Abstract
To address therapeutic challenges in childhood relapsed ALL, a phase 1 study combining a survivin mRNA antagonist, EZN-3042, with re-induction chemotherapy was developed for pediatric patients with second or greater bone marrow relapses of B lymphoblastic leukemia. EZN-3042 was administered as a single agent on days -5 and -2 and then in combination with a 4-drug re-induction platform on days 8, 15, 22 and 29. Toxicity and the biological activity of EZN-3042 were assessed. Six patients enrolled at dose level 1 (EZN-3042 2.5 mg/kg/dose). Two dose limiting toxicities were observed: one patient developed a grade 3 GGT elevation and another patient developed grade 3 gastrointestinal bleeding. Downmodulation of survivin mRNA and protein was assessed after single agent dosing and decreased expression was observed in 2 of 5 patients with sufficient material for analysis. While some biological activity was observed, the combination of EZN-3042 with intensive re-induction chemotherapy was not tolerated at a dose that led to consistent downregulation of survivin expression. The trial was terminated following the completion of dose level 1, after further clinical development of this agent was halted.
Keywords: Relapsed ALL, survivin, antisense
Introduction
Relapsed acute lymphoblastic leukemia (ALL) in children presents a significant challenge and accounts for more deaths than any other malignancy. 1,2 The outcome following relapse is poor, particularly when relapses involve the marrow and occur very early (within 18 months of initial diagnosis), or in cases of second or greater recurrence. 2–9 Therefore, new approaches are needed to improve outcomes for this population and to more effectively prevent relapses in patients with newly diagnosed high risk ALL in the future.
To discover the underlying biological pathways responsible for drug resistance and provide rational opportunities for both prevention of relapse and effective salvage therapy, we and others have tracked the evolution of genetic lesions from diagnosis to relapse using matched diagnosis/relapse pairs of marrow samples from children enrolled on clinical protocols. 10–13 As a result of these studies, survivin (BIRC5) emerged as an attractive target due to its upregulation at relapse in the majority of samples studied. 10,11 Survivin is universally overexpressed in a wide spectrum of human tumors and ranks among the top five most tumor-specific genes in the human genome based on its expression relative to normal cells. 14–17 Previously, we and others have demonstrated that survivin inhibition results in increased apoptosis and augmented responses to conventional chemotherapy in preclinical studies in ALL cell lines and xenograft models. 18–20
Several antagonists have been developed for clinical use. One such survivin mRNA antagonist, developed by Enzon Pharmaceuticals, is EZN-3042. EZN-3042 is a locked nucleic acid antisense oligonucleotide (LNA-ON) that uses third generation antisense technology to optimize biological stability and potency. 21 EZN-3042 is a highly effective inhibitor of survivin expression in cancer cells in vitro (IC50 <1 nM with transfection). 21 Based on the preclinical studies and preliminary clinical experience with EZN-3042 in adults, 22 a phase 1 trial (T2009-007) was initiated through the Therapeutic Advances in Childhood Leukemia and Lymphoma (TACL) Consortium (http://Clinicaltrials.gov NCT01186328). The primary objective of this trial was to determine the safety and tolerability of administering EZN-3042, as a single agent and in combination with chemotherapy, for children with relapsed B lymphoblastic leukemia and to determine the biological activity of this agent. The first experience with a survivin antagonist in pediatric patients was terminated early due to our inability to integrate the agent with reinduction therapy due to toxicity and the lack of further pursuit of this particular agent.
Materials and Methods
Study Population
Patients ages 1–21 years with second or greater marrow relapses (M3 marrow; > 25% blasts) of B lymphoblastic leukemia with or without concomitant extramedullary disease were eligible for this study. This included patients who had undergone prior allogeneic hematopoietic stem cell transplantation (HCT), as long as they were at least 120 days from the time of transplant and had no active graft-versus-host disease. Patients with refractory leukemia after first or greater relapse and a single re-induction attempt were also eligible with a limitation of one patient with refractory disease in each cohort of 3 patients. Patients with mature B-ALL and Down syndrome were excluded. Institutional review boards at participating TACL centers approved the study. Informed consent was obtained from patients or from parents/legal guardians. An independent Data Safety Monitoring Committee at Children’s Hospital Los Angeles monitored study progress.
Study Design and Dose Escalation
All patients received one course of therapy as detailed in Table I. During the 6-day prephase, the survivin antagonist, EZN-3042 was administered by 2 hour IV infusion on days -5 and -2 following the dose escalation schema in Table II. Re-induction chemotherapy began on day 1 and EZN-3042 was next administered in combination with the 4-drug re-induction platform on days 8, 15, 22 and 29. The starting dose for EZN-3042 was 2.5 mg/kg/dose, which was one dose level below the 5 mg/kg/dose MTD in an ongoing adult phase 1 trial at the time this study first opened. Patients were enrolled in groups of 3 using a standard 3+3 design. If the pediatric MTD was exceeded at the first dose level, then the dose of EZN-3042 was to be reduced by another level to dose level 0 (1.5 mg/kg/dose). The trial was also designed to enroll 6 additional patients at the MTD to improve the precision of estimates of the biological activity of EZN-3042.
Table I.
Details of Protocol Therapy
| Drug and Dosage | Day |
|---|---|
| EZN-3042 (dose assigned at study entry) | −5, −2, 8, 15, 22, 29 |
| Vincristine 1.5 mg/m2 IV | 1, 8, 15, 22 |
| Prednisone 40 mg/m2/day PO | 1–29 |
| Pegaspargase 2500 IU/m2 IV | 2, 9, 16, 23 |
| Doxorubicin 60 mg/m2 IV | 1 |
| Intrathecal cytarabine | 0 |
| Intrathecal methotrexate | 15 and 36 (CNS negative) |
| Triple intrathecal chemotherapy | 8, 15, 22, 29 (CNS positive) |
Table II.
Dose Escalation Schema
| Dose Level | Dose | Days |
|---|---|---|
| 0 | 1.5 mg/kg | −5, −2, 8, 15, 22, 29 |
| 1 (starting dose) | 2.5 mg/kg | −5, −2, 8, 15, 22, 29 |
| 2 | 5 mg/kg | −5, −2, 8, 15, 22, 29 |
| 3 | 6.5 mg/kg | −5, −2, 8, 15, 22, 29 |
Toxicity was graded using the CTCAE criteria, version 4.0 (http://ctep.cancer.gov). DLT was defined as any of the following events that were at least possibly, probably or definitely attributable to EZN-3042. Non-hematological DLT was defined as any grade 3 or grade 4 toxicity attributable to EZN-3042 with the specific exclusion of: grade 3 nausea and vomiting; grade 3 transaminase (AST/ALT) elevation that returned to grade ≤1 or baseline prior to the next scheduled dose; grade 3 or 4 fever or infection; grade 3 or 4 electrolyte abnormalities that were transient (<24 hours) and not associated with clinical sequelae; or alopecia. Hematological DLT was defined as the absence of peripheral blood count recovery (ANC > 500/μL and platelet count > 20,000/μL) within 6 weeks of starting systemic chemotherapy (protocol day 1) by marrow aplasia; not marrow infiltration, in patients who achieved remission.
Disease Assessment
At the completion of combination therapy (day 36), a bone marrow aspirate was obtained to assess disease status. Complete blood counts (CBCs) were also obtained at baseline and following exposure to single agent EZN-3042 (Table III). Cerebral spinal fluid (CSF) examination occurred at study entry and with each scheduled dose of intrathecal chemotherapy. Minimal residual disease (MRD) was determined by flow cytometry (optional participation) at the end of re-induction (day 36) in a dedicated reference laboratory at the University of Washington using standard methodology.
Table III.
White Blood Cell Count Response to Single Agent EZN-3042
| Patient Number | WBC/μL on Day -6 | WBC/μL on Day 0 | ABC/μL on Day -6 | ABC/μL on Day 0 |
|---|---|---|---|---|
| 1 | 9300 | 27,600 | 5022 | 24,012 |
| 2 | 20,000 | 118,340* | 8800 | 110,056* |
| 3 | 4510 | 12,470 | 1398 | 7856 |
| 4 | 240 | 430 | 0 | 0 |
| 5 | 16,070 | 25,550 | 4339 | 12,520 |
| 6 | 3000 | 3300 | 0 | 0 |
Sample drawn on day 1 instead of day 0.
WBC = white blood cell count; ABC = peripheral absolute blast count.
Correlative Studies
To evaluate primary target engagement of EZN-3042 monotherapy, survivin mRNA and protein expression were quantitated in enriched marrow blasts before treatment was started (day -6) and on day 0 (48 hours following the day -2 dose of EZN-3042) using quantitative reverse transcription PCR (RT-PCR) and Enzyme Linked Immunosorbent (ELISA) assays, respectively, at NYU Langone Medical Center. This time point was chosen as this was when the maximal decrease in survivin expression was observed after knockdown in preclinical studies. 18 To optimize yield and minimize any potential artifacts associated with sample storage prior to shipment, fresh marrow samples were processed at local centers. Namely, leukemic blasts were purified by Ficoll-Paque extraction and frozen in shipping media. Upon thawing, marrow samples were enriched by flow cytometry to ensure >90% blast purity. 18 Total RNA was extracted from flow-sorted blasts using the RNEasy Mini Kit (Qiagen, Valencia, CA) and two-step RT-PCR was performed using I-Script II cDNA Synthesis kit (Biorad, Hercules, CA) and Perfecta SYBR Green FastMix (Quanta Biosciences, Gaithersburg, MD). Synthesis of PCR products was monitored by the DNA Engine Opticon System (MJ Research, Waltham, MA). Data were plotted relative to β2 microglobulin expression comparing the mRNA prior to and after scheduled doses of EZN-3042 on days -5 and -2. The PCR primers used were as follows: β2 microglobulin (5’-ATGTGTCTGGGTTTCATCCATCC-3’and 5’-AGTCACATGGTTCACACGGCA-3’), survivin main isoform (5’-CCACCGCATCTCTACATTCA-3’ and 5’-TATGTTCCTCTATGGGGTCG-3’), survivin 2B isoform (main forward and 5’-AGTGCTGGTATTACAGGCGT-3’), survivin ΔEX3 isoform (main forward and 5’-TTTCCTTTGCATGGGGTC-3’), and survivin 2α isoform (5’-CAGTGTTTCTTCTGCTTCAAGG 3’ and 5’-GCAACCCTCCCATACTAAGTGTC 3'). For ELISA assays, flow-sorted blasts were lysed at a concentration of 10 million cells/mL of buffer [1mM EDTA, 0.5% triton X-100, 6M urea and protease inhibitor cocktail (Roche, Basel, Switzerland] and total protein concentration determined by DC protein assay (Bio-Rad). ELISA assays were performed according to manufacturer’s protocol using Human Total Survivin DuoSet IC (DYC647-2, R&D Systems). Data were analyzed using the MasterPlex computer software 4 parameter logistics (4-PL) curve fit.
Pharmacokinetic (PK) Studies
Blood samples were collected at the following time points to determine the PK profile of EZN-3042: 15–30 minutes prior to the first dose, at the end of the initial 2-hour (hr) infusion, 30 minutes, 1 hr, 2 hrs, 4 hrs, 6 hrs, 24 hrs after the end of the first infusion, at day 0 (120 hrs after initial infusion) and prior to the day 8 dose of EZN-3042 (312 hrs after the initial infusion).
Statistical Considerations
The primary endpoint for dose escalation was the occurrence of a DLT as defined above. Patients were evaluable for toxicity assessment if they terminated treatment for toxicity or intolerability, or they experienced a DLT, or they received 85% of the required doses of EZN-3042 and other agents without a DLT. Patients who received any prescribed combination therapy (day 0 and later) were considered evaluable for response. The primary measures of biological activity of EZN-3042 were the reduction in survivin mRNA and protein expression in leukemic blasts before treatment and after 2 doses of EZN-3042 were administered on days -5 and day -2 (baseline vs. day 0). Given the small patient number at the time of study closure, the change in survivin expression is reported using descriptive analyses.
Results
Patients and Accrual
A total of 6 patients enrolled on this study between January 31, 2011 – November 11, 2011. Patient characteristics are detailed in Table IV. Two patients had undergone prior allogeneic HCT and one patient had refractory disease with a partial response to prior reinduction therapy at the time of study entry. One DLT was observed in the initial cohort of 3 patients, which prompted cohort expansion to 6 patients according to the study design. A second cohort of 3 patients subsequently accrued and 1 additional DLT was observed. The trial subsequently scheduled to re-open at dose level 0, where each scheduled dose of EZN-3042 would have been decreased to 1.5 mg/kg. The trial, however, was prematurely terminated when further clinical development of this agent was stopped, prior to any accruals at dose level 0, a dose significantly below the MTD in the adult trial with this agent. 22
Table IV.
Patient Summary
| Patient Number | Gender | Age at Study Entry (yrs) | Disease Status at Study Entry | Prior HCT | Dose Level | DLT | Disease Response on Day 36 | Survivin Down Modulation | Survival |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 13 | Second marrow relapse/ testicular disease | No | 1 | No | SD | No | Died from disease |
| 2 | F | 11 | Second marrow relapse/ CNS2 | No | 1 | No | PD | Yes | Died from disease |
| 3 | M | 5 | Second isolated marrow relapse/third overall relapse | No | 1 | Yes Grade 3 GGT elevation |
PD | Yes | Died from disease |
| 4 | M | 8 | Second isolated marrow relapse | No | 1 | No | CR MRD 0.06% | Insufficient material | Alive in remission |
| 5 | M | 21 | Second isolated marrow relapse/Ph+ ALL | Yes | 1 | Yes Grade 3 GI hemorrhage |
NE | No | Died from toxicity |
| 6 | F | 9 | Second isolated marrow relapse | Yes | 1 | No | CRp MRD 5.9% | No | Alive in remission |
CR = complete remission, CRp = complete remission without platelet recovery, PD = progressive disease, SD = stable disease, NE = not evaluable, MRD = minimal residual disease
Toxicity
Single agent therapy with EZN-3042 on days -5 and -2 was well tolerated and no non-hematologic toxicities were reported. The most frequent grade 3 or higher non-hematologic toxicities, occurring in 3 or more patients (50%) during combination therapy (days 1–36) were hepatic, metabolic and infectious. Two DLTs occurred among the 6 patients treated on dose level 1 (2.5 mg/kg/dose). One patient developed reversible grade 3 GGT elevation on protocol day 42, 14 days after the last scheduled dose of EZN-3042. This patient also developed a grade 3 AST and ALT elevation, which persisted for 10 days and then returned to pre-study baseline. Of note, this patient had grade 1 AST and ALT elevation at the time of study entry.
The second DLT which was observed was grade 3 gastrointestinal bleeding on day 16. At the time of the initial onset of the bleeding, the patient had C. difficile enterocolitis and was thrombocytopenic, but did not have a coagulopathy. This patient was unable to complete protocol therapy due to toxicity and expired due to fungal sepsis (Candida Lusitaneae).
Response to Therapy
Among the 5 patients assessable for response on day 36, 1 patient achieved complete remission (CR), 1 patient achieved complete remission without platelet recovery (CRp), 1 patient had stable disease (SD) and 2 patients had progressive disease (PD). Among the patients achieving morphological CRs, both had detectable MRD. The 2 responding patients achieving CR and CRp remained alive in CR following SCT with follow-up durations of 5 months and 14 months, respectively. Although response to single agent EZN-3042 on days -5 and -2 was not formally assessed, CBCs were monitored from study entry (baseline) through day 0 and no decline in WBC was observed for any of the patients (Table III).
Pharmacokinetics and Pharmacodynamics
Five patients provided samples for pharmacokinetic (PK) analysis. The maximum plasma concentration of EZN-3042 at the 2.5 mg/kg dose had median value of 7359 ng/mL (range 3774 to 10075 ng/mL). Median half-life from the end of infusion was 0.51 hrs (range 0.29 to 0.58 hrs). Terminal half-life (t1/2) was estimated to be 5.3 hrs (95% CI 4.2 hrs to 7.2 hrs), although this is likely an underestimate because no samples between 6 hrs and 24 hrs were drawn to confirm log-linear elimination. Median AUC0-24 was 14.6 mg-h/mL (range 7.1 to 18.7 mg-h/mL).
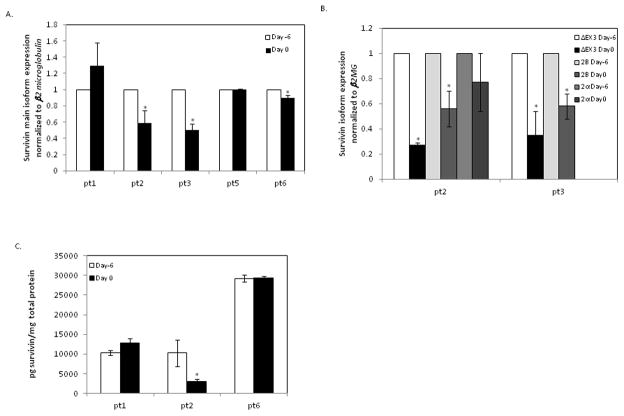
Of the 6 patients enrolled on the study, mRNA and protein analysis could be completed in 5 and 3 patients, respectively, due to limitations arising from sample collection and recovery. We saw a significant decrease in survivin transcript expression in 2 of 5 patients (pt 2 and 3, 40% and 50% decrease respectively) where samples were available (Figure 1A). There are multiple splice variants of the survivin gene with unique properties and function and the expression levels of survivin isoforms were also measured in the patients who showed a decrease in survivin transcript expression (pt 2 and pt 3). The ΔEX3 and 2B splice variants were also downregulated, but not the 2α isoform, which is not targeted by EZN-3042 (Figure 1B) thereby confirming specific targeting of survivin transcripts by EZN-3042. Protein expression correlated with transcript expression in 3 patients, including one patient with downregulation, who had sufficient material for analysis (Figure 1C).
Figure 1. Survivin Expression Following Treatment with EZN-3042.
Messenger RNA and protein expression analysis of patient samples before (day -6) and after (day 0) pre-phase treatment with EZN-3042 is shown. Quantitative RT-PCR (A, B) and ELISA (C) assays were performed on bone marrow samples from patients on days -6 and 0. mRNA expression for survivin main form (A) and survivin splice variants (B) is plotted relative to the pretreatment expression level at day -6. Absolute protein concentration for survivin (C) on days -6 and 0 were determined by standard curve and expressed as pg of target protein per mg of total protein. Each data point was measured in triplicate (* p <0.05 pre-post treatment).
Discussion
This phase 1 trial was the first pediatric experience with the survivin mRNA antagonist, EZN-3042 and also the first experience combining EZN-3042 with a multiagent chemotherapy platform. Third generation antisense compounds have changes in the chemical structure of oligonucleotides, which result in higher affinity for mRNA and higher potency in mRNA down-modulation in general and improved tissue stability, offering a potential benefit compared to earlier generation antagonists. 21 Although EZN-3042 was well tolerated as a single agent, dose limiting toxicities of transaminitis and hemorrhage were observed when it was combined with a 4-drug chemotherapy platform. Transaminitis could have resulted in part from the platform chemotherapy regimen, as the incidence of grade 3 or higher ALT elevation was 14.5% on the COG AALL01P2 study where this platform was previously used alone, 7 however, hepatic toxicities were the most frequently reported drug-related adverse events in the phase 1 study of EZN-3042 in adults 22 and antisense oligonucleotides have been shown to accumulate in the liver. 21 The biological activity of EZN-3042 was assessed in patients on this study by measuring the downregulation of survivin mRNA and protein after single agent exposure. We observed biological activity in 2 of 5 patients even at a low dose of EZN-3042, but higher doses could not be investigated due to the toxicity observed with combination therapy.
Our limited experience with EZN-3042 in pediatric patients with relapsed ALL showed similarities to the phase 1 trial in adults with refractory lymphomas and solid tumors, where patients also received ENZ-3042 twice weekly during week 1 and then weekly thereafter with or without docetaxel. The most common toxicities observed in adults were AST and ALT elevation, occurring in 42% and 38% of patients who received single agent EZN-3042, respectively. The transaminitis was reversible and the agent was otherwise generally well tolerated both alone and in combination with docetaxel. The best response observed with single agent EZN-3042 (n=24) was stable disease in 5 patients and the best response for EZN-3042 (2.5 mg/kg) in combination with docetaxel (n=11) was a confirmed partial response in 1 patient with prostate cancer. 22 Limited pharmacodynamic studies for survivin expression in hair follicles and tumor biopsies showed no consistent downmodulation of survivin mRNA or protein. While the median half-life of ENZ-3042 was longer in adults (1.85 hours) than in the small number of pediatric patients treated on our study, the implications of this finding for the dosing regimen utilized are complex, as tissue half-life may be more relevant with antisense oligonucleotides than plasma half-life. Pharmacokinetic studies of antisense oligonucleotides have demonstrated rapid clearance after systemic administration, followed by accumulation in tissues, especially the liver and kidneys, with a much longer tissue half -life of several days. 23,24
In summary, preclinical data have identified survivin as a key target in relapsed ALL and downregulation of survivin expression has been shown to sensitize tumor cells to conventional chemotherapy. Based on these observations, a phase 1 trial was developed combining EZN-3042 with a traditional chemotherapy platform for pediatric patients with relapsed ALL. While we saw biological activity in 2 of 5 patients using a low dose of EZN-3042 administered on 2 occasions, toxicity precluded increasing the dose of this agent in this heavily pretreated group of patients. Thus while the study was halted prematurely when further pursuit of the agent was terminated by the industry sponsor, it is unlikely that the lower dose mandated by study design would have led to further downmodulation of survivin. Moreover, the collateral toxicity associated with treating multiply relapsed ALL potentially limits integration of novel agents. Alternative strategies, which target survivin indirectly, are presently being pursued in this population. 25 Our experience in this trial both supports the paradigm of using new genomic discoveries to inform clinical trial design as well as highlighting some of the complexities of moving new agents forward in a group of patients known to experience significant toxicities with conventional agents.
Acknowledgments
The authors acknowledge Enzon Pharmaceuticals, Inc for providing EZN-3042 and for their financial support for this trial.
Footnotes
Conflict of interest for authors: None
References
- 1.Reismuller B, Attarbaschi A, Peters C, et al. Long-term outcome of initially homogenously treated and relapsed childhood acute lymphoblastic leukaemia in Austria--a population-based report of the Austrian Berlin-Frankfurt-Munster (BFM) Study Group. Br J Haematol. 2009 Feb;144(4):559–570. doi: 10.1111/j.1365-2141.2008.07499.x. [DOI] [PubMed] [Google Scholar]
- 2.Roy A, Cargill A, Love S, et al. Outcome after first relapse in childhood acute lymphoblastic leukaemia - lessons from the United Kingdom R2 trial. Br J Haematol. 2005 Jul;130(1):67–75. doi: 10.1111/j.1365-2141.2005.05572.x. [DOI] [PubMed] [Google Scholar]
- 3.Einsiedel HG, von Stackelberg A, Hartmann R, et al. Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Munster Group 87. J Clin Oncol. 2005 Nov 1;23(31):7942–7950. doi: 10.1200/JCO.2005.01.1031. [DOI] [PubMed] [Google Scholar]
- 4.Ko RH, Ji L, Barnette P, et al. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: a Therapeutic Advances in Childhood Leukemia Consortium study. J Clin Oncol. 2010 Feb 1;28(4):648–654. doi: 10.1200/JCO.2009.22.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen K, Devidas M, Cheng SC, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children's Oncology Group study. Leukemia. 2008 Dec;22(12):2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker C, Waters R, Leighton C, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010 Dec 11;376(9757):2009–2017. doi: 10.1016/S0140-6736(10)62002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raetz EA, Borowitz MJ, Devidas M, et al. Reinduction platform for children with first marrow relapse of acute lymphoblastic Leukemia: A Children's Oncology Group Study[corrected] J Clin Oncol. 2008 Aug 20;26(24):3971–3978. doi: 10.1200/JCO.2008.16.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saarinen-Pihkala UM, Heilmann C, Winiarski J, et al. Pathways through relapses and deaths of children with acute lymphoblastic leukemia: role of allogeneic stem-cell transplantation in Nordic data. J Clin Oncol. 2006 Dec 20;24(36):5750–5762. doi: 10.1200/JCO.2006.07.1225. [DOI] [PubMed] [Google Scholar]
- 9.Tallen G, Ratei R, Mann G, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. 2010 May 10;28(14):2339–2347. doi: 10.1200/JCO.2009.25.1983. [DOI] [PubMed] [Google Scholar]
- 10.Bhojwani D, Kang H, Moskowitz NP, et al. Biologic pathways associated with relapse in childhood acute lymphoblastic leukemia: a Children's Oncology Group study. Blood. 2006 Jul 15;108(2):711–717. doi: 10.1182/blood-2006-02-002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogan LE, Meyer JA, Yang J, et al. Integrated genomic analysis of relapsed childhood acute lymphoblastic leukemia reveals therapeutic strategies. Blood. 2011 Nov 10;118(19):5218–5226. doi: 10.1182/blood-2011-04-345595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullighan CG, Phillips LA, Su X, et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008 Nov 28;322(5906):1377–1380. doi: 10.1126/science.1164266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang JJ, Bhojwani D, Yang W, et al. Genome-wide copy number profiling reveals molecular evolution from diagnosis to relapse in childhood acute lymphoblastic leukemia. Blood. 2008 Nov 15;112(10):4178–4183. doi: 10.1182/blood-2008-06-165027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fangusaro JR, Caldas H, Jiang Y, Altura RA. Survivin: an inhibitor of apoptosis in pediatric cancer. Pediatr Blood Cancer. 2006 Jul;47(1):4–13. doi: 10.1002/pbc.20805. [DOI] [PubMed] [Google Scholar]
- 15.Kanwar RK, Cheung CH, Chang JY, Kanwar JR. Recent advances in anti-survivin treatments for cancer. Current medicinal chemistry. 2010;17(15):1509–1515. doi: 10.2174/092986710790979935. [DOI] [PubMed] [Google Scholar]
- 16.Kelly RJ, Lopez-Chavez A, Citrin D, Janik JE, Morris JC. Impacting tumor cell-fate by targeting the inhibitor of apoptosis protein survivin. Molecular cancer. 2011;10:35. doi: 10.1186/1476-4598-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velculescu VE, Madden SL, Zhang L, et al. Analysis of human transcriptomes. Nat Genet. 1999 Dec;23(4):387–388. doi: 10.1038/70487. [DOI] [PubMed] [Google Scholar]
- 18.Morrison DJ, Hogan LE, Condos G, et al. Endogenous knockdown of survivin improves chemotherapeutic response in ALL models. Leukemia. 2012 Feb;26(2):271–279. doi: 10.1038/leu.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park E, Gang EJ, Hsieh YT, et al. Targeting survivin overcomes drug resistance in acute lymphoblastic leukemia. Blood. 2011 Aug 25;118(8):2191–2199. doi: 10.1182/blood-2011-04-351239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyner JW, Jemal AM, Thayer M, Druker BJ, Chang BH. Targeting survivin and p53 in pediatric acute lymphoblastic leukemia. Leukemia. 2012 Apr;26(4):623–632. doi: 10.1038/leu.2011.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sapra P, Wang M, Bandaru R, Zhao H, Greenberger LM, Horak ID. Down-modulation of survivin expression and inhibition of tumor growth in vivo by EZN-3042, a locked nucleic acid antisense oligonucleotide. Nucleosides, nucleotides & nucleic acids. 2010 Feb;29(2):97–112. doi: 10.1080/15257771003597733. [DOI] [PubMed] [Google Scholar]
- 22.Tolcher AW, Patnaik A, Papadopoulos KP, et al. Results of a phase 1, open-label, dose-escalation study evaluating the safety and tolerability of EZN-3042, a survivin mRNA antagonist, administered with or without docetaxel in adult patietns with advanced solid tumors or lymphoma. Proceedings of the 102nd Annual Meeting of AACR; 2011. p. LB-409 (abstract). [Google Scholar]
- 23.Mansoor M, Melendez AJ. Advances in antisense oligonucleotide development for target identification, validation, and as novel therapeutics. Gene regulation and systems biology. 2008;2:275–295. doi: 10.4137/grsb.s418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu RZ, Grundy JS, Geary RS. Clinical pharmacokinetics of second generation antisense oligonucleotides. Expert opinion on drug metabolism & toxicology. 2013 Feb;9(2):169–182. doi: 10.1517/17425255.2013.737320. [DOI] [PubMed] [Google Scholar]
- 25.Bhatla T, Wang J, Morrison DJ, et al. Epigenetic reprogramming reverses the relapse-specific gene expression signature and restores chemosensitivity in childhood B-lymphoblastic leukemia. Blood. 2012 Apr 11; doi: 10.1182/blood-2012-01-401687. [DOI] [PMC free article] [PubMed] [Google Scholar]