Abstract
Preeclampsia is a serious and common hypertensive complication of pregnancy, affecting ~5 to 8 % of pregnancies. The underlying cause of preeclampsia is believed to be placental ischemia, which causes secretion of pathogenic factors into the maternal circulation. While a number of these factors have been identified, it is likely that others remain to be elucidated. Here, we have utilized a relevant preclinical rodent model of placental ischemia-induced hypertension, the reduced uterine perfusion pressure (RUPP) model, to determine the effect of chronic placental ischemia on the underlying chorionic tissue and placental villi. Tissue from control and RUPP rats were isolated on gestational day 19 and mRNA from these tissues was subjected to microarray analysis to determine differential gene expression. At a statistical cutoff of p <0.05, some 2,557 genes were differentially regulated between the two groups. Interestingly, only a small subset (22) of these genes exhibited changes of greater than 50 % versus control, a large proportion of which were subsequently confirmed using qRT-PCR analysis. Network analysis indicated a strong effect on inflammatory pathways, including those involving NF-κB and inflammatory cytokines. Of the most differentially expressed genes, the predominant gene classes were extracellular remodeling proteins, pro-inflammatory proteins, and a coordinated upregulation of the prolactin genes. The functional implications of these novel factors are discussed.
Introduction
One of the most common obstetrical complications worldwide is preeclampsia, a disorder marked by new-onset hypertension and proteinuria. The incidence of preeclampsia in the United States is ~5 to 8 %, and it remains to be one of the leading causes of premature birth and maternal/fetal morbidity (Roberts et al. 2003; Sibai et al. 2005). There is currently no fully effective intervention for the management of preeclampsia. Treatment is largely confined to magnesium sulfate for seizure prophylaxis and anti-hypertensives, which typically fail to fully control blood pressure or stem the progression of the disorder (Turner 2010). Ultimately, the definitive resolution of the disorder comes only through delivery of the placenta, which was one of the more suggestive clues as to the origins of the disease (Hladunewich et al. 2007). Despite years of research, the initiating causes of preeclampsia remain unknown. However, there is a growing belief that the origins of the disease lie in underperfusion of the placenta itself. In normal gestation, fetal-derived cytotropho-blasts invade the maternal spiral arteries which feed the placenta, replace the vascular endothelium, and cause dramatic vasodilation to allow for adequate blood flow to the developing tissue. In preeclampsia, however, the cytotrophoblast invasion is incomplete, and the vessels fail to fully distend-ultimately resulting in inadequate delivery of blood to the placenta and chronic ischemia (Khong and Brosens 2010). In turn the placenta produces pathogenic factors which are secreted into the maternal blood stream, and are responsible for the symptomatic manifestations of the disorder.
A great deal of research over the last decade has been focused on identifying and studying these placental-derived factors, and several important disease-associated proteins have been identified. The VEGF antagonist sFlt-1, for instance, has been shown to be elevated in the maternal circulation of preeclampsia patients and when infused directly into various animal models, causes symptoms which are reminiscent of preeclampsia itself (Bergmann et al. 2010; Bridges et al. 2009; Maynard et al. 2003). Inflammatory cytokines, specifically TNF-α likewise are found at elevated levels in patients and also reproduce some of the symptoms of the human disorder in animal models (Kupferminc et al. 1994; LaMarca et al. 2005). One recent novel factor identified is an agonistic autoantibody to the AT1 receptor, which again partially mimics the disease when introduced into pregnant rodents (LaMarca et al. 2009; Wallukat et al. 2003). Importantly, none of these factors are found universally in preeclampsia patients, and it remains possible that still-unknown placental factors remain to be identified in the preeclampsia population.
Indeed, a number of laboratories have examined placental tissue from preeclamptic patients in an effort to elucidate new functional pathways in the preeclamptic placenta. These studies have variously looked at the maternal interface (Meng et al. 2012; Winn et al. 2009), the villous tissue (Centlow et al. 2011; Cox et al. 2011; Reimer et al. 2002), or the whole placenta (Goyal et al. 2010), each of which has demonstrated novel new differentially regulated pathways in the preeclamptic placenta. One limitation of these studies, however, is that due to the nature of their design, they are unable to differentiate between causative differences in gene expression versus those that are a direct result of placental ischemia. Here, we have utilized a model of placental ischemia, which mechanically constricts the vessels feeding the placenta, to examine the direct role of placental ischemia on the expression of potentially pathogenic factors. Through microarray analysis, qRT-PCR, and bioinformatic approaches, we identify several potential new biomarkers and targets for therapy in preeclampsia.
Materials and Methods
Animals
Timed pregnant Sprague–Dawley rats (Harlan, Inc., Indianapolis, IN) were received on gestational day 11. All protocols were approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee, and followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Rats were maintained on a 12:12 h light:dark cycle, at 23 °C constant temperature and were provided food and water ad libitum.
Experimental treatment
For reduced uterine perfusion pressure (RUPP) rats, on gestational day 14, animals were suggested to aortic and bilateral ovarian artery constriction. Briefly, rats were anesthetized and maintained on 3 % isoflurane, and a midline abdominal incision was made. After externalization of both uterine horns, one single 0.023 mm silver surgical clip was placed on the abdominal aorta above the iliac bifurcation. One 0.100 mm silver surgical clip was placed on both the left and right ovarian arteries which supply the uterus to prevent compensatory flow. Total reabsorption of pups on gestational day 19 led to exclusion of the animal from the study. Control groups were anesthetized on gestational day 14 to verify pregnancy. Sham surgeries were not undertaken, as sham surgeries have previously demonstrated no significant effect on pup size, blood pressure, or vascular reactivity (Alexander et al. 2001; Sedeek et al. 2008; Walsh et al. 2009).
Measurement of mean arterial pressure
On gestational day 18, rats were anesthetized as above and implanted with indwelling carotid catheters consisting of V-3 tubing (SCI) which were tunneled under the skin and eternalized at the back of the neck. The following day, rats were placed in individual restraining cages and acclimatized. Mean arterial pressure was measured consciously for 1 h via Cobe III pressure transducers (CDX Sema) and data was collected and analyzed using receivers, amplifiers, and PowerLab software from ADI. Each experimental group had n = 6.
Tissue harvest
Rats were anesthetized as above. The uterus was externalized, thorough a ventral midline incision, and blood was collected by cannulation of the abdominal aorta. Records were made of the viable and reabsorbed pups present in each animal, and individual pups and placentas were weighed and recorded. Two random placentas from each animal were selected for RNA isolation. The selected placentas were immediately dissected by removal of the myometrium and trophospongium layers. The intervillous space was rinsed gently in cold PBS, and placental villi-containing segments were excised from the labyrinth. The tissue was immediately immersed in RNAlater (Ambion), refrigerated overnight and then kept at −80 °C until preparation of RNA.
Microarray experiments
Whole genome transcript analysis was performed using Affymetrix platforms. RNA was isolated using TRIzol® and Invitrogen PureLink Kit (Life Technologies) and evaluated for quality and integrity (Bio-Rad Experion™ System). RNA samples were processed per manufactures directions for specific application [GeneChip® 1.0 ST] using Affymetrix equipment (Scanner 3000 7G System). Hybridized chips were automatically washed, stained, and scanned at the UMMC Institutional Molecular and Genomics Core using Affymetrix equipment. Data obtained from these gene expression studies are deposited in the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) with the GEO accession number GSE50596.
Analysis of microarray data was performed using software provided by Affymetrix (Affymetrix® Expression Console™ Software) and commercially available Gene-Sifter™ software platform (http://www.genesifter.net) and/or Array Star 5 (DNASTAR, Inc, WI). In brief, differentially expressed genes evaluated by t test using two methods: (1) FWER (family-wise error rate) procedure, p <0.05 and fold-change ± 1.2 or greater; and/or (2) Benjamani and Hochberg FDR (false discovery rate) which corrects for multiple comparison, using p <0.05, and fold-change ± 1.2 or greater. Two-way ANOVA using a p <0.01 or lower was performed to identify interaction effects between groups. Gene networks and functional analysis were evaluated through the use of Ingenuity Pathways Analysis (Ingenuity® Systems, www.ingenuity.com). n = 6 for both groups.
qRT-PCR
mRNA levels were confirmed by means of qRT-PCR. cDNA was produced from 2 μg total RNA by Superscript II in concert with oligo dT primers (Invitrogen). Measurements were made from n = 6 in each experimental group. Primers for all genes of interest were designed using Primer-BLAST (NCBI), and were supplied by Invitrogen. Real time amplification was carried out with SYBR-Green Supermix (Bio-Rad) with a Bio-Rad CFX-96 qRT-PCR system undergoing 40 rounds of amplification. Relative fold change was determined by means of the 2ΔΔCt method, with n = 6 in both groups. Specificity of amplification was confirmed by melt curve analysis and resulting PCR products were observed visually on 3 % agarose gels to confirm the presence of a single product.
Results
RUPP animals exhibit hypertension and placental/fetal growth restriction
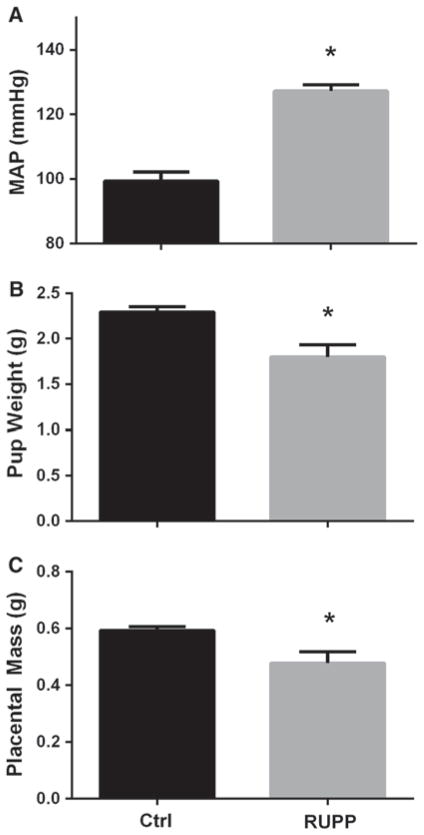
To verify the efficacy of the RUPP procedure, the mean arterial pressure, fetal weight, and placental weight of the control and RUPP animals were determined on gestational day 19. As seen in Fig. 1a, RUPP animals exhibited a significant elevation in blood pressure when compared to the control group (99 ± 3 vs 127 ± 2 mmHg, p <0.05), in line with observations from previous studies. In agreement with previous work in this model, fetal and placental mass were significantly reduced using RUPP treatment. RUPP animals demonstrated a ~20 % reduction in fetal mass (2.29 ± 0.6 vs 1.8 0.13 g, p <0.05), as seen in Fig. 1b. As seen in Fig. 1c, RUPP animals also exhibited a ~19 % decrease in placental mass (0.59 ± 0.01 vs 0.48 ± 0.04 g, p <0.05). Together, these data show that the RUPP animals were hypertensive and exhibit both fetal and placental growth restriction.
Fig. 1.
Physiological response to chronic RUPP. As seen in (a), on gestational day 19, RUPP animals exhibit a significant increase in MAP compared to their normal pregnant controls (99 ± 3 vs 127 ± 2 mmHg, p <0.05). This was accompanied by reductions in both (b) fetal weight (2.29 ± 0.6 vs 1.8 0.13 g, p <0.05), and in placental mass (0.59 ± 0.01 vs 0.48 ± 0.04 g, p <0.05). n = 6 in both groups
mRNA analysis of placental villous tissue
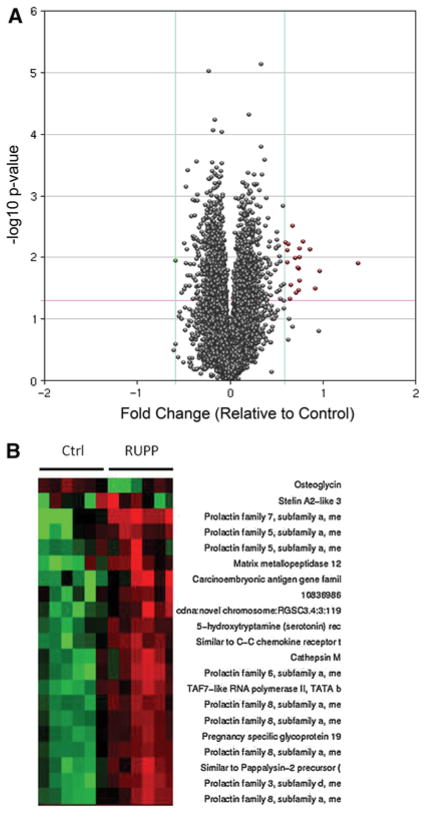
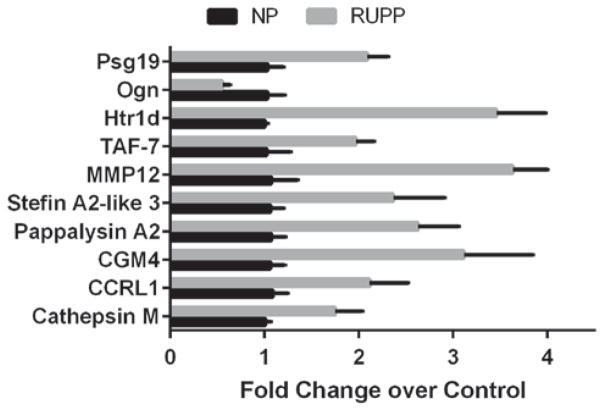
To determine the effects of chronic placental ischemia on gene expression, placental villi-containing sections of the labrynth were subjected to RNA isolation and microarray analysis to determine relative gene expression versus control animals. At a statistical cutoff of p <0.05, there were 2,557 genes differentially expressed, a volcano plot of which is demonstrated in Fig. 2a. However, the changes in gene expression in the vast majority of these genes were relatively mild, with only 22 demonstrating greater than a 50 % change in expression, as demonstrated in the HEAT map in Fig. 2b and summarized in Table 1. A large sampling of the most differentially expressed genes was further confirmed by means of qRT-PCR analysis, the results of which are presented Fig. 3. While the magnitude of change tended to be significantly greater when monitored by means of qRT-PCR, the direction of change was consistent in all of the mRNAs measured.
Fig. 2.
Microarray analysis of gene expression in response to placental ischemia. a Volcano plot of differentially expressed mRNA as determined by microarray analysis at a statistical cutoff of p <0.05. 2557 genes were differentially expressed at this significance level. b HEAT map of 22 genes exhibiting >50 % change of expression as indicated by microarray analysis. n = 6 in each group
Table 1.
Summary of all genes demonstrating >50 % change in expression by microarray in RUPP chorionic tissue versus normal pregnant control animals
| Ratio | Direction | Gene identifier | Gene name |
|---|---|---|---|
| 2.6 | Up | NM_022530 | Prolactin family 7, subfamily a, member 3 |
| 1.95 | Up | NM_053963 | Matrix metallopeptidase 12 |
| 1.89 | Up | NM_012525 | Carcinoembryonic antigen gene family 4 Similar to CEACAM1 |
| 1.81 | Up | NM_138527 | Prolactin family 5, subfamily a, member 1 |
| 1.72 | Up | NM_001135877 | TAF7-like RNA polymerase II, TATA box binding protein (TBP)-associated factor |
| 1.68 | Up | 10836986 | KDM3A |
| 1.68 | Up | NM_020079 | Prolactin family 8, subfamily a, member 3 |
| 1.67 | Up | 10850090 | ROBO2 |
| 1.67 | Up | Stfa2l3 | Stefin A2-like 3 |
| 1.67 | Up | NM_033233 | Prolactin family 3, subfamily d, member 4 |
| 1.66 | Up | LOC680069 | Similar to Pappalysin-2 precursor (Pregnancy-associated plasma protein-A2) (PAPP-A2) (Pregnancy-associated plasma protein-E1) |
| 1.63 | Up | NM_022176 | Prolactin family 6, subfamily a, member 1 |
| 1.62 | Up | AF090348 | Similar to C–C chemokine receptor type 11 (C–C CKR-11) (CC-CKR-11) (CCR-11) (Chemokine receptor-like 1) (CCRL1) (CCX CKR) |
| 1.6 | Up | NM_021580 | Prolactin family 8, subfamily a, member 4 |
| 1.58 | Up | NM_181378 | Cathepsin M |
| 1.56 | Up | NM_001044702 | Prolactin family 5, subfamily a, member 2 |
| 1.54 | Up | NM_134385 | Prolactin family 8, subfamily a, member 9 |
| 1.53 | Up | NM_022846 | Prolactin family 8, subfamily a, member 2 |
| 1.53 | Up | NM_019126 | Pregnancy specific glycoprotein 19 |
| 1.52 | Up | NM_012852 | 5-hydroxytryptamine (serotonin) receptor 1D |
| 1.5 | Down | NM_001106103 | Osteoglycin |
| 1.5 | Up | NM_053587 | S100 calcium binding protein A9 |
Fig. 3.
qRT-PCR of mRNA targets identified by microarray. To verify mRNA expression data from earlier microarray experiments, samples were probed using qRT-PCR to verify magnitude of gene expression. While change in magnitude tended to be greater in the qRT-PCR experiments versus the microarray data, the direction of change remained consistent across all targeted genes. n = 6 in both groups
Pathway analysis
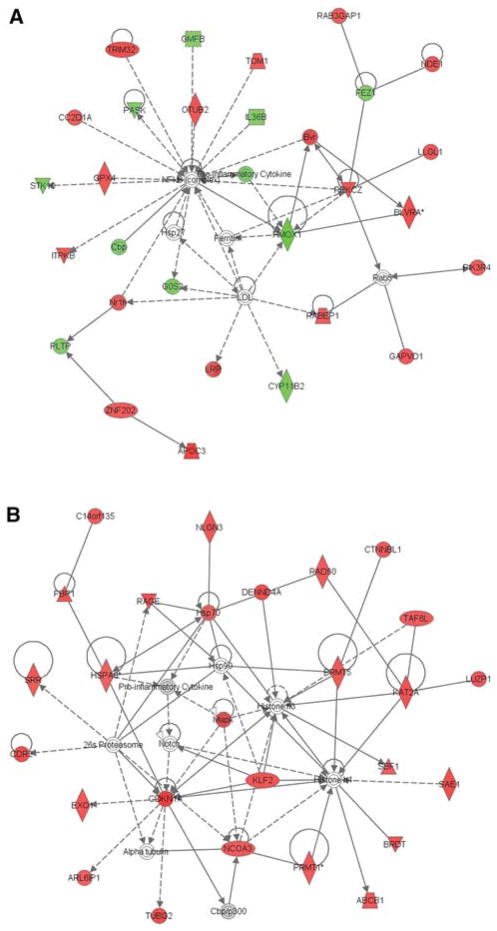
Gene network analysis was performed with ingenuity pathways analysis, with a statistical cutoff of p <0.02. While a number of networks were identified by the software, the two most significantly up-regulated pathways both were linked to cell death (Fig. 4a, b). Interestingly, Pathway 1 is clustered around NF-κB activation (Fig. 4a), while Pathway 2 is associated with inflammatory cytokine production and notch signaling (Fig. 4b).
Fig. 4.
Ingenuity pathway analysis of top differentially affected pathways from the microarray data. Pathway analysis was performed with a statistical cutoff of p <0.02. The top two differentially affected pathways were a involved in NF-κB regulation, and b production of inflammatory cytokines associated with notch signaling
Discussion
While the underlying origins of preeclampsia remain obscure, a number of pathogenic pathways have been identified which are thought to cause the symptomatic phase of the disorder to a greater or lesser degree. What has become clear is that placental ischemia brought about by insufficient maternal vascular remodeling lies at the root of the most preeclampsia cases. The ischemic placenta is then responsible for the production of pathogenic factors which cause the maternal symptomatic phase of the disorder. Of the identified pathways activated by placental ischemia, sFlt-1 and sEng-mediated angiogenic imbalance and activation of inflammatory/autoimmune pathways have been of particular interest. However, experimental induction of either of these pathways fails to adequately recapitulate the blood pressure and fetal growth restriction which is seen either with experimental chronic placental ischemia or in the more severe forms of the disorder in humans. It is possible that other, still undefined, pathways are induced by placental ischemia and could be playing a role in the clinical symptoms of the preeclamptic patient.
A number of groups have undertaken gene expression studies to identify novel targets for either biomarkers or clinical intervention. A number of these have, quite correctly, focused on the maternal/placental interface as a possible source for differentially expressed genes which could be responsible for inadequate trophoblasts migration and thus improper maternal spiral artery remodeling. These studies have identified a number of differentially regulated factors in the preeclamptic placenta which could potentially have a role in mediating trophoblasts migration and function (Enquobahrie et al. 2008; Meng et al. 2012; Winn et al. 2009). Other studies have looked exclusively at the placental chorionic villi and found significant alterations of a number of pathways, including inflammatory pathways (Centlow et al. 2011; Founds et al. 2009), cell adhesion (Reimer et al. 2002), and cell mobility and hematological function (Founds et al. 2009). While these studies have proved insightful and provided a number of novel potential areas of research, they are all confounded by the symptomatic spectrum and genetic background of the patient population. Here, we have attempted to remove some of this variability and look directly at the effects of placental ischemia on the chorionic tissue in a rodent model which recapitulates closely the maternal symptomatic phase of the disorder, with the assumption that the deep tissue is likely to be the most ischemic given its separation from the maternal blood supply.
Unsurprisingly, in response to placental ischemia there was a significant increase in a large number of genes—some 2,500 at a statistical cutoff of p <0.05. Interestingly, the magnitude change of the vast majority of these targets was relatively modest, with only 22 showing a 50 % or greater change in expression over control levels. It is also interesting to note that of the 22 genes in this group, only one, osteoglycin, was down-regulated, suggesting a generalized increase in gene expression. Interestingly, a number of these most differentially expressed genes have been identified as differentially regulated in human patient populations previously, including pappalysin-A2 (Buimer et al. 2008; Winn et al. 2009), PAI-1, and MMP12 (Founds et al. 2009). A number of the most differentially expressed genes, however, had not been previously implicated in placental ischemia. Though it had been previously observed in extravillous intermediate trophoblasts in normal pregnancy, CEACAM-1 this is the first report of its induction by placental ischemia (Bamberger et al. 2000). CEACAM-1, a cell surface molecule with both adhesion and co-receptor activity, has been shown to be an important morphogenic effector of VEGF-induced angiogenesis (Ergun et al. 2000). Furthermore, it was shown that CEACAM-1 is an important regulator of VEGF-dependent vascular permeability both in vivo and in vitro (Nouvion et al. 2010). It is possible then that its upregulation is in response to decreased bioavailable VEGF previously described in this model. Likewise, L-selectin, an adhesion molecule which is typically thought of as a homing receptor for leukocyte recruitment to endothelium, also has an important role in early establishment of cytotrophoblast cell columns and possibly in trophoblast migration to the maternal vasculature (Prakobphol et al. 2006). Its upregulation could also be an attempt to compensate for poor placental blood flow by inducing further vascular development and maternal vascular invasion.
One consistent theme in the differentially expressed genes is a role in inflammation and immune modulation, in particular through leukocyte recruitment. CEACAM-1 also has a role as a co-receptor for both lymphoid and myeloid cells (Gray-Owen and Blumberg 2006). As mentioned above, L-selectin is an important modulator of leukocyte extravasation and migration (Wedepohl et al. 2012). Likewise, the decoy chemokine receptor CCRL1 is involved in the steady-state recruitment of leukocytes, possibly by modulating the localized concentrations of active chemokines (Heinzel et al. 2007). S100A9, when complexes with S100A8, is believed to promote inflammation by co-activating toll-like receptor 4 (TLR4) and the receptor for advanced glycation end products (RAGE). Furthermore, this signaling axis has been shown to regulate vascular inflammation in experimental vascular injury models, specifically by promoting leukocyte recruitment (Croce et al. 2009). It is also worth noting that a previous microarray analysis of chorionic tissue from preeclampsia patients indicated an increase in S100A8, which is necessary for S100A9 function (Founds et al. 2009). Additionally, MMP-12, in addition to being an important component of the macrophage contribution to angiogenesis, also has demonstrated activity as a pro-inflammatory factor in the lung in vivo (Nenan et al. 2005, 2007; Nucera et al. 2011). Also of note is the induction of two pregnancy specific glycoproteins (PSGs). PSGs, which have previously been observed at elevated levels in preeclampsia patients, have been shown to induce macrophage secretion of inflammatory cytokines in vitro and regulate T-cell function (Bebo and Dveksler 2005; Snyder et al. 2001). Taken together, this expression profile suggests several novel inflammatory mediators which are being induced by chronic placental ischemia. Future studies inhibiting these pathways could provide interesting insight into the role of the inflammatory response and the individual factors in mediating the symptomatic phase of the disorder.
Perhaps, the most striking pattern seen in the subset of highly differentially expressed genes is a dramatic upregulation of the prolactin genes. In fact of the 23 most differentially expressed genes, nine of them are prolactin variants. The rodent prolactin genes are significantly different in organization from their human counterpart. While human prolactin is restricted to two nearly identical genes, the rodent prolactin system consists of no less than 23 discrete genes arranged in a single locus, which arose through gene duplication (Alam et al. 2006). Despite this difference, there is a clear and consistent widespread upregulation of a diverse array of these genes, pointing to a common mechanism of regulation in response to placental ischemia. Interestingly, a correlation between preeclampsia and increased prolactin expression was noted as early as the 1970s, and extensive characterization by the Soares laboratory has demonstrated differential temporal and tissue regional localization of a number of these transcripts in the placenta during pregnancy (Ain et al. 2003; Alam et al. 2008; Orwig and Soares 1999; Redman et al. 1975; Soares et al. 2007; Wang et al. 2000; Wiemers et al. 2003). Several recent studies have indicated a link between preeclampsia and elevated prolactin. In the first, a microarray study by Reimer et al. (2002) found significantly increased prolactin expression in chorionic tissue from preeclampsia patients. Even more suggestive, several recent studies have demonstrated significant elevations of prolactin in serum, urine, and amniotic fluid of preeclampsia patients compared to their normal pregnant controls (Leanos-Miranda et al. 2008, 2013). The data from this study suggest a direct link between placental ischemia and production of prolactin, and indicate that prolactin production is likely a consequence, rather than a cause, of preeclampsia.
Importantly, besides its commonly known function in the regulation of maternal milk production, prolactin in both the human and rat has other intriguing effects. Full length prolactin proteins are a target for proteases like MMPs and cathepsins, specifically cathepsin D (Baldocchi et al. 1993; Piwnica et al. 2004). These cleaved prolactin fragments have a distinctly different biological activity than the full length protein, and have been termed “vasoinhibins”. These proteins have clear pro-apoptotic, anti-angiogenic, and anti-proliferative biological activity (Clapp et al. 2006). They have also been shown to directly antagonize the activity of VEGF, the reduction of which by sFlt-1 has been shown to be an important factor in the symptomatic phase of preeclampsia (D’Angelo et al. 1995, 1999; Struman et al. 1999). The recent studies examining prolactin expression in preeclampsia patients have identified the presence of these vasoinhibins, and they appear to be directly correlated with disease severity(Leanos-Miranda et al. 2008, 2013). While the transcriptional data presented here cannot confirm the proteolytically produced vasoinhibins, it is interesting to note that vasoinhibins have also been shown to induce production of adhesion proteins and induce chemokines of the CC family (Piwnica et al. 2004). These factors all have paralogous relatives in the most differentially regulated genes identified in this study, i.e., CEACAM1, CCRL1, Selectin, and PAI-1. It is not clear if the two proteases, cathepsin M and MMP-12, will exhibit proteolytic activity on full length prolactin, and biochemical characterization of their interaction should prove interesting. The preponderance of the data suggests, though not definitively, that prolactin-derived vasoinhibins are being produced in response to placental ischemia and are affecting gene transcription in the ischemic placenta.
The current study extends the work of previous microarray studies into the molecular mechanisms of preeclampsia. The advantage of this approach is in dissecting the secondary effects of placental ischemia from possibly confounding primary causes in the patient population. The transcriptional profile elicited by chronic placental ischemia demonstrates a number of novel inflammatory mediators and significant prolactin production, and is strongly suggestive of vasoinhibin fabrication. Future studies looking at the production of these fragments in response to experimental placental ischemia, as well as further characterization of the effects of vasoinhibins on hypertension, proteinuria, and vascular dysfunction during pregnancy could be enlightening, and provide novel diagnostic and therapeutic targets in the preeclampsia patient.
Acknowledgments
Support for this research was provided by NIH Grants K99HL116774 (EMG), R01Hl108618, and P01HL51971 (JPG). The work performed through the UMMC Molecular and Genomics Facility is supported, in part, by funds from the National Institute of General Medical Sciences of the National Institutes of Health, including Mississippi INBRE (P20GM103476), Center for Psychiatric Neurosciences (CPN)-COBRE (P30GM103328), and Obesity, Cardiorenal and Metabolic Diseases COBRE (P20GM104357).
Contributor Information
Eric M. George, Email: egeorge@umc.edu, Departments of Physiology and Biophysics, University of Mississippi Medical Center, 2500 N. State St., Jackson, MS 39216, USA
Michael R. Garrett, Departments of Pharmacology and Toxicology, University of Mississippi Medical Center, 2500 N. State St., Jackson, MS 39216, USA
Joey P. Granger, Email: jgranger@umc.edu, Departments of Physiology and Biophysics, University of Mississippi Medical Center, 2500 N. State St., Jackson, MS 39216, USA
References
- Ain R, Tash JS, Soares MJ. Prolactin-like protein-A is a functional modulator of natural killer cells at the maternal-fetal interface. Mol Cell Endocrinol. 2003;204:65–74. doi: 10.1016/s0303-7207(03)00125-4. [DOI] [PubMed] [Google Scholar]
- Alam SM, Ain R, Konno T, Ho-Chen JK, Soares MJ. The rat prolactin gene family locus: species-specific gene family expansion. Mamm Genome Off J Int Mamm Genome Soc. 2006;17:858–877. doi: 10.1007/s00335-006-0010-1. [DOI] [PubMed] [Google Scholar]
- Alam SM, Konno T, Sahgal N, Lu L, Soares MJ. Decidual cells produce a heparin-binding prolactin family cytokine with putative intrauterine regulatory actions. J Biol Chem. 2008;283:18957–18968. doi: 10.1074/jbc.M801826200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander BT, Cockrell K, Cline FD, Llinas MT, Sedeek M, Granger JP. Effect of angiotensin II synthesis blockade on the hypertensive response to chronic reductions in uterine perfusion pressure in pregnant rats. Hypertension. 2001;38:742–745. doi: 10.1161/01.hyp.38.3.742. [DOI] [PubMed] [Google Scholar]
- Baldocchi RA, Tan L, King DS, Nicoll CS. Mass spectrometric analysis of the fragments produced by cleavage and reduction of rat prolactin: evidence that the cleaving enzyme is cathepsin D. Endocrinology. 1993;133:935–938. doi: 10.1210/endo.133.2.8344226. [DOI] [PubMed] [Google Scholar]
- Bamberger AM, Sudahl S, Loning T, Wagener C, Bamberger CM, Drakakis P, Coutifaris C, Makrigiannakis A. The adhesion molecule CEACAM1 (CD66a, C-CAM, BGP) is specifically expressed by the extravillous intermediate trophoblast. Am J Pathol. 2000;156:1165–1170. doi: 10.1016/S0002-9440(10)64985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebo BF, Jr, Dveksler GS. Evidence that pregnancy specific glycoproteins regulate T-cell function and inflammatory autoimmune disease during pregnancy. Curr Drug Targets Inflamm Allergy. 2005;4:231–237. doi: 10.2174/1568010053586255. [DOI] [PubMed] [Google Scholar]
- Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Christofori G, Gross V, Gonzalves A, Grone HJ, Ahmed A, Weich HA. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med. 2010;14:1857–1867. doi: 10.1111/j.1582-4934.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges JP, Gilbert JS, Colson D, Gilbert SA, Dukes MP, Ryan MJ, Granger JP. Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am J Hypertens. 2009;22:564–568. doi: 10.1038/ajh.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buimer M, Keijser R, Jebbink JM, Wehkamp D, van Kampen AH, Boer K, van der Post JA, Ris-Stalpers C. Seven placental transcripts characterize HELLP-syndrome. Placenta. 2008;29:444–453. doi: 10.1016/j.placenta.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Centlow M, Wingren C, Borrebaeck C, Brownstein MJ, Hansson SR. Differential gene expression analysis of placentas with increased vascular resistance and pre-eclampsia using whole-genome microarrays. J Pregnancy. 2011;2011:472354. doi: 10.1155/2011/472354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp C, Aranda J, Gonzalez C, Jeziorski MC, Martinez de la Escalera G. Vasoinhibins: endogenous regulators of angiogenesis and vascular function. Trends Endocrinol Metab TEM. 2006;17:301–307. doi: 10.1016/j.tem.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Cox B, Sharma P, Evangelou AI, Whiteley K, Ignatchenko V, Ignatchenko A, Baczyk D, Czikk M, Kingdom J, Rossant J, Gramolini AO, Adamson SL, Kislinger T. Translational analysis of mouse and human placental protein and mRNA reveals distinct molecular pathologies in human preeclampsia. Mol Cell Proteomics MCP. 2011;10(M111):012526. doi: 10.1074/mcp.M111.012526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce K, Gao H, Wang Y, Mooroka T, Sakuma M, Shi C, Sukhova GK, Packard RR, Hogg N, Libby P, Simon DI. Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation. 2009;120:427–436. doi: 10.1161/CIRCULATIONAHA.108.814582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo G, Struman I, Martial J, Weiner RI. Activation of mitogen-activated protein kinases by vascular endothelial growth factor and basic fibroblast growth factor in capillary endothelial cells is inhibited by the antiangiogenic factor 16-kDa N-terminal fragment of prolactin. Proc Natl Acad Sci USA. 1995;92:6374–6378. doi: 10.1073/pnas.92.14.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo G, Martini JF, Iiri T, Fantl WJ, Martial J, Weiner RI. 16K human prolactin inhibits vascular endothelial growth factor-induced activation of Ras in capillary endothelial cells. Mol Endocrinol. 1999;13:692–704. doi: 10.1210/mend.13.5.0280. [DOI] [PubMed] [Google Scholar]
- Enquobahrie DA, Meller M, Rice K, Psaty BM, Siscovick DS, Williams MA. Differential placental gene expression in preeclampsia. Am J Obstet Gynecol. 2008;199(566):511–561. doi: 10.1016/j.ajog.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergun S, Kilik N, Ziegeler G, Hansen A, Nollau P, Gotze J, Wurmbach JH, Horst A, Weil J, Fernando M, Wagener C. CEA-related cell adhesion molecule 1: a potent angiogenic factor and a major effector of vascular endothelial growth factor. Mol Cell. 2000;5:311–320. doi: 10.1016/s1097-2765(00)80426-8. [DOI] [PubMed] [Google Scholar]
- Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009;30:15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal R, Yellon SM, Longo LD, Mata-Greenwood E. Placental gene expression in a rat ‘model’ of placental insufficiency. Placenta. 2010;31:568–575. doi: 10.1016/j.placenta.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006;6:433–446. doi: 10.1038/nri1864. [DOI] [PubMed] [Google Scholar]
- Heinzel K, Benz C, Bleul CC. A silent chemokine receptor regulates steady-state leukocyte homing in vivo. Proc Natl Acad Sci USA. 2007;104:8421–8426. doi: 10.1073/pnas.0608274104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladunewich M, Karumanchi SA, Lafayette R. Pathophysiology of the clinical manifestations of preeclampsia. Clin J Am Soc Nephrol. 2007;2:543–549. doi: 10.2215/CJN.03761106. [DOI] [PubMed] [Google Scholar]
- Khong Y, Brosens I. Defective deep placentation. Best Pract Res Clin Obstet Gynaecol. 2010;25:301–311. doi: 10.1016/j.bpobgyn.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor-alpha is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol. 1994;170:1752–1757. discussion 1757–1759. [PubMed] [Google Scholar]
- LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46:82–86. doi: 10.1161/01.HYP.0000169152.59854.36. [DOI] [PubMed] [Google Scholar]
- LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, Wallukat G, Wenzel K, Cockrell K, Martin JN, Jr, Ryan MJ, Dechend R. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension. 2009;54:905–909. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanos-Miranda A, Marquez-Acosta J, Cardenas-Mondragon GM, Chinolla-Arellano ZL, Rivera-Leanos R, Bermejo-Huerta S, Romero-Arauz JF, Alvarez-Jimenez G, Ramos-Leon JC, Ulloa-Aguirre A. Urinary prolactin as a reliable marker for preeclampsia, its severity, and the occurrence of adverse pregnancy outcomes. J Clin Endocrinol Metab. 2008;93:2492–2499. doi: 10.1210/jc.2008-0305. [DOI] [PubMed] [Google Scholar]
- Leanos-Miranda A, Campos-Galicia I, Ramirez-Valenzuela KL, Chinolla-Arellano ZL, Isordia-Salas I. Circulating angiogenic factors and urinary prolactin as predictors of adverse outcomes in women with preeclampsia. Hypertension. 2013;61:1118–1125. doi: 10.1161/HYPERTENSIONAHA.111.00754. [DOI] [PubMed] [Google Scholar]
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Investig. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng T, Chen H, Sun M, Wang H, Zhao G, Wang X. Identification of differential gene expression profiles in placentas from preeclamptic pregnancies versus normal pregnancies by DNA microarrays. OMICS. 2012;16:301–311. doi: 10.1089/omi.2011.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenan S, Boichot E, Lagente V, Bertrand CP. Macrophage elastase (MMP-12): a pro-inflammatory mediator? Mem Inst Oswaldo Cruz. 2005;100(Suppl 1):167–172. doi: 10.1590/s0074-02762005000900028. [DOI] [PubMed] [Google Scholar]
- Nenan S, Lagente V, Planquois JM, Hitier S, Berna P, Bertrand CP, Boichot E. Metalloelastase (MMP-12) induced inflammatory response in mice airways: effects of dexamethasone, rolipram and marimastat. Eur J Pharmacol. 2007;559:75–81. doi: 10.1016/j.ejphar.2006.11.070. [DOI] [PubMed] [Google Scholar]
- Nouvion AL, Oubaha M, Leblanc S, Davis EC, Jastrow H, Kammerer R, Breton V, Turbide C, Ergun S, Gratton JP, Beauchemin N. CEACAM1: a key regulator of vascular permeability. J Cell Sci. 2010;123:4221–4230. doi: 10.1242/jcs.073635. [DOI] [PubMed] [Google Scholar]
- Nucera S, Biziato D, De Palma M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int J Dev Biol. 2011;55:495–503. doi: 10.1387/ijdb.103227sn. [DOI] [PubMed] [Google Scholar]
- Orwig KE, Soares MJ. Transcriptional activation of the decidual/trophoblast prolactin-related protein gene. Endocrinology. 1999;140:4032–4039. doi: 10.1210/endo.140.9.6954. [DOI] [PubMed] [Google Scholar]
- Piwnica D, Touraine P, Struman I, Tabruyn S, Bolbach G, Clapp C, Martial JA, Kelly PA, Goffin V. Cathepsin D processes human prolactin into multiple 16K-like N-terminal fragments: study of their antiangiogenic properties and physiological relevance. Mol Endocrinol. 2004;18:2522–2542. doi: 10.1210/me.2004-0200. [DOI] [PubMed] [Google Scholar]
- Prakobphol A, Genbacev O, Gormley M, Kapidzic M, Fisher SJ. A role for the L-selectin adhesion system in mediating cytotrophoblast emigration from the placenta. Dev Biol. 2006;298:107–117. doi: 10.1016/j.ydbio.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Redman CW, Bonnar J, Beilin LJ, McNeilly AS. Prolactin in hypertensive pregnancy. Br Med J. 1975;1:304–306. doi: 10.1136/bmj.1.5953.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer T, Koczan D, Gerber B, Richter D, Thiesen HJ, Friese K. Microarray analysis of differentially expressed genes in placental tissue of pre-eclampsia: up-regulation of obesity-related genes. Mol Hum Reprod. 2002;8:674–680. doi: 10.1093/molehr/8.7.674. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension. 2003;41:437–445. doi: 10.1161/01.HYP.0000054981.03589.E9. [DOI] [PubMed] [Google Scholar]
- Sedeek M, Gilbert JS, LaMarca BB, Sholook M, Chandler DL, Wang Y, Granger JP. Role of reactive oxygen species in hypertension produced by reduced uterine perfusion in pregnant rats. Am J Hypertens. 2008;21:1152–1156. doi: 10.1038/ajh.2008.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- Snyder SK, Wessner DH, Wessells JL, Waterhouse RM, Wahl LM, Zimmermann W, Dveksler GS. Pregnancy-specific glycoproteins function as immunomodulators by inducing secretion of IL-10, IL-6 and TGF-beta1 by human monocytes. Am J Reprod Immunol. 2001;45:205–216. doi: 10.1111/j.8755-8920.2001.450403.x. [DOI] [PubMed] [Google Scholar]
- Soares MJ, Konno T, Alam SM. The prolactin family: effectors of pregnancy-dependent adaptations. Trends Endocrinol Metab TEM. 2007;18:114–121. doi: 10.1016/j.tem.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Struman I, Bentzien F, Lee H, Mainfroid V, D’Angelo G, Goffin V, Weiner RI, Martial JA. Opposing actions of intact and N-terminal fragments of the human prolactin/growth hormone family members on angiogenesis: an efficient mechanism for the regulation of angiogenesis. Proc Natl Acad Sci USA. 1999;96:1246–1251. doi: 10.1073/pnas.96.4.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JA. Diagnosis and management of pre-eclampsia: an update. Int J Womens Health. 2010;2:327–337. doi: 10.2147/IJWH.S8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallukat G, Neichel D, Nissen E, Homuth V, Luft FC. Agonistic autoantibodies directed against the angiotensin II AT1 receptor in patients with preeclampsia. Can J Physiol Pharmacol. 2003;81:79–83. doi: 10.1139/y02-160. [DOI] [PubMed] [Google Scholar]
- Walsh SK, English FA, Johns EJ, Kenny LC. Plasma-mediated vascular dysfunction in the reduced uterine perfusion pressure model of preeclampsia: a microvascular characterization. Hypertension. 2009;54:345–351. doi: 10.1161/HYPERTENSIONAHA.109.132191. [DOI] [PubMed] [Google Scholar]
- Wang D, Ishimura R, Walia DS, Muller H, Dai G, Hunt JS, Lee NA, Lee JJ, Soares MJ. Eosinophils are cellular targets of the novel uteroplacental heparin-binding cytokine decidual/trophoblast prolactin-related protein. J Endocrinol. 2000;167:15–28. doi: 10.1677/joe.0.1670015. [DOI] [PubMed] [Google Scholar]
- Wedepohl S, Beceren-Braun F, Riese S, Buscher K, Enders S, Bernhard G, Kilian K, Blanchard V, Dernedde J, Tauber R. L-selectin—a dynamic regulator of leukocyte migration. Eur J Cell Biol. 2012;91:257–264. doi: 10.1016/j.ejcb.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Wiemers DO, Ain R, Ohboshi S, Soares MJ. Migratory trophoblast cells express a newly identified member of the prolactin gene family. J Endocrinol. 2003;179:335–346. doi: 10.1677/joe.0.1790335. [DOI] [PubMed] [Google Scholar]
- Winn VD, Gormley M, Paquet AC, Kjaer-Sorensen K, Kramer A, Rumer KK, Haimov-Kochman R, Yeh RF, Overgaard MT, Varki A, Oxvig C, Fisher SJ. Severe preeclampsia-related changes in gene expression at the maternal-fetal interface include sialic acid-binding immunoglobulin-like lectin-6 and pappalysin-2. Endocrinology. 2009;150:452–462. doi: 10.1210/en.2008-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]