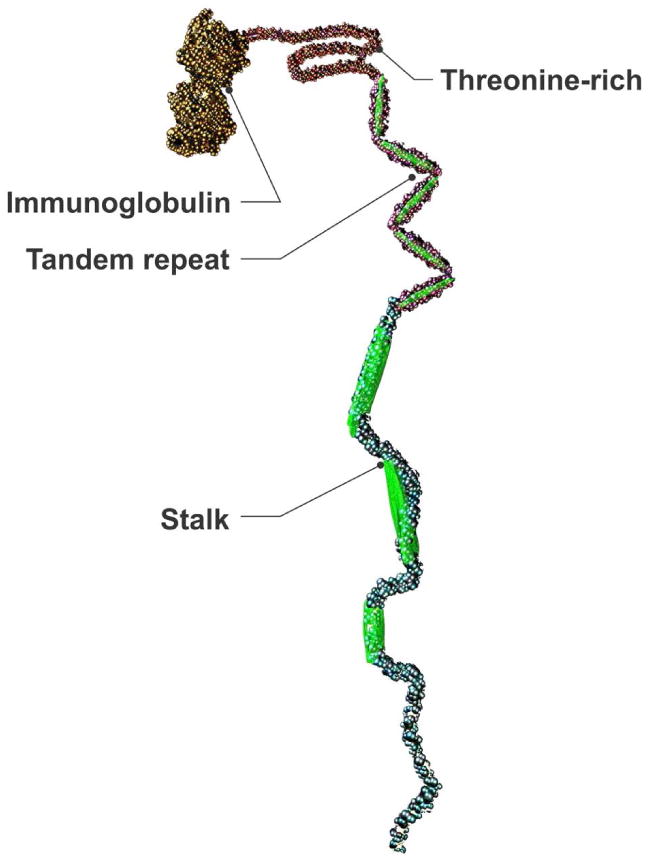
Schematic representation of Candida albicans Als cell surface prtotein.
Table 2.
Cell surface functional amyloids of Candida albicans influence cellular phenotypic characteristics of the fungus.
| Characteristics of Amyloids | Does this apply to Candida albicans? |
|---|---|
| Hydrophobic | yes (Klotz et al. 1985) |
| Surface active | yes (Klotz 1989) |
| Avidity of C. albicans amyloid for self | yes (Garcia et al. 2012) |
| Aggregation of cells | yes (Klotz and Penn 1987) |
| Repetitive motif | yes (Lipke et al. 2011) |
Fungal Amyloid in Human Infection
The recent observation that Candida spp. elaborate amyloid in human disease and that these fibrils are “recognized” by the host resulting in the deposition of host serum amyloid P component (SAP) onto the fungi has opened a new chapter in candidiasis (12, 13). The study involved 25 autopsy patients with invasive candidiasis of the intestines. Many, but not all of the patients had neutropenia near the time of death and some had candidemia, detected either antemortem or at autopsy (14). Multiple specimens from each patient demonstrated yeast cells and hyphae entering the intestines through a point of ulceration of the bowel epithelium into the muscularis layer. The amyloidophilic dyes Congo red and thioflavin-T demonstrated the presence of amyloid on all morphologies of invading Candida (Figure 2 and 3). A monoclonal antibody directed against SAP bound to the same surfaces as the dyes (Figure 4). Accompanying in vitro work established the fact that SAP has a specific interaction with the fungi once functional amyloid is expressed on the fungal surface.
Figure 2. Thioflavin-T binds to fungal cell surface amyloids.
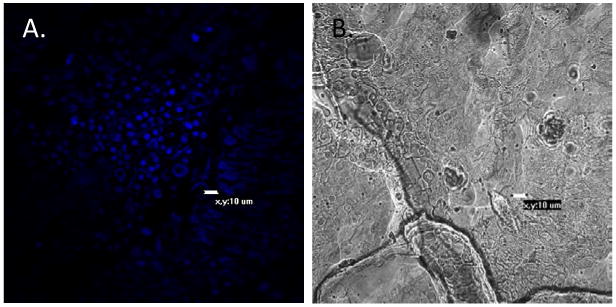
Tissue was stained with 100 nM thioflavin-T. A demonstrates Candida cells in human intestinal tissue binding the fluorescent thioflavin-1; B, same view, light microscopy.
Figure 3. Congo red and thioflavin-S bind to yeast surface amyloids in situ.
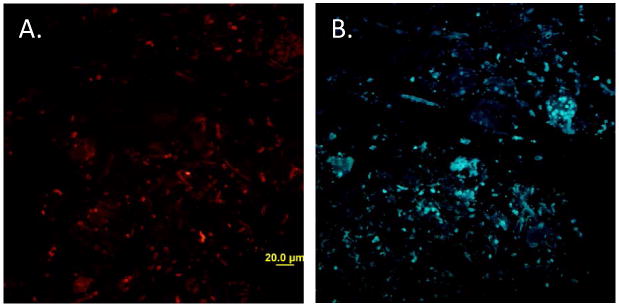
Tissue sections were stained with A) 0.1% Congo red and B) 0.1% thioflavin S.
Figure 4. Serum amyloid P component binds to Candida in human tissue.
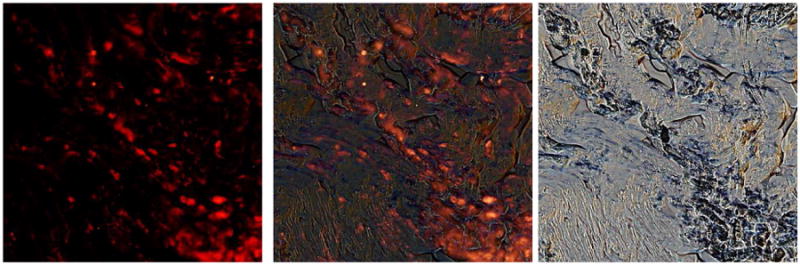
Tissue sections were incubated with anti-SAP, washed and probed with TRITC-conjugated anti-rabbit antibody. The center panel is an overlay of the immunofluorescence image (left) and the brightfield (right). Serum amyloid P component deposits co-localize with fungi in the tissue.
This interaction of SAP with Candida spp. was a surprising finding and not previously known to occur. The story is intriguing because the presence of SAP is a characteristic finding of all the amyloidoses. Serum amyloid P component is a pentraxin, a pentameric protein that circulates at relatively stable concentrations of 20-35mg/L. (CRP, the acute phase reactant, has homologous structures to SAP and is also a pentraxin with a dynamic range of 0.05-500mg/L.) Human SAP, however, is not an acute phase reactant. It binds to amyloid whether it is intracellular or extracellular and is universally present where amyloid is deposited (15). In amyloidoses SAP contributes to the mass effect of the amyloid deposition that leads to tissue and organ dysfunction. Removal of SAP from amyloid desposits in patients results in improvement (16). It is easy to envision how pentameric SAP might cover up amyloid fibrils and mask recognition of amyloid. Perhaps this is one of the reasons that amyloid in humans demonstrates little or no host response—it is seemingly inert. We found in autopsy patients that there was little or no inflammation in response to the fungi present in tissue irrespective of the peripheral white blood cell counts—perhaps the SAP masked the functional amyloid. This masking property of SAP has been proposed as the mechanism by which SAP bound to gram-negative LPS and prevented classical pathway complement activation (17). It also might be why SAP may ameliorate asthma exacerbations caused by Aspergillus. Conidia of the aforementioned fungus are armored with functional amyloid fibers on their cell surface (18) and they penetrate deep into the airways where SAP could conceivably interact with the fungi (19).
Conclusion
As discussed, there are multiple examples of cell surface amyloids on microbes. These functional amyloids are critical for the success of the microbe allowing attachment to non-biological surfaces, forming cellular aggregates and adhering to host tissue. They are also necessary for the success of biofilms and also in infection of the host. These unique structures are utilized for the benefit of the microbe but sometimes to the detriment of the host as seen in some infectious diseases. Amyloids, with their avidity for binding to “self” could serve as probes to identify pathogens in tissue (20) or perhaps as anti-microbials (21).
Footnotes
All authors contributed to the conception and design, manuscript preparation, read and approved the final manuscript.
All authors abide by the Association for Medical Ethics (AME) ethical rules of disclosure.
Competing interests: none declared.
Conflict of interests: none declared.
References
- 1.Marshall K, Serpell L. Structural integrity of beta-sheet assembly. Biochem Soc Trans. 2009;37:671–6. doi: 10.1042/BST0370671. [DOI] [PubMed] [Google Scholar]
- 2.Watt B, van Niel G, Raposo G, Marks M. PMEL: a pigment cell-specific model for functional amyloid formation. Pigment Cell Melanoma Res. 2013 doi: 10.1111/pcmr.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco L, Evans M, Smith D, Badtke M, Chapman M. Diversity, biogenesis and function of microbial amyloids. Trends Microbiol. 2012;20:66–73. doi: 10.1016/j.tim.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen P, Nielsen J, Otzen D, Nielsen PH. Amyloid-like adhesins produced by floc-forming and filamentous bacteria in activated sludge. Appl Environmental Microbio. 2008;74:1517–26. doi: 10.1128/AEM.02274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Otzen D, Nielsen PH. We seek them here, we find them there: functional bacterial amyloid. 2008 doi: 10.1007/s00018-007-7404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz K, Syed A, Stephenson R, Rickard A, Boles B. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xicohtencatl-Cortes J, Saldana Z, Deng W, et al. Bacterial macroscopic rope-like fibers with cytopathic and adhesive properties. J Biological Chem. 2010;285:32336–42. doi: 10.1074/jbc.M110.162248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannister LH, Mitchell GH, Butcher GA, Dennis ED, Cohen S. Structure and development of the surface coat of erthrocytic merozoites of Plasmodium knowlesi. Cell Tissue Res. 1986;245:281–90. doi: 10.1007/BF00213933. [DOI] [PubMed] [Google Scholar]
- 9.Low A, Chandrashekaran I, Adda C, et al. Merozoite surface protein 2 of Plasmodium falciparum: expression, structure, dynamics, and fibril formation of the conserved N-terminal domain. Biopolymers. 2007;87:12–22. doi: 10.1002/bip.20764. [DOI] [PubMed] [Google Scholar]
- 10.Adda C, Murphy V, Sunde M, et al. Plasmodium falciparum merozoite suface protein 2 is unstructured and forms amyloid like fibrils. Mol Biochem Parasitol. 2009;166:159–71. doi: 10.1016/j.molbiopara.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klotz SA, Lipke PN, editors. The perfect adhesive. Badajoz, Spain: Formatex; 2010. [Google Scholar]
- 12.Gilchrist KB, Garcia MC, Sobonya RE, Lipke PN, Klotz SA. New features of invasive candidiasis in humans: amyloid formation by the fungi and deposition of serum amyloid P component by the host. J Infect Dis. 2112;206:1473–8. doi: 10.1093/infdis/jis464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pepys MB. Invasive candidiasis: new insights presaging new therapeutic approaches? J Infect Dis. 2012;206:1339–41. doi: 10.1093/infdis/jis521. [DOI] [PubMed] [Google Scholar]
- 14.Thorn JL, Gilchrist KB, Sobonya RE, Gaur NK, Lipke PN, Klotz SA. Postmortem candidemia: marker of disseminated disease. J Clin Pathol. 2010;63:337–40. doi: 10.1136/jcp.2009.070607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pepys MB, Rademacher TW, Amatayakul-Chantler S, et al. Human serum amyloid P component is an invariant constituent of amyloid deposits and has a uniquely homogeneous glycostructure. Proc Natl Acad Sci USA. 1994;91:5602–6. doi: 10.1073/pnas.91.12.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pepys MB. Pathogenesis, diagnosis and treatment of systemic amyloidosis. Phil Trans R Soc Lond. 2001;356:203–11. doi: 10.1098/rstb.2000.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Haas C, van Leeuwen M, van Bommel T, Verhoef J, van Kessel K, van Strijp J. Serum amyloid P component bound to gram-negative bacteria prevents lipopolysaccharide-mediated classical pathway complement activation. Infect Immun. 2000;68:1753–9. doi: 10.1128/iai.68.4.1753-1759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paris S, Debeaupuis J, Crameri R, et al. Conidial hydrophobins of Aspergillus fumigatus. Appl Environmental Microbio. 2003;69:1581–8. doi: 10.1128/AEM.69.3.1581-1588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreira A, Cavassani K, Hullinger R, et al. Serum amyloid P attenuates M2 macrophage activation and protects against fungal spore-induced allergic airway disease. J Allergey Clin Immunol. 2010;126:712–21. doi: 10.1016/j.jaci.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Garcia MC, Lysak N, Singh S, Sobonya RE, Klotz SA, Lipke PN. A matter of life and death? Detection of C. albicans cell wall amyloids. 4th ASM Conference on Beneficial Microbes; 2012. [Google Scholar]
- 21.Scorciapino M, Pirri G, Vargiu A, et al. A novel dendrimeric peptide with antimicrobial properties: stucture-function analysis of SB056. Biophys J. 2012;102:1039–48. doi: 10.1016/j.bpj.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]


