Abstract
Context
Pediatric disorders characterized by behavioral and emotional dysregulation pose diagnostic and treatment challenges because of high comorbidity, suggesting that they may be better conceptualized dimensionally rather than categorically. Identifying neuroimaging measures associated with behavioral and emotional dysregulation in youth may inform understanding of underlying dimensional vs. disorder-specific pathophysiology.
Objective
Identify, in a large cohort of behaviorally and emotionally dysregulated youth, neuroimaging measures that: 1) are associated with behavioral and emotional dysregulation pathological dimensions (behavioral and emotional dysregulation measured with the Parent General Behavior Inventory 10 Item Mania Scale [PGBI-10M], mania, depression, anxiety); or 2) differentiate diagnostic categories(BPSD, ADHD, anxiety, disruptive behavior disorders (DBD)).
Design
Multi-site neuroimaging study(February 2011–April 2012).
Setting
Academic medical centers: Case Western Reserve University, Cincinnati Children’s Hospital, University of Pittsburgh.
Patients
Referred sample of behaviorally and emotionally dysregulated youth(n=85) from the Longitudinal Assessment of Manic Symptoms study and healthy youth (n=20).
Main Outcome Measures
Region-of-interest analyses examined relationships among prefrontal-ventral striatal reward circuitry during a reward paradigm (Win, Loss, control conditions), symptom dimensions, and diagnostic categories.
Results
Regardless of diagnosis, higher PGBI-10M scores were associated with greater left middle prefrontal cortical (mPFC; r=0.28), and greater levels of anxiety with greater right dorsal anterior cingulate cortical (dACC; r=0.27), activity to Win. The 20 highest (t=2.75) and 20 lowest (t=2.42) PGBI-10M scoring youth showed significantly greater left mPFC activity to Win than 20 healthy youth. DBD were associated with lower left ventrolateral prefrontal cortex(VLPFC) activity to Win (t=2.68) (all ps<0.05, corrected).
Conclusions
Greater PGBI-10M-related left mPFC activity, and greater anxiety-related right dACC activity, to Win may reflect heightened reward sensitivity and greater attention to reward in behaviorally and emotionally dysregulated youth, regardless of diagnosis. Reduced left VLPFC activity to Win may reflect reward insensitivity in youth with DBD. Despite a distinct reward-related neurophysiology in DBD, findings generally support a dimensional approach to studying neural mechanisms in behaviorally and emotionally dysregulated youth.
Pediatric disorders characterized by behavioral and emotional dysregulation, including bipolar spectrum disorders(BPSD)(1), major depressive disorder(2–3), attention deficit/hyperactivity disorder(ADHD)(4–7), disruptive behavior disorders(DBD)(8–9), and anxiety disorders(10–11), pose clinical challenges for diagnosis and treatment, particularly due to high comorbidity rates(12–15). These disorders may thus be better conceptualized as comprising a set of dimensions of behavioral and emotional dysregulation pathology that cut across conventionally-defined diagnostic categories. This dimensional approach to studying pediatric behavioral and emotional dysregulation parallels the Research Domain Criteria(RDoC), which aims to elucidate physiological dimensions reflecting the range of pathology severity across categorically-defined diagnoses(16).
The Longitudinal Assessment of Manic Symptoms(LAMS) study is an ongoing multi-site study of youth with a variety of behavioral and emotional dysregulation diagnoses, several of which include manic-like symptoms(eText). The main purpose of LAMS is to assess relationships among the longitudinal course of symptoms, clinical, and functional outcomes in these youth. In addition to using commonly-used dimensional symptom measures of emotional dysregulation in youth(rating scales of mania, depression, and anxiety), LAMS also uses the Parent General Behavior Inventory-10 Item Mania Scale(PGBI-10M), a parent self-report dimensional measure of behavioral and emotional dysregulation behaviors in youth, that includes measurement of manic-like behaviors associated with difficulty regulating positive mood and energy(17–18). PGBI-10M scores were positively and significantly associated with higher scores on the Drive and Fun-seeking subscales of the Behavioral Activation Scale in youth seeking outpatient services, suggesting that PGBI-10M also captures information regarding reward sensitivity in youth(Youngstrom, personal communication;eText). Initial screening results from LAMS found that, irrespective of diagnosis, high PGBI-10M scores(≥12) were common(in 43% of these youth), and associated with worse overall functioning and higher rates of a variety of psychiatric disorders(19–20).
To improve understanding of pathophysiological processes underlying pediatric disorders characterized by behavioral and emotional dysregulation, neuroimaging studies should thus seek to identify: 1)neuroimaging biomarkers associated with symptom dimensions characterized by behavioral and emotional dysregulation(e.g., mania, depression, anxiety, PGBI-10M), irrespective of diagnosis; versus 2)neuroimaging biomarkers associated with distinct diagnostic categories(e.g., BPSD, ADHD, anxiety disorders, DBD). The significant positive associations between PGBI-10M and reward sensitivity measures further suggest that neuroimaging studies of reward processing in behaviorally and emotionally dysregulated youth may, in particular, yield biomarkers of underlying pathophysiologic processes.
Neuroimaging studies in healthy adults highlight key roles of ventral striatum(VS) and different prefrontal cortical regions in reward processing: VS is activated during changes in expected or obtained reward(21–23); orbitofrontal cortex(OFC;Brodmann Area[BA]11) and ventrolateral prefrontal cortex(VLPFC;BA47) track reward value and arousal during anticipation of rewarding stimuli(24–25); dorsal anterior cingulate cortex(dACC;BA24/32) is involved in attention during reward-related decision making(26); and middle prefrontal cortex(mPFC;BA10) is implicated in risky decision making in potentially rewarding contexts(27–28). Several studies reported abnormally increased reward sensitivity(29–31), and abnormally elevated reward–related VS(21, 30), OFC, and VLPFC activity(30, 32–33), in adults with bipolar disorder. Abnormal reward-related neural activity has also been shown in BPSD youth(34), and youth with other diagnoses characterized by behavioral and emotional dysregulation, including ADHD(35–36), anxiety disorders(37), and DBD(38–39).
In the present study, we examined a large cohort of LAMS youth. Our primary aim was to identify specific neuroimaging measures associated with severity of different dimensions of behavioral and emotional dysregulation pathology in these youth, irrespective of diagnosis. Our secondary aim was to identify neuroimaging measures associated with distinct diagnostic categories in these youth. We employed a number guessing reward paradigm(40)(Win, Loss, and control blocks) that has been used in neuroimaging studies of mood-disordered adolescents and adults(30, 41) and reliably activates key reward neural circuitry regions: dACC, mPFC, OFC, VLPFC, and VS(30, 42).
Using multiple regression analyses, we aimed to evaluate two separate hypotheses related to our primary and secondary aims.
Given the above studies showing that behaviorally and emotionally dysregulated adults and youth across different diagnostic categories display abnormal prefrontal cortical-VS activity to reward(Win vs. control) versus healthy control participants, our primary hypothesis was as follows:
Primary Hypothesis (Dimensional): Across all LAMS youth, irrespective of diagnosis, the magnitude of prefrontal cortical-VS activity to Win(>control) would be significantly associated with greater severity of symptoms reflecting behavioral and emotional dysregulation(PGBI-10M, mania, depression, anxiety).
Secondary Hypothesis (Categorical): Patterns of prefrontal cortical-VS activity to Win(>control) would differentiate current diagnostic categories in LAMS youth. The paucity of studies comparing reward circuitry activity in youth with different diagnostic categories did not allow us to specify the specific patterns of neural activity associated with each diagnostic category.
We recruited a comparison group of healthy youth(HY) to examine the extent to which significant relationships between neural activity and symptom dimensions(or diagnostic categories) represented abnormal neural activity in LAMS youth.
Methods
Participants
107 youth (10–17 years; eTable 1) from the original LAMS study participated in the neuroimaging component of the second phase of the LAMS study. Neuroimaging participants were recruited from three LAMS sites: Case Western Reserve University(CWRU; n=32); Cincinnati Children’s Hospital(CCH; n=37); and University of Pittsburgh Medical Center/Western Psychiatric Institute and Clinic(UPMC; n=38). 22 age- and sex ratio-matched HY recruited from all three sites (8–16 years;eText) participated in this study for analyses comparing LAMS youth with HY. Parents/guardians provided written informed consent, and children provided written informed assent prior to study participation. Participants received monetary compensation and a framed picture of their structural neuroimaging scan. See eText for exclusion criteria.
Due to data loss and excessive head movement(>4 mm, as in previous studies(41)) during scanning, data from 22 LAMS youth and 2 HY were excluded, leaving data from 85 LAMS youth (Age: M=13.65, SD=1.96, Range=9.89–17;46 males; CWRU n= 25;CCH n=31; UPMC n=29; eTables 2–3) and 20 HY (Age: M=13.31, SD=2.36, Range=8.03–16.92; 12 males; CWRU n=6, CCH n=2; UPMC n=12; eTable 2). Participants excluded for movement were more likely to be male and have lower IQ scores(eTable 1).
52 of the 85 LAMS youth were taking at least one psychotropic medication(eTable 2). Of those 52 LAMS youth, 29 were taking one class, 15, two classes, 6, three classes, and 2, four classes, of psychotropic medications. Given ethical problems with stopping medication for research participation, LAMS youth were permitted to use prescribed medication(s) before, and on the day of, scanning.
Symptom Assessment
LAMS youth completed several symptom assessment measures. Parents/guardians completed the PGBI-10M(eText) at baseline and 6-monthly intervals from study entry throughout both phases of LAMS. PGBI-10M score nearest the scanning session (days between PGBI-10M assessment and scan date: M=15.54; SD=35.01; Range=87 before, to 143 days after, scan date) was included as a measure of most recent PGB1-10M score(eTable 2). PGBI-10M scores were very stable over three assessment points(i.e., over one year) close to the scan day(eText).
On the scan day, both parents and children completed the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Mania Rating Scale(K-MRS)(43), to assess hypo/mania severity, and the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Present Episode Depression Rating Scale(K-DRS)(44) to assess depressive symptom severity(eTable 2). Interviewers made final decisions on summary scores based upon all available information if parent and child responses differed. Participants completed the Screen for Child Anxiety Related Emotional Disorders(SCARED) on the scan day to assess youth anxiety symptoms over the last 6 months(45)(eTable 2).
Diagnostic Categories
This final sample of 85 LAMS youth had a variety of current unmodified DSM-IV diagnoses, confirmed by a licensed child psychiatrist or psychologist: ADHD (n=27), anxiety disorders (n=7), BPSD (n=33), and DBD (n=17) (eText).
Reward Paradigm
A block-design reward fMRI task(40) examined reward-related neural circuitry(Figure 1; eText for paradigm details).
Figure 1.
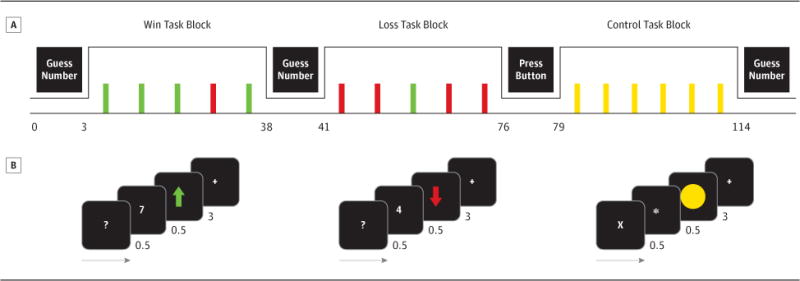
Reward task adapted from(40). Participants guessed whether a card(value: 1–9) was higher/lower than 5, then viewed the number, outcome(Win=green arrow, Loss=red arrow), and fixation cross. Control trials: Participants pressed a button to “X”, then viewed an asterisk, circle, and fixation cross.
Neuroimaging Data Analysis
Creation of a single a priori anatomically-defined bilateral ROI mask to test main hypotheses
Statistical Parametric Mapping software (SPM8 http://www.fil.ion.ucl.ac.uk/spm) was used to preprocess and analyze fMRI data (eText). Based on previous neuroimaging findings highlighting the roles of several regions in reward processing in healthy adults(30, 33), several anatomically-defined regions of interest(ROIs) were selected a priori to be included in a single ROI mask for testing our two main hypotheses: dACC(BA24/32), mPFC(BA10), OFC(BA11), VLPFC(BA47), and VS(bilateral spheres centered on −9, 9, −8 and 9, 9, −8; radius=8mm based on meta-analyses(46–47)). One anatomically-defined bilateral mask containing all five of these bilateral individual ROIs was then created from the WFU PickAtlas(48) for hypothesis testing. By using one large ROI mask we avoided conducting multiple statistical tests over several small ROIs.
Identifying neural activity to each stimulus contrast
After creating the mask, we then established which regions in the entire a priori anatomically-defined bilateral ROI mask showed significant activity to the two different reward task conditions: Win>control and Loss>control. We ran separate t-tests for each of the two contrasts using a voxelwise p<.025 to correct for the two parallel tests(Win>control, Loss>control), and a cluster level alpha of p<.05, corrected with a cluster forming threshold(49). Significant clusters of activity were then saved as stimulus contrast-related masks for use in the multiple regression analyses used to test Hypotheses 1 and 2.
Statistical Approach to test a priori hypotheses
We performed two sets of voxelwise multiple regression analyses(one for each hypothesis) to determine which a priori dimensional(primary hypothesis) and categorical(secondary hypothesis) variables were significantly associated with neural activity to Win>control and Loss>control, after accounting for demographic(age, IQ, sex), scan site, signal:noise ratio(SNR;see below), and medication status(taking versus not taking psychotropic medication) variables of no interest. To avoid model overfitting and balance Type I and II errors, we adopted the following approach. First, we examined the univariate relationship between each of our variables(i.e., variables of interest and variables of no interest) and neural activity using a p<0.05 voxelwise and p<.05 clusterwise significance threshold. Variables that demonstrated a significant relationship were then added to a final multiple regression model containing all such variables. This allowed us to identify those variables that remained significant in the final multiple regression model, after accounting for all other variables of interest and variables of no interest. This procedure was repeated twice, once for the primary hypothesis involving the four dimensional symptom measures(K-DRS, K-MRS, PGBI-10M, SCARED) and variables of no interest, and once for the secondary hypothesis involving diagnostic categories(BPSD, ADHD, DBD, anxiety disorders) and variables of no interest.
Finally, in order to determine the extent to which any observed relationships between dimensional measures and neural activity represented abnormalities in neural activity, we compared neural activity of LAMS youth with that of the HY. To do this, we identified the 20 highest and 20 lowest scoring LAMS youth on the dimensional measure of interest, and each of these two groups was compared with the 20 HY. For these analyses, we examined group differences in those neural regions showing the associations between the dimensional measure and neural activity, using a voxelwise threshold of p<0.025 to control for the two between-group pairwise comparisons (20 highest scoring LAMS youth vs. HY, and 20 lowest scoring LAMS youth vs. 20 HY; p<0.05, corrected threshold). All three groups were matched on group means for age, IQ, and on sex ratio. Comparing the two groups of LAMS youth with the HY thereby allowed us to determine whether the pattern of neural activity was associated with the dimensional construct per se(ie., if the highest scoring LAMS sample, but not the lowest scoring LAMS sample, differed significantly from HY in this pattern of neural activity), or was associated with psychopathology more generally(i.e., if both LAMS samples differed from HY in this pattern of neural activity).
We conducted similar analyses regarding our secondary hypothesis. That is, when a significant relationship with a diagnostic category was identified in the multiple regression analysis, a follow-up analysis was conducted to further examine the extent to which this represented a pattern of abnormal neural activity versus HY. Here, similar analyses were performed as for our primary hypothesis, but this time comparing the 20 LAMS youth with, and 20 LAMS youth without, the diagnosis in question with the 20 HY. Here, all three groups were matched on group means for age, IQ, and on sex ratio.
Analyses of multi-site neuroimaging data: strategies to reduce inter-site signal variability
We implemented several recommended measures to reduce inter-scan site variability: using global signal normalization in first-level analyses(50)(eText), monitoring scanner signal stability over time(eText, eTable 4), using scan site and SNR as covariates when appropriate(described above), and examining whether the main findings were paralleled by similar patterns of neural activity-behavioral relationships at each individual site(eText, eTable 9).
Exploratory Analyses
Exploratory wholebrain(voxelwise p<.001;clusterwise corrected p<.05) analyses were conducted to Win>control and Loss>control contrasts to determine the extent to which patterns of wholebrain activity to these two stimulus contrasts were similar to patterns of neural activity in our a prior bilateral ROI mask.
Results
In all LAMS youth, Win>control significantly activated bilateral dACC(BA32), left mPFC, and bilateral VLPFC(p<.025, corrected p<.05;Figure 2A;color bars:t-values;Table 1). Loss>control significantly activated bilateral dACC(BA32) and right VLPFC(p<.025, corrected p<.05;Table 1). Exploratory wholebrain analyses revealed similar activation patterns(eTable 5).
Figure 2.
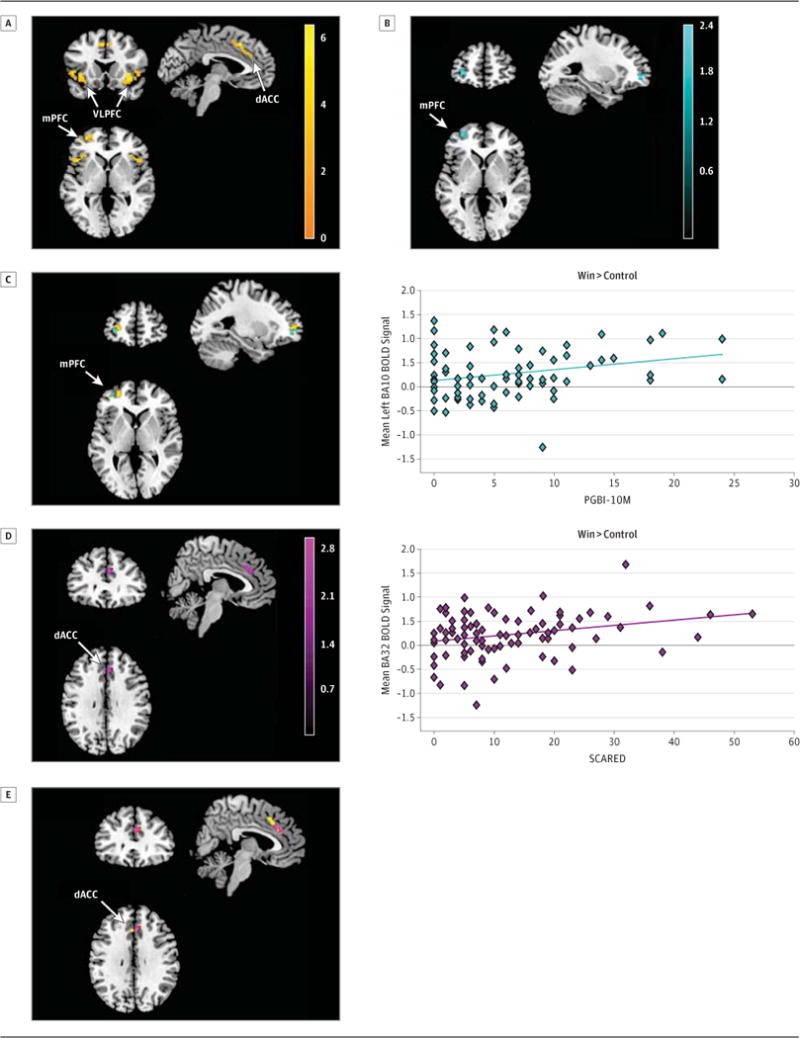
Entire bilateral ROI mask analysis to Win>control in LAMS youth (n=85)
(A)Left mPFC, bilateral dACC, and bilateral VLPFC activity (orange)
(B)Left mPFC activity and PGBI-10M(teal)(r=.28)
(C)Overlap between left mPFC activity in (A) and (B).
(D)Bilateral dACC activity and SCARED (purple) (r=.27).
(E)Overlap between dACC activity in (A) and (D).
Table 1. Reward-related neural activity in all LAMS youth (N=85).
Region of interest analyses using voxelwise p<.025 and p<.05, AlphaSim corrected. Each row in the table represents the peak voxel within the specified region. Abbreviations: BA = Brodmann area; dACC=dorsal anterior cingulate cortex; df=degrees of freedom; k = cluster size in voxels; MNI=Montreal Neurological Institute coordinates; mPFC=middle prefrontal cortex; puncorrected=uncorrected voxelwise p value; t=t-test statistical value; VLPFC=ventrolateral prefrontal cortex
| MNI Coordinates | Statistic | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Region | BA | k | x | y | z | Test Statistic (df) | puncorrected |
| Loss>Control | |||||||
| Left dACC | 32 | 76 | −6 | 17 | 46 | t(82)=5.94 | <0.001 |
| Right dACC | 32 | 76 | 3 | 20 | 46 | t(82)=5.62 | <0.001 |
| Right VLPFC | 47 | 24 | 30 | 20 | −8 | t(82)=4.56 | <0.001 |
| Win>Control | |||||||
| Left dACC | 32 | 46 | −3 | 20 | 46 | t(82)=6.16 | <0.001 |
| Right dACC | 32 | 58 | 3 | 20 | 46 | t(82)=6.35 | <0.001 |
| Left mPFC | 10 | 38 | −39 | 47 | 1 | t(82)=5.37 | <0.001 |
| Left VLPFC | 47 | 56 | −45 | 17 | 1 | t(82)=4.71 | <0.001 |
| Right VLPFC | 47 | 57 | 30 | 20 | −5 | t(82)=5.98 | <0.001 |
Primary Hypothesis (Dimensional)
Initial univariate analyses revealed that the following symptom dimensional variables showed significant positive relationships(p<0.05, corrected) with Win>control neural activity: PGBI-10M and left mPFC (23 voxels), and SCARED and right dACC(BA32, 20 voxels). For Loss>control, no significant relationships with any of the four dimensional measures were observed. Thus we did not perform further analyses for Loss>control.
Univariate analyses revealed the following significant relationships(p<0.05, corrected) to Win>control neural activity and variables of no interest: a positive relationship between age and bilateral dACC(Left=25 voxels, Right=22 voxels) and right VLPFC (13 voxels); sex and left dACC (15 voxels) and right VLPFC (35 voxels), with females>males; and a negative relationship between SNR and right VLPFC (40 voxels). Medication status(taking versus not taking psychotropic medication) was not significantly associated with Win>control neural activity.
We added these three variables of no interest(age, sex, SNR) as covariates to a multiple regression model containing the two significant dimensional measures(PGBI-10M, SCARED). The two relationships between dimensional measures and neural activity observed in univariate analyses remained significant when these three covariates were added to the model(both p<0.05, voxelwise, p<0.05, corrected within the Win>control activity mask): PGBI-10M and left anteriolateral mPFC (20 voxels; Pearson r=0.28, p=0.009; Spearman r=0.23; p=0.031 on extracted left anteriolateral mPFC BOLD signal values; Figure 2B–C, color bars= t-values); and SCARED and right ventral dACC (21 voxels; Pearson r=0.27, p=0.011; Spearman r=0.21, p=0.05; Figure 2D–E, color bars= t-values; Table 2). See eText for associations between the three covariates and neural activity from this model.
Table 2. Symptom measures associated with reward-related neural activity in all LAMS youth (N=85).
Regression analyses of Win>control neural activity using a voxelwise p<.05 and p<0.05, AlphaSim corrected. Each row in the table represents the peak voxel within the specified region. Abbreviations: BA=Brodmann area; dACC=dorsal anterior cingulate cortex; df=degrees of freedom; k=cluster size in voxels; MNI=Montreal Neurological Institute coordinates; mPFC=middle prefrontal cortex; puncorrected=uncorrected voxelwise p value; PGBI-10M=Parent General Behavior Inventory 10 Item Mania scale; r=Pearson’s correlation coefficient; SCARED=Screen for Child Anxiety Related Emotional Disorders (child rating).
| MNI Coordinates | Statistic | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Region | BA | k | x | y | z | Test Statistic (df) | puncorrected |
| PGBI-10M | |||||||
| Left mPFC | 10 | 20 | −33 | 53 | −2 | r(79)=0.28 | .008 |
| SCARED | |||||||
| Right dACC | 32 | 21 | 3 | 32 | 28 | r(79)=.27 | .002 |
Regarding the comparison with HY, HY showed significantly less left anteriolateral mPFC activity than both LAMS youth with high PGBI-10M scores[ 20 voxels, t(36)=2.75; voxelwise p<.025; p<.05, corrected; Cohen’s d=.92] and LAMS youth with low PGBI-10M scores[ 11 voxels; t(36)=2.42; p<.025, p<.05, corrected; d=.81](Figure 3, color bars= t-values; eTable 6). There were no significant differences in right ventral dACC activity to Win>control among the 20 LAMS youth with highest SCARED score, 20 LAMS youth with the lowest SCARED score, and 20 HY, although the highest SCARED score group scored significantly higher on the SCARED than both the lowest SCARED group and HY(eTable 7).
Figure 3.
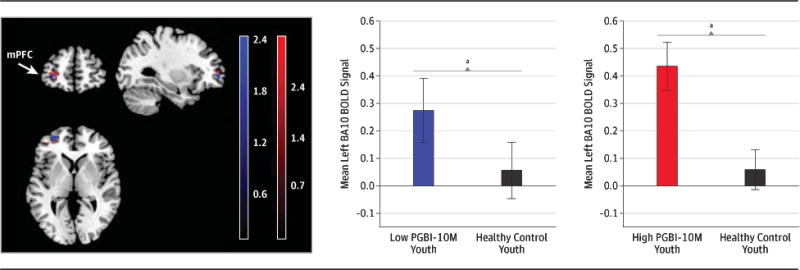
Group contrast of Win>control activity in left mPFCclusters determined in analyses depicted in Figure 2(A). Relative to healthy youth (n=20) (black) LAMS youth with high PGBI-10M scores (n=20) (red) and LAMS youth with low PGBI-10M scores (n=20) (blue) had greater left mPFC activity.
Secondary Hypothesis (Categorical)
Initial univariate analyses revealed that of the three categorical disorders tests, only DBD showed a significant relationship(p<0.05, corrected) with significant clusters of activity to Win>control in left VLPFC (21 voxels). There were no significant relationships between any diagnostic category and neural activity to Loss>control. Thus, we did not perform further analyses to Loss>control.
When the three variables of no interest showing significant relationships with Win>control neural activity described above(age, sex, SNR) were added to the multiple regression model, youth with DBD continued to show significantly reduced activity (p<0.05 voxelwise, p<0.05, corrected within the Win>control activity mask) than youth without these disorders in left lateral VLPFC (19 voxels; t(83)=2.68, p=0.009; With DBD: M=−0.40, SD=0.46; Without DBD: M=0.28, SD=0.35).
Regarding the comparison with HY, HY had significantly less left lateral VLPFC activity than either LAMS youth with DBD[ t(32)=3.69; p<.05, corrected; d=1.30] or LAMS youth without DBD [t(36)=3.70; p<.05, corrected, d=1.23] (eTable 8).
To examine whether the association between DBD and neural activity was independent of the associations between the two dimensional measures(PGBI-10M; SCARED), we constructed a final multiple regression model with each of these three variables as well as the significant covariates identified above(age, sex, SNR). The two positive relationships between dimensional measures and neural activity remained significant when DBD was added to the model(both p<0.05, corrected within the Win>control activity mask): PGBI-10M and left anteriolateral mPFC (24 voxels); and SCARED and bilateral ventral dACC(Left=13 voxels; Right=21 voxels). Likewise, youth with DBD continued to show significantly reduced activity(p<0.05, corrected) than youth without these disorders in left lateral VLPFC (19 voxels).
Comment
The overall goal of the present study was to identify measures of activity in reward processing neural circuitry that were related to behavioral and emotional dysregulation in a large cohort of youth with a variety of different diagnoses. We aimed to determine the extent to which these neuroimaging measures were either associated with dimensions of behavioral and emotional dysregulation, irrespective of diagnosis, or instead differentiated diagnostic categories. In support of our dimension-focused primary hypothesis, greater PGBI-10M score, a stable measure of behavioral and emotional dysregulation within 6 months of scanning, was associated with greater left anteriolateral mPFC activity to Win. Greater anxiety on the scanning day was associated with greater right ventral dACC activity to Win. In support of our secondary diagnostic category-focused hypothesis, youth with DBD had lower left VLPFC activity to Win than youth without these disorders. Importantly, these findings remained after including both dimensional measures and DBD in the same multiple regression model, and even after accounting for demographic and SNR variables that showed significant relationships with Win-related neural activity. Overall, LAMS youth activated bilateral dACC to Win(>control) and Loss(>control), suggesting that LAMS youth attended to both Win and Loss contexts, given the role of the dACC in attentional processing(51). Our findings that greater right ventral dACC, part of the ACC affective subdivision(51), activity to Win was associated with greater anxiety suggest that more anxious youth may have attended preferentially to Win. There were no significant right ventral dACC activity differences among high anxious LAMS youth, low anxious LAMS youth, and HY, however. This may be due to the greater power of a dimensional, rather than a categorical(e.g., between-group) approach for detecting brain-behavioral relationships(52). All LAMS youth also activated bilateral VLPFC to Win and right VLPFC to Loss, suggesting that both contexts were evaluated as salient, given the role of the VLPFC in evaluation of emotionally-salient contextual information(53). The right-sided focus of VPLFC activity to Loss may, however, reflect the right hemisphere’s role in processing withdrawal-related emotional contexts(54).
Left mPFC was activated only to Win. Given the putative role of the left PFC in approach-related emotion processing(55), the role of the mPFC in risky decision making in potentially rewarding contexts(27–28), and the relationship between PGBI-10M and BAS subscales shown in a diagnostically heterogeneous cohort of youth(Youngstrom, personal communication), the positive relationship between left mPFC activity to Win and PGBI-10M in LAMS youth suggests that activity in this region may be a biomarker of behavioral and emotional dysregulation and heightened reward sensitivity in rewarding contexts in these youth. Our additional finding that both the 20 LAMS youth with the highest and lowest PGBI-10M scores showed significantly greater left mPFC activity to Win than 20 age, IQ, and sex-matched HY suggests that elevated left mPFC activity to Win may represent an abnormal pathophysiologic process in LAMS youth. These findings parallel previous reports of elevated left prefrontal cortical activity to reward across different mood disordered individuals versus healthy control participants(30, 33), and reports of heightened reward sensitivity in individuals with bipolar disorder(21, 30, 32). Our present finding thus suggests that elevated left prefrontal activity may reflect heightened sensitivity to reward-related cues and may be a biomarker of pathophysiologic processes associated with behavioral and emotional dysregulation and heightened reward sensitivity across different diagnoses in youth.
Of all diagnostic categories examined, only DBD showed disorder-specific abnormalities in reward circuitry: significantly reduced left VLPFC activity to Win in LAMS youth with, versus those without, these disorders. These findings parallel previous reports of impaired functioning within OFC during reward processing in youth with conduct disorder(38–39), and in individuals with higher levels of psychopathic traits(56–57). Given the role of the OFC and VLPFC in evaluation of reward and emotional contexts, these findings suggest that youth with DBD may evaluate rewarding contexts as less salient than youth without these disorders. This, in turn, may be associated with reduced reward sensitivity and result in the socially inappropriate behaviors characteristic of these youth(58). Additionally, both LAMS youth with and without DBD showed significantly greater left VLPFC activity to Win relative to age, IQ, and sex-matched HY, possibly because both LAMS subgroups had comorbid mood disorders. Further studies are needed to clarify the extent to which DBD may be associated with impaired functioning in reward circuitry in youth without behavioral and emotional dysregulation.
No significant clusters of activity in VS or OFC were shown by LAMS youth to Win, suggesting prefrontal cortical-level attention and evaluative decision-making processing, rather than subcortical-level prediction error encoding or OFC-centered valuation, of reward in these youth. This may reflect the relatively high degree of certainty that participants had of obtaining reward(and thus low levels of prediction error and valuation) during Win blocks. Interestingly, mania and depression were not associated with significant activity in a priori ROIs. These findings suggest that elevated left mPFC and bilateral dACC activity during reward processing may represent pathophysiologic processes underlying behavioral and emotional dysregulation, reward sensitivity, and anxiety, but are not associated with the types of behaviors specifically measured by mania and depression rating scales.
There were limitations to the study. We adopted an ROI approach in our analyses, given findings associating activity in specific ROIs during reward processing in healthy individuals(30, 33). Exploratory whole brain analyses, however, showed that patterns of wholebrain activity to the Win and Loss contrasts were similar to patterns of neural activity in our a prior bilateral ROI mask. Most participants were medicated(n=52), and of those, 23 were taking more than one class of psychotropic medications. While we did not have enough statistical power to assess how using one versus multiple psychotropic medications influenced reward-related neural activity, univariate regression analyses revealed no significant effect of medication status(taking versus not taking psychotropic medication) on Win>control neural activity. The use of atypical antipsychotic medication, used by approximately 27% of LAMS youth, may have influenced reward-related neural activity through dopamine receptor blocking(59). We were unable to specifically examine this relationship, however, due to low statistical power for assessing potential individual medication confounds arising from: 1)use of different types of atypical antipsychotics, which have different neurobiological mechanisms; and 2)interactions between atypical antipsychotics and different classes of psychotropic medications, such as antidepressants, which may also influence dopaminergic activity. We did not include a measure of pubertal status, which has been associated with medial prefrontal activity(dACC)(42) during reward outcome. The next phase of LAMS neuroimaging will include a self-report of pubertal status(60). While an event-related design may have been more powerful for identifying interactions between groups and neural activity, our block design may have been more powerful for obtaining robust and statistically powerful neuroimaging findings(61).
Youth were scanned at multiple sites, but inter-scanner differences were minimized by monitoring SNR monthly at each scan site; using global signal normalization during fMRI processing; and including scan site and SNR as covariates in analyses when appropriate. When SNR was included as a covariate, it was associated with a different pattern of neural activity from the main clinical measures of interest(eText). Additionally, neural activity-behavioral relationships at each site were very similar to the main dimensional and categorical findings over all sites. The advantages of multi-site neuroimaging(increased statistical power and a participant population from a variety of different environments) are likely to outweigh potential limitations. Although the range of PGBI-10M scores (0–24) captured both low and high levels of behavioral and emotional dysregulation in LAMS youth, the mean PGBI-10M score was low (6.09). Further studies should replicate our findings and aim to examine youth with higher mean scores on this scale. Finally, PGBI-10M scores were not collected for HY, so we were unable to compare scores between LAMS and HY.
There is a pressing need for objective biomarkers reflecting underlying pathophysiologic processes in psychiatric disorders in youth. The large cohort of symptomatically at-risk youth in LAMS provided a unique opportunity to examine the extent to which measures of function within neural circuitry supporting reward processing reflected dimensions of pathology regardless of diagnosis or was associated with specific diagnostic categories. Our findings support a dimensional approach to the study of neural mechanisms in behaviorally and emotionally dysregulated youth, paralleling the focus of the NIMH RDoC. We also found evidence for distinct neurophysiologic processes during reward processing in youth with DBD. The combination of dimensional and diagnostic categorical approaches may identify biomarkers that can ultimately help identify and guide treatment for youth with, or at risk for, behavioral and emotional dysregulation pathology.
Supplementary Material
Acknowledgments
Supported by the National Institute of Mental Health grants 2R01 MH73953-06A1 (Dr. Boris Birmaher and Dr. Mary L. Phillips, University of Pittsburgh), 2R01 MH73816-06A1 (Dr. Scott Holland, Children’s Hospital Medical Center), 2R01 MH73967-06A1 (Dr. Robert Findling, Case Western Reserve University), and 2R01 MH73801-06A1 (Dr. Mary Fristad, Ohio State University).
References
- 1.Leibenluft E, Charney DS, Pine DS. Researching the pathophysiology of pediatric bipolar disorder. Biological Psychiatry. 2003;53(11):1009–20. doi: 10.1016/s0006-3223(03)00069-6. [DOI] [PubMed] [Google Scholar]
- 2.Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, et al. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. Journal of Child Psychology and Psychiatry. 2006;47(10):1031–40. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abela JRZ, Hankin BL. Handbook of depression in children and adolescents. Guilford Press; 2007. [Google Scholar]
- 4.Barkley R. Deficient emotional self-regulation: a core component of attention-deficit/hyperactivity disorder. J ADHD Relat Disord. 2010;1(2):5–37. [Google Scholar]
- 5.Maedgen JW, Carlson CL. Social functioning and emotional regulation in the attention deficit hyperactivity disorder subtypes. Journal of Clinical Child Psychology. 2000;29(1):30–42. doi: 10.1207/S15374424jccp2901_4. [DOI] [PubMed] [Google Scholar]
- 6.Musser ED, Backs RW, Schmitt CF, Ablow JC, Measelle JR, Nigg JT. Emotion regulation via the autonomic nervous system in children with attention-deficit/hyperactivity disorder (ADHD) Journal of Abnormal Child Psychology. 2011;39(6):841–52. doi: 10.1007/s10802-011-9499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clinical Psychology Review. 2005;25(2):183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Cole PM, Zahn-Waxler C. Emotional dysregulation in disruptive behavior disorders. 1992 [Google Scholar]
- 9.O’Brien BS, Frick PJ. Reward dominance: Associations with anxiety, conduct problems, and psychopathy in children. Journal of Abnormal Child Psychology. 1996;24(2):223–40. doi: 10.1007/BF01441486. [DOI] [PubMed] [Google Scholar]
- 10.Cole PM, Michel MK, Teti LOD. The development of emotion regulation and dysregulation: A clinical perspective. Monographs of the Society for Research in Child Development. 1994;59(2–3):73–102. [PubMed] [Google Scholar]
- 11.Hirshfeld DR, Rosenbaum JF, Biederman J, Bolduc EA, Faraone SV, Snidman N, et al. Stable behavioral inhibition and its association with anxiety disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 1992;31(1):103–11. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: a review of the past 10 years. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44(9):846–71. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- 13.Arnold LE, Demeter C, Mount K, Frazier TW, Youngstrom EA, Fristad M, et al. Pediatric bipolar spectrum disorder and ADHD: comparison and comorbidity in the LAMS clinical sample. Bipolar Disorders. 2011;13(5–6):509–21. doi: 10.1111/j.1399-5618.2011.00948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold LE, Mount K, Frazier T, Demeter C, Youngstrom EA, Fristad MA, et al. Pediatric bipolar disorder and ADHD: Family history comparison in the LAMS clinical sample. Journal of affective disorders. 2012 doi: 10.1016/j.jad.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kowatch RA, Youngstrom EA, Danielyan A, Findling RL. Review and meta-analysis of the phenomenology and clinical characteristics of mania in children and adolescents. Bipolar Disorders. 2005;7(6):483–96. doi: 10.1111/j.1399-5618.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 16.Insel TR, Cuthbert BN, Garvey MA, Heinssen RK, Pine DS, Quinn KJ, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 17.Youngstrom E, Meyers O, Demeter C, Youngstrom J, Morello L, Piiparinen R, et al. Comparing diagnostic checklists for pediatric bipolar disorder in academic and community mental health settings. Bipolar Disorders. 2005;7(6):507–17. doi: 10.1111/j.1399-5618.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- 18.Youngstrom EA, Frazier TW, Demeter C, Calabrese JR, Findling RL. Developing a Ten Item Mania Scale from the Parent General Behavior Inventory for Children and Adolescents. The Journal of clinical psychiatry. 2008;69(5):831. doi: 10.4088/jcp.v69n0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Findling RL, Youngstrom EA, Fristad MA, Birmaher B, Kowatch RA, Arnold LE, et al. Characteristics of children with elevated symptoms of mania: the Longitudinal Assessment of Manic Symptoms (LAMS) study. The Journal of clinical psychiatry. 2010;71(12):1664. doi: 10.4088/JCP.09m05859yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horwitz SMC, Demeter C, Pagano ME, Youngstrom EA, Fristad MA, Arnold LE, et al. Longitudinal Assessment of Manic Symptoms (LAMS) Study: background, design and initial screening results. The Journal of clinical psychiatry. 2010;71(11):1511. doi: 10.4088/JCP.09m05835yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abler B, Herrnberger B, Grön G, Spitzer M. From uncertainty to reward: BOLD characteristics differentiate signaling pathways. BMC neuroscience. 2009;10(1):154. doi: 10.1186/1471-2202-10-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38(2):329–37. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 23.Pagnoni G, Zink CF, Montague PR, Berns GS. Activity in human ventral striatum locked to errors of reward prediction. Nature neuroscience. 2002;5(2):97–8. doi: 10.1038/nn802. [DOI] [PubMed] [Google Scholar]
- 24.Kahnt T, Heinzle J, Park SQ, Haynes JD. The neural code of reward anticipation in human orbitofrontal cortex. Proceedings of the National Academy of Sciences. 2010;107(13):6010. doi: 10.1073/pnas.0912838107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Progress in neurobiology. 2008;86(3):216–44. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences. 2002;99(1):523. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrence NS, Jollant F, O’Daly O, Zelaya F, Phillips ML. Distinct roles of prefrontal cortical subregions in the Iowa Gambling Task. Cerebral Cortex. 2009;19(5):1134–43. doi: 10.1093/cercor/bhn154. [DOI] [PubMed] [Google Scholar]
- 28.Xue G, Lu Z, Levin IP, Weller JA, Li X, Bechara A. Functional dissociations of risk and reward processing in the medial prefrontal cortex. Cerebral Cortex. 2009;19(5):1019–27. doi: 10.1093/cercor/bhn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: Relations of the BIS/BAS scales with symptoms. Journal of Psychopathology and Behavioral Assessment. 2001;23(3):133–43. doi: 10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nusslock R, Almeida JRC, Forbes EE, Versace A, Frank E, LaBarbara EJ, et al. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disorders. 2012;14(3):249–60. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urosevic S, Abramson LY, Harmon-Jones E, Alloy LB. Dysregulation of the behavioral approach system (BAS) in bipolar spectrum disorders: Review of theory and evidence. Clinical Psychology Review. 2008;28(7):1188–205. doi: 10.1016/j.cpr.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bermpohl F, Kahnt T, Dalanay U, Hägele C, Sajonz B, Wegner T, et al. Altered representation of expected value in the orbitofrontal cortex in mania. Human brain mapping. 2010;31(7):958–69. doi: 10.1002/hbm.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caseras XLN, Murphy K, Wise R, Phillips ML. Ventral-striatum activity to reward anticipation distinguishes bipolar I from bipolar II disorder and is positively associated with reward sensitivity across bipolar and healthy adults. American Journal of Psychiatry. In press. [Google Scholar]
- 34.Dickstein DP, Finger EC, Skup M, Pine DS, Blair JR, Leibenluft E. Altered neural function in pediatric bipolar disorder during reversal learning. Bipolar Disorders. 2010;12(7):707–19. doi: 10.1111/j.1399-5618.2010.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009 doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 36.Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61(5):720–4. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 37.Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K, et al. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. The American journal of psychiatry. 2012;169(2):205. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubia K, Smith A, Halari R, Matsukura F, Mohammad M, Taylor E, et al. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. American Journal of Psychiatry. 2009;166(1):83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- 39.Finger EC, Marsh AA, Blair KS, Reid ME, Sims C, Ng P, et al. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. American Journal of Psychiatry. 2011;168(2):152–62. doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forbes E, Brown S, Kimak M, Ferrell R, Manuck S, Hariri A. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular psychiatry. 2009;14(1):60. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 2009;166(1):64. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, et al. Healthy adolescents’ neural response to reward: Associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(2):162–72. e5. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. Journal of Child and Adolescent Psychopharmacology. 2003;13(4):463–70. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- 44.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 45.Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, et al. The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(4):545–53. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- 46.Di Martino A, Scheres A, Margulies D, Kelly A, Uddin L, Shehzad Z, et al. Functional connectivity of human striatum: a resting state FMRI study. Cerebral Cortex. 2008;18(12):2735–47. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 47.Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cerebral Cortex. 2006;16(10):1508–21. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- 48.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 49.Ward B. AlphaSim. National Institute of Mental Health; Bethesda, MD: 2002. [Google Scholar]
- 50.Eklund A, Andersson M, Josephson C, Johannesson M, Knutsson H. Does parametric fMRI analysis with SPM yield valid results?-an empirical study of 1484 rest datasets. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.03.093. [DOI] [PubMed] [Google Scholar]
- 51.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in cognitive sciences. 2000;4(6):215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 52.MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychological methods. 2002;7(1):19. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- 53.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry. 2003 doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 54.Davidson RJ, Ekman P, Saron CD, Senulis JA, Friesen WV. Approach-withdrawal and cerebral asymmetry: Emotional expression and brain physiology: I. Journal of personality and social psychology. 1990;58(2):330. [PubMed] [Google Scholar]
- 55.Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biological psychology. 2010;84(3):451–62. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Blair R. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in cognitive sciences. 2007;11(9):387–92. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 57.Blair R. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363(1503):2557–65. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matthys W, Vanderschuren LJMJ, Schutter DJLG. The neurobiology of oppositional defiant disorder and conduct disorder: Altered functioning in three mental domains. Development and psychopathology. 2012;1(1):1–15. doi: 10.1017/S0954579412000272. [DOI] [PubMed] [Google Scholar]
- 59.Yatham LN, Goldstein JM, Vieta E, Bowden CL, Grunze H, Post RM, et al. Atypical antipsychotics in bipolar depression: potential mechanisms of action. Journal of Clinical Psychiatry. 2005;66(Suppl 5):40–8. [PubMed] [Google Scholar]
- 60.Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–33. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- 61.Amaro E, Barker GJ. Study design in fMRI: Basic principles. Brain and cognition. 2006;60(3):220–32. doi: 10.1016/j.bandc.2005.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


