Abstract
Background:
The outcomes of corticosteroid injection for trigger finger are well documented only with short-term follow-up. The purpose of this investigation was to determine the long-term effectiveness of a single injection and to examine predictors of success up to ten years after injection.
Methods:
This case series analyzed 366 first-time corticosteroid injections in flexor tendon sheaths from January 2000 to December 2007 with a minimum follow-up duration of five years. Two hundred and forty patients (66%) were female, 161 patients (44%) had multiple trigger fingers, and eighty-eight patients (24%) had diabetes at the time of injection. The primary outcome of treatment failure was defined as subsequent injection or surgical trigger finger release of the affected digit. Medical records were reviewed, and any patients without documented failure or a return office visit in 2012 to 2013 were contacted by telephone regarding symptom recurrence and the need for additional treatment. Kaplan-Meier analyses with log-rank test and Cox regression analysis assessed the effect of baseline patient and disease characteristics on injection success.
Results:
Forty-five percent of patients demonstrated long-term treatment success after a single injection. In the final regression model, the interaction of sex and the number of trigger fingers was the single predictor of treatment success. Exploring this association revealed a ten-year success rate of 56% for female patients presenting for the first time with a trigger finger compared with 35% in male patients presenting for the first time with a trigger finger, 39% in female patients with multiple trigger fingers, and 37% in male patients with multiple trigger fingers. Eighty-four percent of treatment failures occurred within the first two years following injection. Patient age, symptom type, and undifferentiated diabetes status were not predictive of treatment success.
Conclusions:
Female patients presenting with their first trigger finger have the highest rate of long-term treatment success after a single corticosteroid injection. Patients who continue to experience symptom relief two years after injection are likely to maintain long-term success.
Level of Evidence:
Therapeutic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Trigger finger is one of the most common hand disorders treated by orthopaedic surgeons, with a lifetime risk estimated at 2.6% in the general population and 4% to 10% in patients with diabetes1,2. Conservative treatments, including activity modification, splinting, physiotherapy, and non-steroidal anti-inflammatory drugs (NSAIDs), have been described, but the mainstay of nonoperative treatment remains corticosteroid injection3-6.
Prior studies have shown the success of corticosteroid injections in the range of 61% to 84% for patients presenting with new onset trigger symptoms after one to three injections6-14. Increased rates of treatment failure have been correlated with younger age, diabetes mellitus, the increased duration of symptoms, and the presence of multiple affected digits8,9,15-17. However, long-term success remains unknown, with the average follow-up duration for these investigations ranging from one to three years and the minimum follow-up duration ranging from four to twelve months. Rozental et al. are unique in considering the time to treatment failure with a Kaplan-Meier survival analysis showing a nearly linear rate of symptom recurrence within the first year17. We are currently unable to counsel patients regarding the risk of long-term symptom recurrence after corticosteroid injection and do not know whether the pattern of time to treatment failure noted by Rozental et al. would continue to gradually deteriorate or plateau beyond one year17.
The purposes of this investigation were to determine the long-term effectiveness of a single corticosteroid injection for trigger finger, to quantify factors impacting treatment success, and to examine the rate of treatment failure in a Kaplan-Meier analysis up to ten years after injection. Our hypothesis was that diabetes mellitus and the presence of multiple trigger fingers would be associated with lower rates of success five or more years after treatment.
Materials and Methods
This retrospective case series was initiated following institutional review board approval. Trigger finger injections performed from January 2000 to December 2007 were eligible for inclusion, providing a minimum potential follow-up duration of five years from the time of study initiation (including telephone follow-up for the purpose of this study). The lack of electronic patient health records prohibited the analysis of injections prior to 2000. Patients were identified from a search of the departmental billing database for Current Procedural Terminology (CPT) codes 20550 (injection; tendon sheath, ligament), 20551 (injection; tendon origin/insertion), and 20600 (arthrocentesis, aspiration, or injection) associated with International Classification of Diseases, Ninth Revision (ICD-9) codes 727.03 (trigger finger) or 727.05 (tenosynovitis; hand, wrist). Corticosteroid injection for trigger finger was confirmed in all cases by manual chart review. Once selected on the basis of corticosteroid injection, no attempt was made to control for prior treatment other than injection. Patients were seen, were diagnosed, and were injected by one of four fellowship-trained hand surgeons at our tertiary medical center (R.D.W., N.C.F., R.H.G., and R.P.C.). The injection protocol at our institution, and the likely method used on patients in this study, involves injection at the A1 pulley angling 45° distally and consists of 1 mL of 40-mg/mL Depo-Medrol (methylprednisolone acetate) and 0.5 to 1 mL of 1% lidocaine without epinephrine.
Only patients with a complete office note in the electronic medical record system were eligible for the study. Patient age, sex, date of injection, digit injected, presenting symptoms graded according to the Green classification18 (Table I), presence of multiple trigger fingers, and presence of diabetes at the time of injection were recorded. The initial study design did not differentiate between insulin-dependent and non-insulin-dependent diabetes; thus, both types were combined under the term “undifferentiated diabetes.” The presence of multiple trigger fingers was defined as more than one symptomatic digit at the time of injection or a history of trigger digits in the past. For patients with multiple documented injections within the seven-year period, only the first injection was considered eligible to ensure that all injections were independent. For patients receiving simultaneous injections in several digits, only the first digit listed in the office note was considered. This decision was made to minimize bias that could be introduced by the selection of digit according to the duration of symptoms, the severity of symptoms, or the digit number. Exclusion criteria after initial chart review included prior injection or surgery of the digit, injection for diagnoses other than trigger finger, or failure to specify which digit was injected. Patients with additional hand conditions such as carpal tunnel syndrome or degenerative joint disease were not excluded from the study.
TABLE I.
The Green Classification of Trigger Finger Symptoms18
| Grade I | Pain or tenderness at the A1 pulley |
| Grade II | Catching but can actively extend digit |
| Grade III | Locking, requiring passive extension |
| Grade IV | Fixed flexion contracture |
The records of the remaining patients were examined for our primary outcome of treatment failure, defined as subsequent injection or surgical trigger finger release of the affected digit. Success was determined when, at the time of subsequent follow-up visits, office notes did not document recurrent or persistent trigger symptoms or any additional treatment for trigger finger for patients. Patients without recorded evidence of treatment failure or a follow-up visit in 2012 to 2013 were contacted by telephone. These patients were verbally queried regarding recurrence of trigger finger symptoms and dates of any further treatment of the affected digit. All study participants contacted by phone provided verbal consent for study participation. This telephone follow-up call was conducted to minimize bias toward higher estimated rates of treatment failure if our outcomes were solely determined from office follow-up, as patients who experience symptomatic resolution are not instructed to schedule routine follow-up visits in our practice. Alternatively, counting all patients who did not return to the office as presumed treatment successes would have biased toward falsely inflated rates of success had these patients not been called.
All patients with treatment failure or at least five years of follow-up were included in the study. Patients were considered lost to follow-up if we were unable to reach them by telephone after four attempts on separate days. Patients were excluded after telephone call attempts if they declined participation, had died, experienced cognitive impairment that would prohibit study consent, or had subsequent office notes detailing an injection without specifying the digit, or if they stated that they received a later treatment but could not recall an approximate date within one month.
Statistical Analysis
The overall data were examined by descriptive statistics for the frequencies in percentages of categorical variables and means and standard deviations for continuous variables. Univariate analysis with the chi-square test for categorical variables or the independent t test for continuous variables was conducted to determine success status according to independent predictors. Kaplan-Meier analyses with the log-rank test were used to assess the difference in time to treatment failure for the categorical baseline variables. Participants without treatment failure were censored and were removed from the Kaplan-Meier analysis at the time point corresponding to their most recent office visit or telephone follow-up, as outcome data were unavailable beyond this point. Cox regression analysis was used to determine the impact of independent variables and all possible interactions between variables on time to treatment failure. Any significant interaction term (p < 0.05) was stratified in the final multivariable Cox regression model.
Exploratory subgroup analysis according to diabetic status (insulin-dependent or non-insulin-dependent) was performed with chi-square analysis testing for difference in success.
Source of Funding
No external funding was provided for this study.
Results
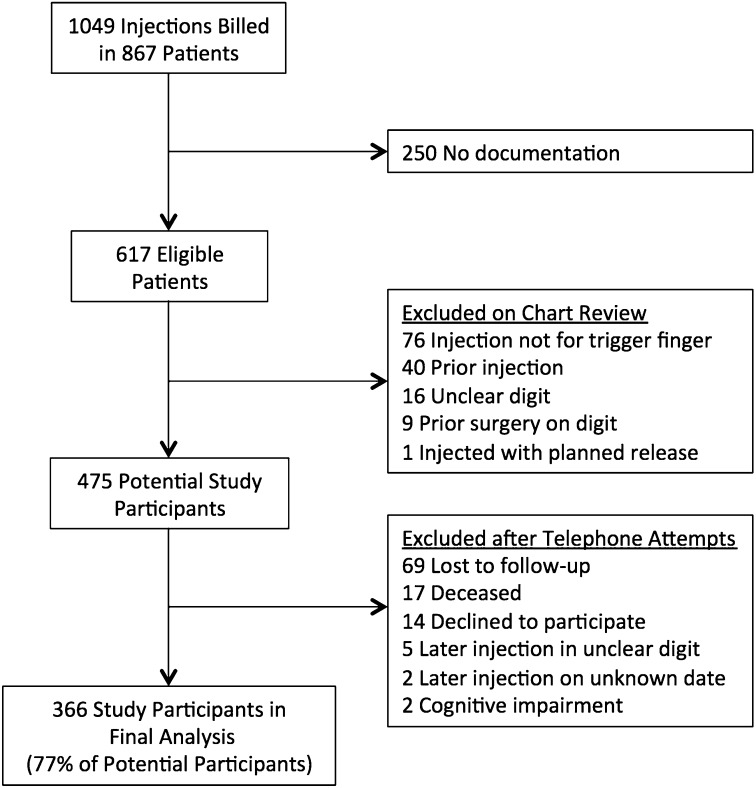
A search of the departmental database identified 1049 injections in 867 patients over the specified date range. An initial screening determined that 617 injections in 617 patients were eligible for the study. One hundred and forty-two patients were subsequently excluded after chart review (injections for diagnoses other than trigger finger, prior injection of the digit, digit injected not specified, prior surgery on the digit, and injection with planned release). Of the remaining 475 potential study participants, 366 completed follow-up with no missing data and were included in the final analysis (366 injections) (Fig. 1).
Fig. 1.
Flow diagram of study enrollment.
Two hundred and forty patients (65.6%) were female, with an average age (and standard deviation) of 59.2 ± 11.4 years (range, nineteen to ninety-two years). One hundred and sixty-one patients (44.0%) had multiple trigger fingers and eighty-eight patients (24.0%) were diabetic at the time of injection. Digits injected included 137 thumbs (37.4%), ninety-eight long fingers (26.8%), eighty-five ring fingers (23.2%), thirty-one index fingers (8.5%), and fifteen small fingers (4.1%). The symptom types of injected digits were seventy Green Grade I (19.1%), 217 Green Grade II (59.3%), and seventy-nine Green Grade III (21.6%). There were no Green Grade-IV digits among the study participants, as statically locked trigger fingers typically undergo early operative release.
Two hundred patients (54.6%) had treatment failure requiring repeat injection or surgical release, and 166 patients (45.4%) did not require treatment between injection and study data collection. Seven patients (1.9%) reported no symptom relief from the initial injection and were counted as immediate treatment failure. Of the 200 patients with treatment failure, 128 (64%) required repeat injection and sixty-five (33%) proceeded to operative trigger release; these sixty-five patients were 18% of all patients in the study. The median time to treatment failure was forty-five months, and the time from injection to study data collection for asymptomatic digits ranged from sixty-two to 158 months. Of the 165 subjects with telephone follow-up, 150 (90.9%) reported treatment success.
Treatment success differed between sexes, with 118 female patients (49.2%) and forty-eight male patients (38.1%) requiring no subsequent injection or surgery (p < 0.05). One hundred and four patients (50.7%) without multiple trigger fingers and sixty-two patients (38.5%) with multiple trigger fingers had treatment success (p < 0.05). Neither patient age (p = 0.45) nor symptom type (p = 0.44) impacted the likelihood of injection success. Undifferentiated diabetes status did not alter outcomes with 125 patients without diabetes (45.0%) and forty-one patients with diabetes (46.6%) reporting treatment success (p = 0.79).
The Kaplan-Meier curve displays overall time to treatment failure for study participants (Fig. 2). Of the treatment failures, 54.5% occurred within one year and 83.6% occurred within two years.
Fig. 2.
Kaplan-Meier curve of overall injection success for all study participants.
The Appendix shows the Kaplan-Meier curve comparing patients with undifferentiated diabetes and those without diabetes. Log-rank analysis failed to show a significant difference in time to treatment failure between these two groups (p = 0.62).
Log-rank analysis comparing sexes showed a non-significant difference (p = 0.06) in time to treatment failure favoring increased success in women. The presence of multiple trigger fingers was associated with shorter time to treatment failure (p < 0.05). However, Cox regression analysis demonstrated a significant interaction between these two variables (p < 0.05). Therefore, an interaction term was stratified in the final multivariable Cox regression model. Diabetes status was also included in the final model because of its hypothesized clinical impact based on prior studies.
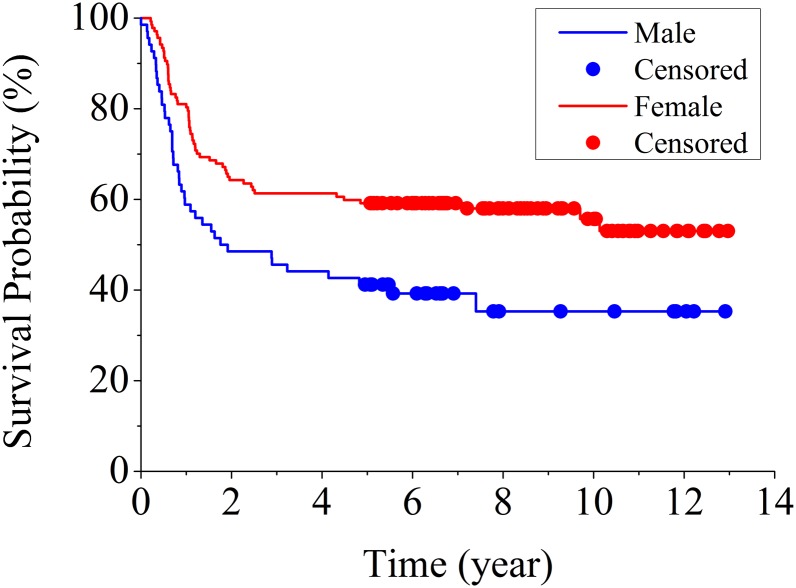
The final multivariable Cox regression model showed a significant difference in time to treatment failure for the interaction term of sex and multiple trigger fingers (p < 0.05) and no difference for undifferentiated diabetes status (p = 0.44). Kaplan-Meier curves were constructed comparing time to treatment failure by sex for both patients with their first trigger digit (Fig. 3) and patients with multiple trigger digits (see Appendix). Table II displays the one, three, five, and ten-year treatment success rates and 95% confidence intervals (CIs) for these groups. Thus, the highest chance of long-term symptomatic relief from a single corticosteroid injection for a trigger finger is in a female patient with a single trigger finger.
Fig. 3.
Kaplan-Meier curve of time to treatment failure by sex for patients with their first trigger finger.
TABLE II.
Treatment Success Rates by Sex and Number of Trigger Fingers
| Single Trigger Finger* |
Multiple Trigger Fingers* |
|||
| Time | Male | Female | Male | Female |
| At one year | 59% (46% to 69%) | 80% (73% to 86%) | 71% (57% to 81%) | 64% (54% to 73%) |
| At three years | 46% (34% to 57%) | 61% (53% to 69%) | 48% (35% to 60%) | 44% (34% to 53%) |
| At five years | 41% (29% to 53%) | 59% (50% to 67%) | 41% (29% to 54%) | 40% (30% to 49%) |
| At ten years | 35% (23% to 48%) | 56% (46% to 64%) | 37% (24% to 49%) | 39% (29% to 48%) |
The values are given as the average success rate, with the 95% CI in parentheses.
Exploratory analysis according to diabetic type revealed that our study included thirty-nine insulin-dependent patients and forty-nine non-insulin-dependent patients. The ultimate success rate was 35.9% for insulin-dependent patients (fourteen patients) and 55.1% for non-insulin-dependent patients (twenty-seven patients).
Discussion
This retrospective case series evaluated the long-term efficacy of corticosteroid injection for trigger finger and attempted to identify factors that may alter treatment success. Overall, 45% of patients experienced long-term treatment success after a single injection. Age, symptom type, and diabetes status were not predictive of treatment success. Our regression analysis identified a significant interaction between sex and number of trigger fingers (first or multiple), indicating that it is inappropriate to consider either variable in isolation as predictors of treatment success. This finding suggests that female patients may have an inherently better prognosis than male patients, but the presence of multiple trigger fingers erases this protective effect. At this time, it is not clear why isolated trigger fingers in female patients responded better to initial injection than the same condition in male patients. Eighty-four percent of treatment failures occurred within the first twenty-four months, suggesting that patients who continue to experience symptom relief two years after treatment are likely to maintain long-term success.
The overall treatment success of the present study was lower than that previously reported in the literature. Rhoades et al. found a 72% success rate after one injection, with a mean follow-up duration of twenty-five months (range, six to sixty months)9. Marks and Gunther reported a success rate of 84% for trigger fingers and 92% for trigger thumbs after a single injection with a mean follow-up duration of forty-one months (range, twelve to 104 months)13. The higher treatment success of these studies may be explained by the shorter minimum follow-up time, as our data show that treatment failures are frequently within the first two years after injection. Other studies permitted a second or third injection several weeks later in patients who failed to respond to the first injection but defined failure more broadly to include any symptom recurrence. Using such methodology, Faunø et al. found a 76% success rate at a follow-up duration of three to fifteen years, with only 35% of patients reporting symptom resolution after one injection12. Similarly, Newport et al. reported a 77% success rate at an average follow-up duration of thirty-five months, with only 49% improving after a single injection8. By defining treatment failure as repeat injection or surgery, our rates of success would be expected to be higher, as mild symptom persistence did not necessitate categorization as failure.
The association between age and treatment success is debated. Faunø and colleagues showed no association between age and treatment success in their long-term study of 104 digits12. However, more recently, Rozental et al. reported increased rates of treatment failure in younger patients17. Considering our data in light of the report by Rozental et al. that concluded follow-up at one year, it is possible that age plays a role in short-term, but not long-term, treatment success. Consistent with prior studies, we found no correlation between symptom type and prognosis following injection8,17. Our finding of multiple trigger fingers as a predictor of poorer outcomes is consistent with several prior works6,8,9,17.
The literature on diabetes and steroid injection for trigger finger continues to evolve. Early studies found no relationship between treatment success and associated disease processes, including diabetes9,12. More recent studies looking specifically at diabetic patients have concluded that this patient population is at heightened risk for symptom recurrence and subsequent intervention. Griggs et al. reported a 50% success rate among a diabetic population at an average follow-up of twenty-seven months with worse outcomes in insulin-dependent patients with diabetes (44%) than in non-insulin-dependent patients with diabetes (71%)2. Stahl and colleagues found 49.2% success in diabetics compared with 76.3% in a control group of non-diabetics at four-month follow-up15. A randomized controlled trial by Baumgarten et al. concluded that patients with diabetes responded less favorably after a two-injection treatment and were more likely to require surgery than patients without diabetes at thirteen to forty-one months follow-up, although they found no difference in outcome between the groups after a single injection16. However, none of these studies controlled for the potential confounding factor of multiple trigger fingers, which had a higher incidence among patients with diabetes in all studies. Our own Cox regression model found no significant difference between patients with diabetes and patients without diabetes despite being adequately powered. We did not design this study to differentiate between patients with insulin-dependent diabetes and those with non-insulin-dependent diabetes, but we did perform an exploratory analysis that suggested that insulin dependence may impact injection success2,15,17. Despite enrolling nearly 400 patients, our study remained underpowered to incorporate diabetic categories according to insulin dependence into our Kaplan-Meier analysis.
Similar to the investigation by Rozental and colleagues17, our Kaplan-Meier analysis demonstrated a nearly linear rate of treatment failure during the first year after injection. However, the longer-term follow-up in our present study indicates that this rate decreases substantially after one year, nearly reaching a plateau by two years. This finding suggests that, in many patients, a single injection may be sufficient to alter local biology of disease and to prevent symptom recurrence indefinitely.
Completion of this study required several methodologic choices and compromises that deserve consideration. First, we only looked at time to repeat injection or surgery, not recurrence of symptoms, which is difficult to assess retrospectively. Therefore, patients with recurrent symptoms who did not seek further treatment are categorized as successes in our study. Although this definition of treatment failure was necessary, we believe that it was an appropriate measure as we captured clinically relevant symptoms sufficient to prompt further treatment. At the same time, we did not control for any adjunct treatments such as splinting, therapy, or NSAIDs. Second, chart review alone selects for patients who returned with symptom recurrence and would bias the study toward higher rates of treatment failure. We attempted to mitigate this by calling patients who did not have sufficient follow-up time in the chart. Nevertheless, sixty-nine patients were lost to follow-up, and thus our study may have underestimated the true rate of treatment success as 91% of those who did not return to the office after injection reported treatment success when contacted by telephone. On the basis of a sensitivity analysis, we estimated that an adjusted success rate for the entire population may approach 53% compared with our calculated 45%, assuming a success rate among the sixty-nine participants lost to follow-up, consistent with those who were contacted by telephone (Table III). Third, we did not consider two or three-injection regimens for initial treatment of trigger finger. By considering only a single injection, our study design allows patients and physicians to estimate the chance that a single injection for trigger finger at initial presentation will resolve the condition to a degree that further injections or surgery will not be needed. Finally, in the event of multiple presenting trigger fingers, we analyzed only data from the first digit noted in the record. This was chosen to capture the chief symptom for the office visit as opposed to secondary trigger fingers identified during examination. Multiple digits from individual patients were not analyzed to keep each trigger digit as a truly independent event during statistical analysis.
TABLE III.
Potential Impact of Patients Lost to Follow-up on Overall Success Rate
| Loss to Follow-Up |
Impact on Overall Data |
|||
| Theoretical Success Rate* | Theoretical Additional Successes† | Adjusted Total Successes† | Adjusted Total Patients† | Adjusted Success Rate* |
| 0% | 0 | 166 | 435 | 38.2% |
| 50% | 34.5 | 200.5 | 435 | 46.1% |
| 90.9%‡ | 62.7 | 228.7 | 435 | 52.6% |
| 100% | 69 | 235 | 435 | 54% |
The values are given as the rates of success in percentages.
The values are given as the number of patients.
The rate of success was based on participants who were contacted by telephone in this study.
Our study had several limitations. We did not have data on treatment sought by patients with an outside provider. Patients were asked by telephone if they required any further treatment, but it is possible that we were still unable to fully account for injections or surgery performed elsewhere secondary to recall bias. We also did not consider adjunctive treatments such as splinting, hand therapy, or oral medication prior to or after injection. However, this should not bias our results as patients are not routinely prescribed such treatments following injection and it is unlikely any patient group independently sought these adjuncts more than others. Additionally, this study did not evaluate outcomes in patients who had received prior injections, which is a group of patients for which optimal treatment remains controversial.
This study lends further support to the use of corticosteroid injection as the initial treatment in patients presenting with new onset trigger finger. It is our hope that patients and clinicians can utilize these data to make more informed treatment decisions and to set reasonable expectations regarding the need for future treatment following injection.
Appendix
Figures showing Kaplan-Meier curves of time to treatment failure by diabetic status and by sex for patients with multiple trigger fingers are available with the online version of this article as a data supplement at jbjs.org.
Footnotes
Investigation performed at the Department of Orthopaedic Surgery, Washington University Orthopedics, Washington University School of Medicine, Saint Louis, Missouri
A commentary by Mark A. Vitale, MD, MPH, is linked to the online version of this article at jbjs.org.
Disclosure: None of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of any aspect of this work. One or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Strom L. Trigger finger in diabetes. J Med Soc N J. 1977November;74(11):951-4. [PubMed] [Google Scholar]
- 2.Griggs SM, Weiss AP, Lane LB, Schwenker C, Akelman E, Sachar K. Treatment of trigger finger in patients with diabetes mellitus. J Hand Surg Am. 1995September;20(5):787-9. [DOI] [PubMed] [Google Scholar]
- 3.Tarbhai K, Hannah S, von Schroeder HP. Trigger finger treatment: a comparison of 2 splint designs. J Hand Surg Am. 2012February;37(2):243-9: 249.e1. Epub 2011 Dec 20. [DOI] [PubMed] [Google Scholar]
- 4.Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006January;31(1):135-46. [DOI] [PubMed] [Google Scholar]
- 5.Colbourn J, Heath N, Manary S, Pacifico D. Effectiveness of splinting for the treatment of trigger finger. J Hand Ther. 2008Oct-Dec;21(4):336-43 Epub 2008 Aug 22. [DOI] [PubMed] [Google Scholar]
- 6.Patel MR, Bassini L. Trigger fingers and thumb: when to splint, inject, or operate. J Hand Surg Am. 1992January;17(1):110-3. [DOI] [PubMed] [Google Scholar]
- 7.Kolind-Sorensen V. Treatment of trigger fingers. Acta Orthop Scand. 1970;41(4):428-32. [DOI] [PubMed] [Google Scholar]
- 8.Newport ML, Lane LB, Stuchin SA. Treatment of trigger finger by steroid injection. J Hand Surg Am. 1990September;15(5):748-50. [DOI] [PubMed] [Google Scholar]
- 9.Rhoades CE, Gelberman RH, Manjarris JF. Stenosing tenosynovitis of the fingers and thumb. Results of a prospective trial of steroid injection and splinting. Clin Orthop Relat Res. 1984November;(190):236-8. [PubMed] [Google Scholar]
- 10.Clark DD, Ricker JH, MacCollum MS. The efficacy of local steroid injection in the treatment of stenosing tenovaginitis. Plast Reconstr Surg. 1973February;51(2):179-80. [DOI] [PubMed] [Google Scholar]
- 11.Anderson B, Kaye S. Treatment of flexor tenosynovitis of the hand (‘trigger finger’) with corticosteroids. A prospective study of the response to local injection. Arch Intern Med. 1991January;151(1):153-6. [PubMed] [Google Scholar]
- 12.Faunø P, Andersen HJ, Simonsen O. A long-term follow-up of the effect of repeated corticosteroid injections for stenosing tenovaginitis. J Hand Surg Br. 1989May;14(2):242-3. [DOI] [PubMed] [Google Scholar]
- 13.Marks MR, Gunther SF. Efficacy of cortisone injection in treatment of trigger fingers and thumbs. J Hand Surg Am. 1989July;14(4):722-7. [DOI] [PubMed] [Google Scholar]
- 14.Freiberg A, Mulholland RS, Levine R. Nonoperative treatment of trigger fingers and thumbs. J Hand Surg Am. 1989May;14(3):553-8. [DOI] [PubMed] [Google Scholar]
- 15.Stahl S, Kanter Y, Karnielli E. Outcome of trigger finger treatment in diabetes. J Diabetes Complications. 1997Sep-Oct;11(5):287-90. [DOI] [PubMed] [Google Scholar]
- 16.Baumgarten KM, Gerlach D, Boyer MI. Corticosteroid injection in diabetic patients with trigger finger. A prospective, randomized, controlled double-blinded study. J Bone Joint Surg Am. 2007December;89(12):2604-11. [DOI] [PubMed] [Google Scholar]
- 17.Rozental TD, Zurakowski D, Blazar PE. Trigger finger: prognostic indicators of recurrence following corticosteroid injection. J Bone Joint Surg Am. 2008August;90(8):1665-72. [DOI] [PubMed] [Google Scholar]
- 18.Green D, Hotchkiss R, Pederson W, Wolfe S, editors. Green’s operative hand surgery. 5th ed London: Churchill Livingstone; 2005. [Google Scholar]