Abstract
Background
In patients with type 2 diabetes (T2D), incretin-based therapies improve glycaemic control with low incidence of hypoglycaemia and without weight gain, both advantages over traditional add-ons to metformin. Dipeptidyl peptidase-4 (DPP-4) inhibitors are administered orally and provide a physiological increase in glucagon-like peptide-1 (GLP-1) levels, while GLP-1 receptor agonists (GLP-1RAs) are injectable and deliver pharmacological levels of GLP-1RA. This review aims to distinguish between GLP-1RAs and DPP-4 inhibitors, and discuss when each may be favoured in clinical practice.
Methods
A MEDLINE search, limited to human clinical trials and using the search criteria ‘GLP-1RA’ or ‘DPP-4 inhibitor’, identified seven head-to-head studies and one relevant post hoc analysis (all a GLP-1RA vs. the DPP-4 inhibitor sitagliptin). In combination with treatment algorithms, product prescribing information and personal clinical experience, these studies were used to compare the efficacy and suitability of GLP-1RAs and DPP-4 inhibitors in patients with T2D.
Results
In head-to-head clinical trials, GLP-1RAs provided greater glycaemic control, weight loss and overall treatment satisfaction vs. the DPP-4 inhibitor sitagliptin. Transient nausea was more frequent with GLP-1RAs and should be addressed through patient education and an incremental dosing approach. Current treatment algorithms recommend incretin-based therapy use after metformin failure, but local guidance may restrict their use.
Conclusion
GLP-1RAs provide superior glycaemic control and weight loss vs. DPP-4 inhibitors in patients with T2D. DPP-4 inhibitors may sometimes be preferred to a GLP-1RA if weight is not a concern, oral administration is a desirable feature or when a GLP-1RA cannot be tolerated.
Review criteria
MEDLINE searches were performed to include publications comparing GLP-1RAs and DPP-4 inhibitors in patients with T2D. MeSH search terms used were ‘GLP-1RA’ or ‘DPP-4 inhibitor’. All phase III trials and post hoc analyses were selected following review of titles, abstracts and whether the trial studied a licensed indication of the agents. Published treatment algorithms, product prescribing information and personal clinical experience are also discussed.
Message for the clinic
Clinical evidence demonstrates that GLP-1RAs provide superior glycaemic control and weight loss compared with DPP-4 inhibitors, suggesting that GLP-1RAs are an appropriate and effective treatment where local guidelines allow.
Because of their oral administration, there are some instances when the use of DPP-4 inhibitors is preferable.
Ultimately, when making treatment decisions, clinicians should consider the individual needs of each patient.
Introduction
Traditional therapies available to patients with type 2 diabetes (T2D) after metformin failure [sulphonylureas (SUs), thiazolidinediones (TZDs)] are often associated with drawbacks such as weight gain, hypoglycaemia or poor long-term efficacy. The incretin-related therapies dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs) not only improve glycaemic control with a low risk of hypoglycaemia but can also have beneficial non-glycaemic effects such as avoidance of weight gain, reduced blood pressure and improvements in beta-cell function and cardiovascular risk biomarkers 1–3.
From personal experience, it appears that there is a misconception among some clinicians that DPP-4 inhibitors are essentially orally administered GLP-1RAs. This review aims to distinguish between the two treatment classes.
Incretin physiology
The incretins are a group of hormones produced by the gastrointestinal system that enhance insulin secretion in a glucose-dependent manner; the combined incretin response accounts for 50–70% of total postprandial insulin production 4,5. The two main human incretins are GLP-1 and glucose-dependent insulinotropic peptide (GIP). In addition to direct insulinotropic action, animal data suggest that incretin hormones may also have protective effects on the beta-cell by enhancing proliferation and resistance to apoptosis 6. GLP-1 also promotes satiety and inhibits glucose-dependent glucagon secretion, as well as reducing hepatic glucose production 6,7. Within the gut, GLP-1 exerts a motility-inhibiting effect and slows gastric emptying 6.
In patients with T2D, the response to GIP is impaired. Unlike GLP-1, GIP infusion in patients with T2D does not amplify the late-phase insulin response to glucose 8,9. Furthermore, the addition of GIP to a concurrent GLP-1 infusion not only provides no further glycaemic benefit but also antagonises GLP-1-induced glucagon suppression 9. Therefore, incretin-based therapeutic intervention has focused on GLP-1. However, native GLP-1 has limited pharmacological value because of its short half-life (1–2 min), attributable to degradation by the peptidase enzyme DPP-4 10. Two strategies have been employed to elevate and sustain GLP-1-mediated effects over prolonged periods: inhibition of DPP-4, which extends the half-life of endogenous GLP-1, and is therefore dependent on endogenous GLP-1 production (DPP-4 inhibitors); and use of GLP-1RAs resistant to DPP-4 degradation that can provide supraphysiological stimulation of the GLP-1R. The therapeutic potential of DPP-4 inhibitors and GLP-1RAs is dependent on their different modes of action.
DPP-4 inhibitors and GLP-1RAs: what is the difference?
DPP-4 inhibitors are small molecular-weight drugs that inhibit ≥ 90% of DPP-4 activity and are orally administered on a once-daily (OD) basis [vildagliptin twice daily (BID)] 11,12. There are currently three Food and Drug Administration (FDA)-approved DPP-4 inhibitors: sitagliptin, saxagliptin and linagliptin. In the European Union (EU), a fourth, vildagliptin, is also available.
The GLP-1RAs are peptide-based therapies and therefore, such as insulin, require subcutaneous injection to avoid degradation by gastrointestinal enzymes. There are currently three approved DPP-4-resistant GLP-1RA therapies: exenatide, a GLP-1-like xenopeptide and two GLP-1RAs – liraglutide, a human GLP-1 analogue, and the recently approved exendin-4-based agent lixisenatide. Exenatide and lixisenatide are synthetic forms of the naturally occurring peptide exendin-4 and both share approximately 50% sequence identity with native GLP-1 13,14. Exenatide is available as a BID or once-weekly (OW) formulation where the latter comprises exenatide encapsulated in microspheres of poly (d,l lactic-co-glycolic acid) for gradual drug delivery 15. Lixisenatide is administered OD 16. Liraglutide is a human GLP-1 analogue that shares 97% amino acid sequence identity with native GLP-1. Liraglutide reversibly binds to albumin, increasing plasma half-life and allowing OD dosing 17. Unlike exenatide and lixisenatide, which are predominantly eliminated by glomerular filtration with subsequent proteolytic degradation, liraglutide is largely metabolised prior to excretion, with no specific organ identified as a major route of elimination 16,18,19.
The main patient-perceived difference between DPP-4 inhibitors and GLP-1RAs is likely to be their mode of administration: oral (DPP-4 inhibitors) vs. injection (GLP-1RAs). Although it is believed that patients generally oppose injectable therapies, evidence suggests that this is not always the case, especially if the injectable therapy has greater efficacy 20–22.
Efficacy of DPP-4 inhibitors and GLP-1RAs in clinical trials
In clinical trials, comparable HbA1c reductions of 0.4–0.7% have been reported with sitagliptin (100 mg OD), vildagliptin (50 mg BID), saxagliptin (5 mg OD), or liniagliptin (5 mg OD) monotherapy for 26 weeks 23. GLP-1RAs (liraglutide 1.2 or 1.8 mg OD, exenatide 10 μg BID, exenatide OW, or lixisenatide 20 μg OD), by comparison, result in HbA1c reductions of 0.6–1.9% following 24/26/30 weeks of treatment as dual (+metformin) or triple (+metformin + SU/TZD) therapy 24–29. Comparing the individual GLP-1RAs, liraglutide 1.8 mg has been shown to provide greater reductions in HbA1c than both exenatide BID (−1.12% vs. −0.79%; p < 0.0001) and exenatide OW (−1.48% vs. −1.28%) in head-to-head studies 30,31.
GLP-1RAs are typically associated with weight loss (1–3 kg after 26/30 weeks), whereas DPP-4 inhibitors are generally weight-neutral, again possibly reflecting the limited increase in GLP-1R stimulation with DPP-4 inhibitors 23,25,32,33. Direct comparisons of the GLP-1RAs have suggested that liraglutide 1.8-mg treatment may result in greater weight loss than exenatide BID [−3.24 vs. −2.87 kg; estimated treatment difference (ETD) −0.38 kg (95% CI −0.99 to 0.23); p = 0.22] and exenatide OW [−3.58 vs. 2.68 kg; ETD 0.90 kg (95% CI 0.39–1.40); p < 0.001] 30,31. Because of their glucose-dependent mechanism of action, the risk of hypoglycaemia is low with both GLP-1RAs and DPP-4 inhibitors. However, the risk of hypoglycaemia is higher when either is used in combination with a SU 24,34–38.
Studies of GLP-1RAs in Asian populations have shown reductions in HbA1c that are comparable to or greater than those seen in global large randomised trials 39. Likewise, clinical evidence suggests that DPP-4 inhibitors exhibit greater HbA1c-lowering efficacy in Asians than in other ethnic populations 40. The mechanisms underlying these effects remain unclear, although they may potentially be caused by differences in pathophysiology of T2D among Asian patients, particularly in relation to body weight 39,40. However, as yet, no head-to-head studies have been conducted that compared the use of GLP-1RAs and DPP-4 inhibitors in Asian populations.
Aim of the review
Numerous clinical trials have compared the efficacy and safety of GLP-1RAs and DPP-4 inhibitors with placebo or oral antidiabetic drugs (OADs); however, few trials directly compare the two treatment classes. This review will focus on the results of trials directly comparing GLP-1RAs and DPP-4 inhibitors with the aim of distinguishing between the two treatment classes, and will also discuss clinical situations when each of the drug classes might be preferable.
Methods
Clinical trials directly comparing GLP-1RAs and DPP-4 inhibitors in patients with T2D were identified through a MEDLINE search using the search criteria ‘GLP-1RA’ or ‘DPP-4 inhibitor’. Nine relevant studies were identified, one of which was excluded because it compared the use of exenatide OW vs. sitagliptin, both as monotherapy, and exenatide OW is not licensed for monotherapy. Trial data, in combination with treatment algorithms, product prescribing information and personal clinical experience, were used to compare the efficacy and safety of GLP-1RAs and DPP-4 inhibitors in patients with T2D.
Results
Seven head-to-head studies and a post hoc analysis met the inclusion criteria. At the time of writing, the one study comparing lixisenatide with sitagliptin (NCT00976937) had not yet reported results.
Clinical performance: head-to-head studies of DPP-4 inhibitors and GLP-1RAs
Few studies have directly compared GLP-1RAs and DPP-4 inhibitors 41. In fact, of the DPP-4 inhibitors, only sitagliptin has been studied in comparison with GLP-1RAs. However, as individual agents within the DPP-4 inhibitor class have achieved similar efficacy in clinical trials, these head-to-head data should represent a fair comparison of GLP-1RAs and DPP-4 inhibitors in general 23,24.
Direct comparisons of exenatide BID with sitagliptin have been limited to two short cross-over clinical studies, a 4-week and 8-week study (Table1) 42,43. In patients with T2D uncontrolled on metformin, exenatide BID treatment provided significantly greater improvements in 24 h and postprandial glucose (PPG) levels vs. sitagliptin (Table1) 42,43. Switching from sitagliptin to exenatide BID reduced 2-h PPG, while switching from exenatide BID to sitagliptin increased 2-h PPG 42. Exenatide BID treatment also significantly slowed gastric emptying and reduced total daily caloric intake vs. sitagliptin, reflected by greater weight loss (Table1). No major hypoglycaemia was reported with either treatment, and common adverse events were mild-to-moderate gastrointestinal complaints (nausea, vomiting, diarrhoea), which were more frequent with exenatide treatment than sitagliptin 42,43.
Table 1.
Summary of glycaemic control and weight change data from GLP-1RA vs. DPP-4 inhibitor trials
| Study | Duration (n) | Treatment | Change in glycaemic control | Change in body weight |
|---|---|---|---|---|
| DeFronzo et al. 42 | 2 weeks (61) | Exen BID + Met | 2-h PPG: −6.2 mmol/l; p < 0.0001* | −0.8 kg; p = 0.006* |
| Sita + Met | 2-h PPG: −2.1 mmol/l | −0.3 kg | ||
| Switch 2 weeks (61) | Exen BID → Sita | 2-h PPG: +4.1 mmol/l | N/A | |
| Sita → Exen BID | 2-h PPG: −4.2 mmol/l | N/A | ||
| Berg et al. 43 | 4 weeks (86) | Exen BID + Met/TZD | 24-h glucose: −2.3 mmol/l; p < 0.001* 2-h PPG: −6.0 mmol/l; p < 0.001* | −1.37 kg; p < 0.05* |
| Sita + Met/TZD | 24-h glucose: −1.6 mmol/l 2-h PPG: −2.5 mmol/l | −0.89 kg | ||
| Bergenstal et al. 44 | 26 weeks (342) | Exen OW + Met | HbA1c: −1.5%; p < 0.0001* | −2.3 kg; p = 0.0002* |
| Sita + Met | HbA1c: −0.9% | −0.8 kg | ||
| Wysham et al. 45 | Switch 26 weeks (130) | Sita → Exen OW | HbA1c: −0.3%; p = 0.001† | −1.1 kg; p = 0.0006† |
| Pratley et al. 46 | 26 weeks (665) | Lira 1.2 mg + Met | HbA1c: −1.24%; p < 0.0001 vs. Sita | −2.9 kg; p < 0.0001 vs. Sita |
| Lira 1.8 mg + Met | HbA1c: −1.5%; p < 0.0001 vs. Sita | −3.4 kg; p < 0.0001 vs. Sita | ||
| Sita + Met | HbA1c: −0.9% | −1.0 kg | ||
| Pratley et al. 22 | 52 weeks (665) | Lira 1.2 mg + Met | HbA1c: −1.29%; p < 0.0001 vs. Sita | −2.8 kg; p < 0.0001 vs. Sita |
| Lira 1.8 mg + Met | HbA1c: −1.51%; p < 0.0001 vs. Sita | −3.7 kg; p < 0.0001 vs. Sita | ||
| Sita + Met | HbA1c: −0.88% | −1.2 kg | ||
| Pratley et al. 48 | Switch 26 weeks (419) | Sita → Lira 1.2 mg | HbA1c: −0.24%; p = 0.006† | −1.64 kg; p < 0.0001† |
| Sita → Lira 1.8 mg | HbA1c: −0.45%; p = 0.0001† | −2.48 kg; p < 0.0001† |
Exenatide BID: 10 μg BID following incremental dosing (5 μg BID for first week). Sitagliptin: 100 mg each morning. Exenatide OW: 2 mg OW. Liraglutide: incremental dosing; 0.6 mg OD for 2 weeks, 1.2 mg OD for 2 weeks, then 1.8 mg at week 4 if required. BID, twice daily; Exen, exenatide; Met, metformin; N/A, data not available; PPG, postprandial glucose; OD, once daily; OW, once weekly; Sita, sitagliptin; TZD, Thiazolidinedione.
Versus comparator treatment arm
versus baseline (preswitch) value.
Exenatide OW has been compared with sitagliptin in a longer 26-week randomised trial of patients with T2D inadequately controlled on metformin alone, in which exenatide OW resulted in significantly greater reductions in HbA1c and body weight compared with sitagliptin (Table1) 44. Again, there were no episodes of major hypoglycaemia and the most common adverse events with both treatments were gastrointestinal. As part of a 26-week study extension, patients were switched from sitagliptin to exenatide OW, resulting in significant further incremental decreases in HbA1c and body weight (Table1) 45.
A 26-week randomised, open-label trial compared the safety and efficacy of liraglutide (1.2 and 1.8 mg) with sitagliptin in patients with T2D uncontrolled on metformin 46. Significantly greater reductions in HbA1c and body weight were achieved with liraglutide 1.8 mg and liraglutide 1.2 mg compared with sitagliptin (Table1). Nausea was more frequent with liraglutide, but was transient in nature, and the proportion of patients experiencing minor hypoglycaemia was low (5%) in all groups. Liraglutide (1.2 and 1.8 mg) provided greater reduction in HbA1c than sitagliptin across the continuum of HbA1c (Figure1) 47. Following a 26-week extension period, during which prior improvements in HbA1c and weight were generally maintained 22, 419 patients switched from sitagliptin to liraglutide 1.2 or 1.8 mg for a further 26 weeks, resulting in significant improvements in HbA1c and body weight (Table1) 48.
Figure 1.
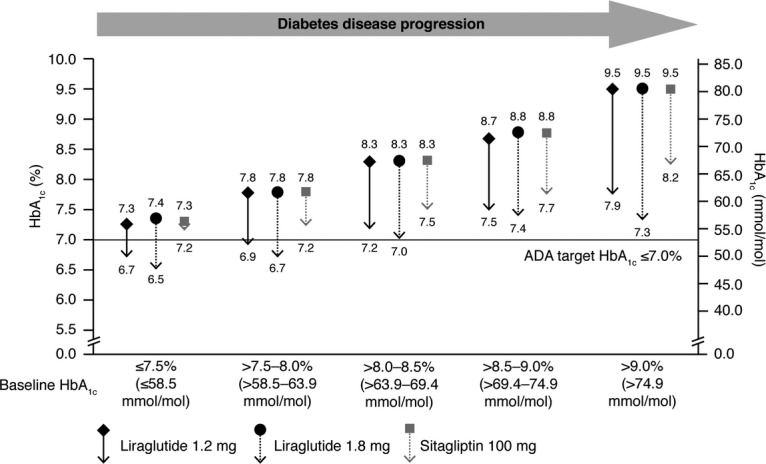
Effect of liraglutide and sitagliptin on HbA1c according to baseline HbA1c following 26 weeks’ treatment. Data from 47, originally presented as an oral at ADA 2010. Data are mean, LOCF, ITT analysis
Based on these trial data, the GLP-1RAs show a consistently superior blood glucose-lowering effect and result in greater weight loss than sitagliptin, with both classes carrying a low risk of hypoglycaemia; this is in accordance with our understanding of the mode of action of the two drug classes. This must be balanced against the requirement to inject a GLP-1RAs and their greater tendency to cause nausea, at least during treatment initiation.
A post hoc analysis of data from the LIRA-DPP-4 and LEAD-6 studies, which compared the efficacy of liraglutide 1.8 mg with that of exenatide BID and sitagliptin when used as add-on to metformin in patients already close to target [baseline HbA1c > 8.0% (63.9 mmol/mol)], supports early liraglutide use as an alternative to sitagliptin 49. Following 26 weeks of treatment, the mean reduction in HbA1c was significantly greater with liraglutide than sitagliptin (−1.01% vs. −0.48%; p < 0.0001), reflected by more than twice as many patients achieving HbA1c targets with liraglutide 1.8 mg [HbA1c < 7.0% (53.0 mmol/mol): 78% vs. 37%, p < 0.0001; HbA1c ≤ 6.5% (47.5 mmol/mol): 53% vs. 19%, p < 0.0001]. Substantially more patients also achieved HbA1c targets with liraglutide 1.8 mg compared with exenatide BID [HbA1c < 7.0% (53.0 mmol/mol): 84% vs. 62%, p = 0.03; HbA1c ≤ 6.5% (47.5 mmol/mol): 65% vs. 35%, p = 0.01] 49.
Patient selection: guidelines and future trends
Clinical guidelines provide criteria to assist medical professionals in determining the most appropriate therapeutic intervention for T2D management. Current recommendations are based on the data available at the time of publication. However, with increasing data supporting the earlier use of incretin therapies, these guidelines may change in the future.
What do the current guidelines recommend?
Treatment algorithms for the management of T2D have been published by the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) 50,51. Clinicians must also consider local guidance when prescribing therapies. For example, in England and Wales, the National Institute for Health and Clinical Excellence (NICE) publishes guidelines that are strongly influenced by the cost effectiveness of a drug.
The AACE has recently released a comprehensive diabetes management algorithm 51. The algorithm includes 11 major classes of medications with therapeutic pathways based on three entry HbA1c ranges [> 7.5% or 58.5 mmol/mol (monotherapy); ≥ 7.5% or 58.5 mmol/mol (dual/triple therapy); > 9.0% or 74.9 mmol/mol (dual, triple or insulin therapy)]. The AACE panel positions GLP-1RAs ahead of DPP-4 inhibitors (but behind metformin) as monotherapy for patients in the lowest HbA1c category. When considering the addition of a second- or third-line agent, GLP-1 RAs are positioned at the top of the hierarchy of agents to use; DPP-4 inhibitors are positioned second for dual therapy and fifth for use in triple therapy (Figure2). In patients on basal insulin who require additional prandial control, GLP-1RAs are again positioned ahead of DPP-4 inhibitors in the AACE algorithm 51. The EASD/ADA position statement recommends GLP-1RA or DPP-4 inhibitor use following the failure of metformin monotherapy 50.
Figure 2.
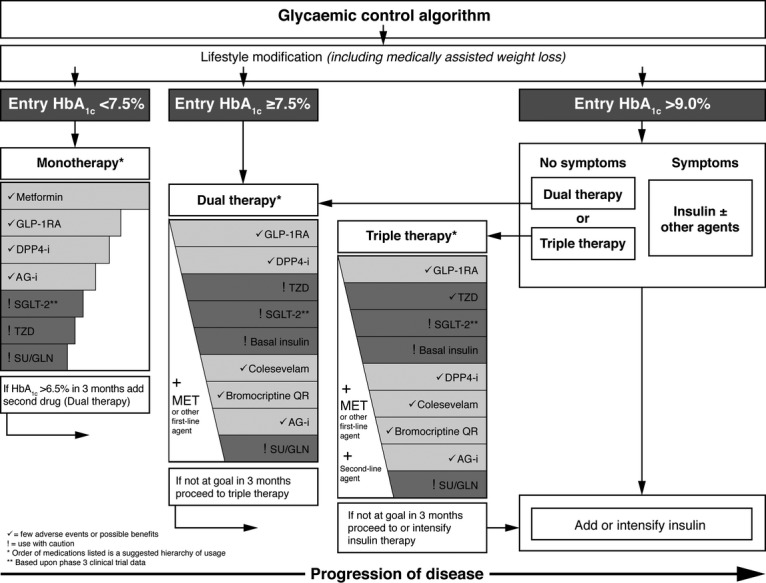
Glycaemic control algorithm for the management of type 2 diabetes developed by the AACE. ©Reprinted with permission from American Association of Clinical Endocrinologists 51. AG-I, alpha-glucosidase inhibitors; DPP-4-i, DPP-4 inhibitor; GLN, glinides; GLP-1-RA, GLP-1 receptor agonist; MET, metformin; SGLT-2, sodium-glucose transporter-2 inhibitors; SU, sulphonylurea; TZD, thiazolidinedione. HbA1c correspondent mmol/mol values: 6.5% = 47.5 mmol/mol; 7.5% = 58.5 mmol/mol; 9.0% = 74.9 mmol/mol
NICE recommends the use of DPP-4 inhibitors as second-line therapy after metformin failure [HbA1c ≥ 6.5% (47.5 mmol/mol)] when SU use is either contraindicated or not tolerated, or there is a significant risk of hypoglycaemia or its consequences 52. NICE prioritises GLP-1RA use in patients where body weight or weight-related comorbidities are a particular concern. For example, liraglutide 1.2 mg and exenatide BID are recommended for use in triple therapy with metformin and SU/TZD if HbA1c ≥ 7.5%, body mass index (BMI) ≥ 35 kg/m2 and the patient has psychological or medical problems associated with high body weight, or if BMI < 35 kg/m2, but weight loss would benefit other significant obesity-related comorbidities 52,53.
In summary, the EASD/ADA position statement and the AACE consensus guidelines support the frequent use of incretin-based therapies (particularly GLP-1RAs) following metformin failure 1,50. However, clinicians should also consider local guidance, which may prioritise incretin-based therapies only in specific patient groups.
DPP-4 inhibitors and GLP-1RAs: contraindications, safety concerns and special populations
Contraindications
The DPP-4 inhibitors and the GLP-1RAs exenatide BID and lixisenatide are only contraindicated in patients with hypersensitivity to any of their excipients, while exenatide OW (US) and liraglutide (US) are also contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia syndrome type 2 (MEN-2) 16,18,19,54–64. There have been postmarketing reports and published case studies relating to skin lesions in patients treated with DPP-4 inhibitors 59,60,62,63,65. Skin lesions have not been reported in an increased incidence in clinical trials with DPP-4 inhibitors, although experience is limited in patients with diabetic skin complications. Therefore, it is recommended that patients treated with DPP-4 inhibitors are monitored for skin disorders 59,60,62,63. Skin and tissue reactions are also an uncommon adverse event observed with GLP-1RAs 16,18,19.
Pancreatitis and pancreatic cancer
There has been concern regarding the risk of pancreatitis with GLP-1RAs and DPP-4 inhibitors, particularly with their long-term use, which may initiate histological changes leading to chronic pancreatitis and, potentially, pancreatic cancer 66. In the liraglutide Phase 3 clinical programme, the rate of pancreatitis was slightly increased compared with comparators, although still lower than expected in a background population with T2D 54,67, and there have been postmarketing reports of acute pancreatitis with sitagliptin, vildagliptin, saxagliptin and exenatide BID 18,59,60,62. Therefore, both DPP-4 inhibitors and GLP-1RAs should be discontinued promptly if pancreatitis is suspected 18,19,54–64. Recently, pancreatic safety with incretin therapies has come under further scrutiny following publication of a report citing increased, potentially precancerous, pancreatic mass in patients treated with sitagliptin or exenatide BID 68. This study analysed a small number of donated human cadaveric pancreata and reported an approximately 40% increase in pancreatic mass. Because of a number of methodological flaws, these findings should be interpreted with caution. For example, substantial differences existed between the two diabetic groups: subjects in the control group were 18 years younger, 67% were female subjects (vs. 25%), two died of diabetic ketoacidosis and five were untreated 68. As such, an editorial in the same issue of the journal questioned whether the increase in pancreas mass in those receiving incretin-based therapy was attributable to their treatment or a function of some of the control group having type 1 diabetes (which is associated with a 48% decrease in pancreatic mass within 10 years of diagnosis) 69. Subsequently, the FDA has advised patients and healthcare practitioners to continue with treatment as before 70. More recently, following a thorough investigation, the European Medicines Agency has stated that currently available data on GLP-1-based therapies do not confirm concerns of an increased risk of pancreatic adverse events 71.
Cardiovascular outcome studies for saxagliptin and alogliptin have recently been published, which together included ∼11,000 DPP-4 inhibitor-treated subjects 72,73. In both the EXAMINE (Examination of Cardiovascular Outcomes with Alogliptin vs. Standard of Care) and the SAVOR-TIMI 53 (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction) randomised, double-blind, placebo-controlled clinical trials, low incidence of pancreatitis and pancreatic cancer was reported, with comparable rates in both active treatment and placebo groups 72,73.
Renal insufficiency and acute renal failure
Renal insufficiency is a common comorbidity in T2D patients and can complicate treatment by elevating plasma levels of therapeutic agents. Although, overall, there is low incidence of acute renal failure with GLP-1RAs, several cases have been reported 74–78. Conversely, cases of acute renal failure resulting from DPP-4 inhibitor therapy are extremely rare 79. Sitagliptin, saxagliptin and vildagliptin are largely renally excreted and a degree of drug accumulation has been reported in patients with renal insufficiency treated with sitagliptin and saxagliptin 58–62. Therefore, in patients with moderate-to-severe renal impairment, dosing adjustment is required when administering sitagliptin (US and EU), saxagliptin (EU and US) and vildagliptin (EU) (Table2) 58–62. Linagliptin, however, has a largely non-renal route of excretion and can be used without dose adjustment in patients at all stages of renal disease 63,64. Exenatide (BID and OW) is predominantly renally excreted and is not recommended in patients with severe renal impairment (Table2) 18,55–57. Lixisenatide may be prescribed without dose adjustment in mild renal impairment, but data are lacking in patients with more advanced disease, and lixisenatide should be used with caution (moderate renal impairment) or not at all (severe impairment) in these populations 16. Liraglutide is metabolised in a similar manner to large proteins and thus is not renally excreted. In the US, liraglutide is approved for use with caution in patients at all stages of renal disease, but is not recommended in patients with moderate-to-severe disease in the EU, because of limited data (Table2) 19,54. The renal safety of incretin-based therapies is currently being assessed in a number of large prospective trials (e.g. NCT01394341, NCT01744236, NCT01835678, NCT01664676).
Table 2.
Summary of label information for DPP-4 inhibitor and GLP-1RA use in the US and EU in patients with renal and hepatic impairment. Please consult the respective SPC/PI for further information
| Ref | Renal impairment | Hepatic impairment | ||||
|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | ||||
| DPP-4 inhibitors | Sitagliptin | EU (SPC) 59 | ✓ | ✓50 mg | ✓25 mg | ✓Mild to moderate. Not studied for severe |
| US (PI) 58 | ✓ | ✓50 mg | ✓25 mg | ✓Mild to moderate. Not studied for severe | ||
| Saxagliptin | EU 60 | ✓ | ✓2.5 mg | ✓2.5 mg with caution | ✓Mild to moderate ×Severe | |
| US 61 | ✓ | ✓2.5 mg | ✓2.5 mg | ✓ | ||
| Vildagliptin | EU 62 | ✓ | ✓50 mg | ✓50 mg | × | |
| US | N/A | N/A | N/A | N/A | ||
| Linagliptin | EU 63 | ✓ | ✓ | ✓ | ✓However, clinical experience is lacking | |
| US 64 | ✓ | ✓ | ✓ | ✓ | ||
| GLP-1RAs | Liraglutide | EU 19 | ✓ | ×Limited clinical experience | ×Limited clinical experience | ×Limited clinical experience |
| US 54 | ✓ | ✓With caution | ✓With caution | ✓With caution because of limited experience | ||
| Exenatide BID | EU 18 | ✓ | ✓With caution* | × | ✓ | |
| US 57 | ✓ | ✓With caution* | × | ✓However, clinical experience is lacking | ||
| Exenatide OW | EU 56 | ✓ | ×Limited clinical experience | × | ✓ | |
| US 55 | ✓ | ✓With caution | × | ✓ | ||
| Lixisenatide | EU 16 | ✓ | ✓With caution | ×Limited clinical experience | ✓ | |
| US | N/A | N/A | N/A | N/A | ||
BID, twice daily; OW, once weekly; SPC, summary of product characteristics; PI, prescribing information. ✓, recommended with no dose adjustment unless stated; ×, not recommended; N/A, not applicable.
Caution when escalating dose from 5 to 10 μg.
Other considerations
Finally, again because of limited data, GLP-1RAs and DPP-4 inhibitors are either not recommended or should be used with caution in elderly (≥ 75 years old), paediatric (< 18 years old), pregnant or breast-feeding patients, or those with hepatic impairment (Table2) 16,18,19,54–64.
When incretin choice may be subjective or based on patient choice
In general, when a patient is already within ∼1.5% of HbA1c target, practitioners often prefer to prescribe DPP-4 inhibitors over GLP-1RAs in the first instance, largely because of ease of incorporation into existing therapy and lower cost. However, practitioners should consider that, in clinical trials, when DPP-4 inhibitors are used as monotherapy or added to existing metformin therapy, reductions in HbA1c are typically < 1% 23,46. A patient's willingness to lose weight may be an important additional factor when choosing an incretin therapy in patients already close to target. Obese or overweight patients with T2D may prefer a GLP-1RA, even when only a small reduction in HbA1c is required, as weight loss may improve their long-term outcomes 80–82. It is important to note that retrospective analyses have suggested that patients with heart failure who experience clinically significant weight loss are at increased risk of mortality. Consequently, because of the decrease in body weight seen with GLP-1RAs, obese patients with heart failure may represent a subset of individuals that requires more careful observation. Less risk for these patients is associated with DPP-4 inhibitors as they do not induce clinically significant weight loss 83.
When patients have very poor glycaemic control on OADs (> 1.5% from target), a GLP-1RA is often recommended over a DPP-4 inhibitor attributable to superior glycaemic efficacy, particularly if the patient is overweight. One major barrier to GLP-1RA treatment is the reluctance of some patients to inject; in such cases, a DPP-4 inhibitor is often the next choice.
Dealing with practical issues: injections and gastrointestinal tolerability
The main advantages of DPP-4 inhibitors compared with GLP-1RAs are less frequent nausea and oral administration. Nausea with GLP-1RAs often occurs early in GLP-1 RA therapy and can be limited using an incremental dosing approach 18,19,57; exenatide OW has only a single dose (2 mg), but it takes 6–10 weeks to achieve steady state plasma levels 55,56. Injecting GLP-RAs at mealtimes may also help some patients, and my personal experience suggests that nausea from liraglutide can often be obviated by eating smaller meals and stopping eating at the first sign of satiation; patients sometimes describe experiencing nausea after meals, but this may actually just be a feeling of ‘fullness’. When nausea is a problem, returning the patient to a lower GLP-1RA dose for a week before repeating the incremental dosing steps can often prove successful. In patients who are reluctant to inject, practitioners can demonstrate that GLP-1RA injection pens are easy and relatively painless to use; a dummy ‘dry’ injection (to the patient and/or the clinician) can illustrate this very well. In addition, personal experience suggests that a patient is often reassured that, with their eyes closed, they often cannot differentiate between a soft pinch on the arm and a dry needle.
Treatment satisfaction data from patient-reported outcome studies suggest that patients are satisfied with injectable therapies if they provide advantages over orally administered treatments 84,85. Overall treatment satisfaction has been reported to be significantly greater for liraglutide 1.8 mg compared with sitagliptin, with similar treatment convenience and flexibility scores 85. Overall treatment satisfaction was also greater for exenatide OW compared with sitagliptin after 26 weeks' treatment 84. Switching from sitagliptin to liraglutide also improved overall treatment satisfaction (p < 0.05 for liraglutide 1.2 mg), while there was no significant change in treatment convenience and flexibility despite the different administration routes 48. In all these examples, the improved treatment satisfaction with the GLP-1RA was observed in concert with improved glycaemic control and greater weight loss compared with sitagliptin therapy.
Together, these results suggest that treatment efficacy is as important to patients as convenience. Patients appear satisfied with an injectable therapy if it provides additional clinical benefits.
Conclusions
The incretin-based therapies improve glycaemic control with a low incidence of hypoglycaemia and without weight gain, both advantages over traditional add-ons to metformin therapy.
DPP-4 inhibitors are simple to incorporate into existing therapy following metformin ± SU failure and are generally weight-neutral with few gastrointestinal side effects. GLP-1RAs offer superior glycaemic control and weight loss compared with sitagliptin, likely because of the supraphysiological levels of the GLP-1RA provided in comparison with the physiological concentrations of GLP-1 and GIP achieved with sitagliptin. Although DPP-4 inhibitors are oral agents, GLP-1RAs are easy to administer and performing the first injection in the office can often alleviate any needle anxiety. In addition, treatment satisfaction data suggest that patients are more satisfied with a GLP-1RA than with sitagliptin and do not mind switching from oral to injectable medication when efficacy is improved.
With this in mind, a GLP-1RA is usually my preferred choice ahead of a DPP-4 inhibitor. However, if weight loss is not particularly desirable and only a small decrease in HbA1c is required to achieve glycaemic target, a DPP-4 inhibitor may be appropriate. Currently, GLP-1RAs are sometimes prioritised over DPP-4 inhibitors in specific patient groups, such as those with obesity-related comorbidities. However, wider use of GLP-1RAs early in disease progression may provide superior glycaemic control and weight loss, and therefore result in more favourable long-term outcomes.
Acknowledgments
The author takes full responsibility for this study, but is grateful to David Harvey, DPhil, of Watermeadow Medical (supported by Novo Nordisk Inc.) for writing assistance.
References
- 1.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control 2009. Endocr Pract. 2009;15:540–59. doi: 10.4158/EP.15.6.540. [DOI] [PubMed] [Google Scholar]
- 2.Davidson MH. Cardiovascular effects of glucagonlike peptide-1 agonists. Am J Cardiol. 2011;108(3 Suppl):33B–41B. doi: 10.1016/j.amjcard.2011.03.046. [DOI] [PubMed] [Google Scholar]
- 3.Jose T, Inzucchi SE. Cardiovascular effects of the DPP-4 inhibitors. Diabetes Vasc Dis Res. 2012;9:109–16. doi: 10.1177/1479164111436236. [DOI] [PubMed] [Google Scholar]
- 4.Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63:492–8. doi: 10.1210/jcem-63-2-492. [DOI] [PubMed] [Google Scholar]
- 5.Nauck M, Stockmann F, Ebert R, et al. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29:46–52. doi: 10.1007/BF02427280. [DOI] [PubMed] [Google Scholar]
- 6.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–9. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- 7.Hare KJ, Vilsbøll T, Asmar M, et al. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes. 2012;59:1765–70. doi: 10.2337/db09-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilsbøll T, Krarup T, Madsbad S, et al. Defective amplification of the late phase insulin response to glucose by GIP in obese type II diabetic patients. Diabetologia. 2002;45:1111–9. doi: 10.1007/s00125-002-0878-6. [DOI] [PubMed] [Google Scholar]
- 9.Mentis N, Vardarli I, Köthe LD, et al. GIP does not potentiate the antidiabetic effects of GLP-1 in hyperglycemic patients with type 2 diabetes. Diabetes. 2011;60:1270–6. doi: 10.2337/db10-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829–35. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 11.Herman GA, Bergman A, Liu F, et al. Pharmacokinetics and pharmacodynamiceffects of the oral DPP-4 inhibitor sitagliptin in middle-aged obese subjects. J Clin Pharmacol. 2006;46:876–86. doi: 10.1177/0091270006289850. [DOI] [PubMed] [Google Scholar]
- 12.He YL, Serra D, Wang Y, et al. Pharmacokinetics and pharmacodynamics of vildagliptin in patients with type 2 diabetes mellitus. Clin Pharmacokinet. 2007;46:577–88. doi: 10.2165/00003088-200746070-00003. [DOI] [PubMed] [Google Scholar]
- 13.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Werner U, Haschke G, Herling AW, et al. Pharmacological profile of lixisenatide: a new GLP-1 receptor agonist for the treatment of type 2 diabetes. Regul Pept. 2010;164:58–64. doi: 10.1016/j.regpep.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 15.DeYoung MB, MacConell L, Sarin V, et al. Encapsulation of exenatide in poly-(D, L-lactide-co-glycolide) microspheres produced an investigational long-acting once-weekly formulation for type 2 diabetes. Diabetes Technol Ther. 2011;13:1145–54. doi: 10.1089/dia.2011.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanofi-aventis. Lixisenatide SPC: Lixisenatide summary of product characteristics. http://ec.europa.eu/health/documents/community-register/2013/20130201125120/anx_125120_en.pdf (accessed May 2013)
- 17.Knudsen LB, Nielsen PF, Huusfeldt PO, et al. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem. 2000;43:1664–9. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- 18.Amylin Pharmaceuticals. Byetta SPC http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000698/WC500051845.pdf (accessed May 2013)
- 19.Novo Nordisk. Victoza SPC http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000698/WC500051845.pdf (accessed July 2012)
- 20.Houlden R, Ross S, Harris S, et al. Treatment satisfaction and quality of life using an early insulinization strategy with insulin glargine compared to an adjusted oral therapy in the management of type 2 diabetes: the Canadian INSIGHT Study. Diabetes Res Clin Pract. 2007;78:254–8. doi: 10.1016/j.diabres.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Nicolucci A, Cucinotta D, Squatrito S, et al. Clinical and socio-economic correlates of quality of life and treatment satisfaction in patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2009;19:45–53. doi: 10.1016/j.numecd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Pratley R, Nauck M, Bailey T, et al. One year of liraglutide treatment offers sustained and more effective glycaemic control and weight reduction compared with sitagliptin, both in combination with metformin, in patients with type 2 diabetes: a randomised, parallel-group, open-label trial. Int J Clin Pract. 2011;65:397–407. doi: 10.1111/j.1742-1241.2011.02656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerich J. DPP-4 inhibitors: what may be the clinical differentiators? Diabetes Res Clin Pract. 2012;90:131–40. doi: 10.1016/j.diabres.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Davidson J. Advances in therapy for type 2 diabetes: GLP-1 receptor agonists and DPP-4 inhibitors. Cleve Clin J Med. 2009;76(Suppl. 5):S28–S38. doi: 10.3949/ccjm.76.s5.05. [DOI] [PubMed] [Google Scholar]
- 25.Madsbad S. Exenatide and liraglutide: different approaches to develop GLP-1 receptor agonists (incretin mimetics) – preclinical and clinical results. Best Pract Res Clin Endocrinol Metab. 2009;23:463–77. doi: 10.1016/j.beem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Rosenstock J, Raccah D, Korányi L, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24-week, randomized, open-label, active-controlled study (GetGoal-X) Diabetes Care. 2013;36:2945–51. doi: 10.2337/dc12-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinget M, Goldenberg R, Niemoeller E, et al. Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal-P) Diabetes Obes Metab. 2013;15:1000–7. doi: 10.1111/dom.12121. [DOI] [PubMed] [Google Scholar]
- 28.Ahrén B, Leguizamo Dimas A, Miossec P, et al. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M) Diabetes Care. 2013;36:2543–50. doi: 10.2337/dc12-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ratner RE, Hanefeld M, Shamanna P, et al. Post-meal pharmacodynamic profile of lixisenatide once daily vs placebo in T2DM insufficiently controlled on SU ± metformin (GetGoal-S) (Abstract D-0743). Proceedings of the World Diabetes Congress; December 4–8, 2011; Dubai, United Arab Emirates. 2011. [Google Scholar]
- 30.Bolli G, Munteanu M, Dotsenko S, et al. Efficacy and safety of lixisenatide once-daily versus placebo in patients with type 2 diabetes mellitus insufficiently controlled on met-formin (GetGoal-F1) [Abstract] Diabetologia. 2011;54(Suppl. 1):S316–S317. [Google Scholar]
- 31.Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 32.Buse JB, Nauck MA, Forst T, et al. Efficacy and safety of exenatide once weekly versus liraglutide in subjects with type 2 diabetes (DURATION-6): a randomised, open-label study. Diabetologia. 2011;54(Suppl. 1):S38. doi: 10.1016/S0140-6736(12)61267-7. (Abstract A75) [DOI] [PubMed] [Google Scholar]
- 33.Stonehouse A, Walsh B, Cuddihy R. Exenatide once-weekly clinical development: safety and efficacy across a range of background therapies. Diabetes Technol Ther. 2011;13:1063–9. doi: 10.1089/dia.2011.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holst JJ. Pharmacology of GLP-1-based therapies. Br J Diab Vasc Dis. 2008;8:S11–S18. [Google Scholar]
- 35.Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–35. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 36.Hermansen K, Kipnes M, Luo E, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733–745. doi: 10.1111/j.1463-1326.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 37.Marre M, Shaw J, Brändle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU) Diabet Med. 2006;26:268–78. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diamant M, Van Gaal L, Stranks S, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet. 2010;375:2234–43. doi: 10.1016/S0140-6736(10)60406-0. [DOI] [PubMed] [Google Scholar]
- 39.Yabe D, Seino Y. Liraglutide in adults with type 2 diabetes: global presepctive on safety, efficacy and patient preference. Clin Med Insights Endocrinol Diabetes. 2011;4:47–62. doi: 10.4137/CMED.S5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YG, Hahn S, Oh TJ, et al. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56:696–708. doi: 10.1007/s00125-012-2827-3. [DOI] [PubMed] [Google Scholar]
- 41.Reid T. Choosing GLP-1 receptor agonists or DPP-4 inhibitors: weighing the clinical trial evidence. Clin Diabetes. 2012;30:3–12. [Google Scholar]
- 42.DeFronzo RA, Okerson T, Viswanathan P, et al. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008;24:2943–52. doi: 10.1185/03007990802418851. [DOI] [PubMed] [Google Scholar]
- 43.Berg JK, Shenouda SK, Heilmann CR, et al. Effects of exenatide twice daily versus sitagliptin on 24-h glucose, glucoregulatory and hormonal measures: a randomized, double-blind, crossover study. Diabetes Obes Metab. 2011;13:982–9. doi: 10.1111/j.1463-1326.2011.01428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bergenstal RM, Wysham C, Macconell L, et al. Efficacy and safety of exenatide once weekly versus sitagliptin or pioglitazone as an adjunct to metformin for treatment of type 2 diabetes (DURATION-2): a randomised trial. Lancet. 2010;376:431–9. doi: 10.1016/S0140-6736(10)60590-9. [DOI] [PubMed] [Google Scholar]
- 45.Wysham C, Bergenstal R, Malloy J, et al. DURATION-2: efficacy and safety of switching from maximum daily sitagliptin or pioglitazone to once-weekly exenatide. Diabet Med. 2011;28:705–14. doi: 10.1111/j.1464-5491.2011.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin in patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447–56. doi: 10.1016/S0140-6736(10)60307-8. [DOI] [PubMed] [Google Scholar]
- 47.Davies M, Pratley R, Montanya E, et al. Liraglutide reduces A1c to a greater extent than sitagliptin regardless of baseline A1c levels. Diabetes. 2010;59(Suppl. 1):A15. (Abstract 57-OR) [Google Scholar]
- 48.Pratley RE, Nauck MA, Bailey T, et al. Efficacy and safety of switching from the DPP-4 inhibitor sitagliptin to the human GLP-1 analog liraglutide after 52 weeks in metformin-treated patients with type 2 diabetes: a randomized, open-label trial. Diabetes Care. 2012;35:1986–93. doi: 10.2337/dc11-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King AB, Montanya E, Pratley R, et al. Liraglutide achieves A1C targets more often than sitagliptin or exenatide when added to metformin in patients with type 2 diabetes and a baseline A1C <8.0% Endocr Pract. 2013;19:64–72. doi: 10.4158/EP12232.OR. [DOI] [PubMed] [Google Scholar]
- 50.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–79. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE comprehensive diabetes management algorithm. Endocr Pract. 2013;19:327–36. doi: 10.4158/endp.19.2.a38267720403k242. [DOI] [PubMed] [Google Scholar]
- 52.National Institute for Health and Clinical Excellence. Clinical guideline 87, the management of type 2 diabetes. 2009. http://www.nice.org.uk/nicemedia/live/12165/44320/44320.pdf (accessed May 2013)
- 53.National Institute for Health and Clinical Excellence. Final appraisal determination: Liraglutide for the treatment of type 2 diabetes mellitus. 2010. http://www.nice.org.uk/nicemedia/live/11895/50663/50663.pdf (accessed May 2013)
- 54.Novo Nordisk. Victoza PI. http://www.novo-pi.com/victoza.pdf (accessed May 2013)
- 55.Amylin Pharmaceuticals. Bydureon PI. http://documents.bydureon.com/Bydureon_PI.pdf (accessed May 2013)
- 56.Amylin Pharmaceuticals. Bydureon SPC. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002020/WC500108241.pdf (accessed May 2013)
- 57.Amylin Pharmaceuticals. Byetta PI. http://documents.byetta.com/Byetta_PI.pdf (accessed May 2013)
- 58.Merck Sharp & Dohme Corp. Januvia PI. http://www.merck.com/product/usa/pi_circulars/j/januvia/januvia_pi.pdf (accessed May 2013)
- 59.Merck Sharp & Dohme Corp. Januvia SPC. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000722/WC500039054.pdf (accessed May 2013)
- 60.Bristol-Myers Squibb. Onglyza SPC. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001039/WC500044316.pdf (accessed May 2013)
- 61.Bristol-Myers Squibb. Onglyza PI. http://packageinserts.bms.com/pi/pi_onglyza.pdf (accessed May 2013)
- 62.Novartis. Galvus SPC. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000771/WC500020327.pdf (accessed May 2013)
- 63.Boehringer Ingelheim Pharmaceuticals, Inc. Trajenta SPC. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002110/WC500115745.pdf (accessed May 2013)
- 64.Boehringer Ingelheim Pharmaceuticals, Inc. Trajenta PI. http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing+Information/PIs/Tradjenta/Tradjenta.pdf (accessed May 2013)
- 65.Nakatani K, Kurose T, Hyo T, et al. Drug-induced generalized skin eruption in a diabetes mellitus patient receiving a dipeptidyl peptidase-4 inhibitor plus metformin. Diabetes Ther. 2012;3:14. doi: 10.1007/s13300-012-0014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nauck MA. A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes Care. 2013;36:2126–32. doi: 10.2337/dc12-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noel RA, Braun DK, Patterson RE, et al. Increased risk of acute pancreatitis observed in patients with type 2 diabetes. Diabetes Care. 2009;32:834–8. doi: 10.2337/dc08-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Butler AE, Campbell-Thompson M, Gurlo T, et al. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013;62:2595–604. doi: 10.2337/db12-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kahn SE. Incretin therapy and islet pathology: a time for caution. Diabetes. 2013;62:2178–80. doi: 10.2337/db13-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Food and Drug Administration. FDA Drug Safety Communication: FDA investigating reports of possible increased risk of pancreatitis and pre-cancerous findings of the pancreas from incretin mimetic drugs for type 2 diabetes. http://www.fda.gov/Drugs/DrugSafety/ucm343187.htm (accessed May 2013)
- 71.European Medicines Agency (EMA) Investigation into GLP-1-based diabetes therapies concluded. Press Release 26 July 2013. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2013/07/WC500146619.pdf (accessed September 2013)
- 72.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary in patients in type 2 diabetes. N Engl J Med. 2013;369:1327–35. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 73.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 74.López-Ruiz A, del Peso-Gilsanz C, Meoro-Avilés A, et al. Acute renal failure when exenatide is co-administered with diuretics and angiotensin II blockers. Pharm World Sci. 2010;32:559–61. doi: 10.1007/s11096-010-9423-8. [DOI] [PubMed] [Google Scholar]
- 75.Kaakeh Y, Kanjee S, Boone K, et al. Liraglutide-induced acute kidney injury. Pharmacotherapy. 2012;32:e7–11. doi: 10.1002/PHAR.1014. [DOI] [PubMed] [Google Scholar]
- 76.Nandakoban H, Furlong TJ, Flack JR. Acute tubulointerstitial nephritis following treatment with exenatide. Diabet Med. 2013;30:123–5. doi: 10.1111/j.1464-5491.2012.03738.x. [DOI] [PubMed] [Google Scholar]
- 77.Weise WJ, Sivanandy MS, Block CA, et al. Exenatide-associated ischemic renal failure. Diabetes Care. 2009;32:e22–e23. doi: 10.2337/dc08-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuehn B. Exenatide and kidney function. JAMA. 2009;302:2644. [Google Scholar]
- 79.Leibovitz E, Gottlieb S, Goldenberg I, et al. Sitagliptin pretreatment in diabetes patients presenting with acute coronary syndrome: results from the Acute Coronary Syndrome Israeli Survey (ACSIS) Cardiovasc Diabetol. 2013;12:53. doi: 10.1186/1475-2840-12-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lean ME, Powrie JK, Anderson AS, et al. Obesity, weight loss and prognosis in type 2 diabetes. Diabet Med. 1990;7:228–33. doi: 10.1111/j.1464-5491.1990.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 81.Aucott LS. Influences of weight loss on long-term diabetes outcomes. Proc Nutr Soc. 2008;67:54–9. doi: 10.1017/S0029665108006022. [DOI] [PubMed] [Google Scholar]
- 82.Fujioka K. Benefits of moderate weight loss in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:186–94. doi: 10.1111/j.1463-1326.2009.01155.x. [DOI] [PubMed] [Google Scholar]
- 83.Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33:187–215. doi: 10.1210/er.2011-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Best JH, Rubin RR, Peyrot M, et al. Weight-related quality of life, health utility, psychological well-being, and satisfaction with exenatide once weekly compared with sitagliptin or pioglitazone after 26 weeks of treatment. Diabetes Care. 2011;34:314–9. doi: 10.2337/dc10-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davies M, Pratley R, Hammer M, et al. Liraglutide improves treatment satisfaction in people with type 2 diabetes compared to sitagliptin, each as add-on to metformin. Diabet Med. 2011;28:333–7. doi: 10.1111/j.1464-5491.2010.03074.x. [DOI] [PubMed] [Google Scholar]


