Abstract
Introductıon: Non-invasive ventilation (NIV) is a preferred treatment in acute respiratory failure after operations. Our aim is to investigate the success of early use of bilevel positive airway pressure (BIPAP) after cardiac or thoracic surgeries to prevent reintubation. Methods: In a prospective randomized study, 254 patients were divided into two groups depending on the time period between extubation and the application of BIPAP. In Group 1 BIPAP was applied after extubation within 48 hours after surgery following fulfilling of acute respiratory failure criterias whereas, in Group 2, BIPAP was applied one hour after extubation for two episodes of 20 minute duration and 3 hours apart. Arterial blood gas values (pH, PaO2, PaCO2) at first and fourth hour after BIPAP were collected. Results: In comparison between groups, no significant differences were observed for arterial blood gas values of pH and PaCO2 at baseline, one and four hours after BIPAP (p > 0.05) however, the PaO2 values at one and four hours after BIPAP were significantly better in Group 1 in comparison to Group 2 (p < 0.001, p < 0.001; respectively). Reintubation rate was 14 patients (11%) in Group 1 and 7 patients (5.5%) in Group 2 (p = 0.103). Conclusıons: The early and prophylactic use of BIPAP after cardiac or thoracic operations did not provide diminished rates in the postoperative complications such as reintubation.
Keywords: Non-invasive ventilaton, bilevel positive airway pressure, hypoxia, postoperative complications, reintubation
Introduction
Postoperative pulmonary dysfunction after cardiac or thoracic surgeries occur due to; impairment in gas exchange and lung mechanics secondary to general anesthesia and mechanical ventilation, use of cardiopulmonary bypass (CPB), hypothermia, activation of the inflammatory cascade, decreased extravascular lung water, atelectasis, pleural opening, possible phrenic nevre injury, pain, prolonged recumbent position and reduction of diaphragmatic movement. Previous studies have shown that a longer duration of mechanical ventilation, difficulty in weaning of the patient, and prolonged duration of hospitalization does occur after almost all abdominal, thoracic or cardiovascular surgeries however, a relation with a higher incidence of mortality was not shown [1-4].
Non-invasive ventilation (NIV) was investigated during respiratory failure treatment in cardiothoracic surgery units to provide beneficial effects in lung and heart functions and these include; (1) to partially compensate for the affected respiratory function by reducing the work of breathing, (2) to improve alveolar recruitment with better gas exchange (oxygenation and ventilation) and (3) to reduce left ventricular afterload by increasing cardiac output and improving haemodynamics. One of the methods of NIV is the use of a bilevel positive airway pressure (BIPAP) and in this method the pressure is higher during inspiration and decreases during expiration. Several randomized, controlled studies were published using different methods of NIV after cardiac surgeries [2,5-7,9-13]. The main concerns during use of NIV are; 1- a reduction in the left ventricular preload and afterload that may cause hypotension, 2- an increase in pulmonary compliance due to recruiting of previously collapsed alveolar units [3,8]. There are not enough well established randomized clinical studies that will enlighten these concerns and the current literature providing evidence for the use of NIV to avoid postextubation respiratory failure and reintubation is limited [9-14]. Our aim is to investigate the success of early use of BIPAP after cardiac or thoracic surgeries to prevent reintubation.
Material and methods
In a prospective randomized study, 273 patients with normal preoperative spirometric study (forced expiratory volume in one second (FEV1) values between 80 and 120% of the avarage value) were evaluated and after exclusion of 19 patients, a total of 254 patients were divided into two groups depending on the time period between extubation and the application of BIPAP. The study was approved by The Hospital Ethical Commitee and an informed written consent was obtained for study protocol prior to surgery from all patients. From a total of 261 patients that were randomized after fulfilling the inclusion criterias, seven of them did not complete the study secondary to problems related to 1- reintubation within four hours after extubation (before completion of the second BIPAP treatment as per study protocol), 2- hemodynamical unstability including hypotension, rhythm disturbances. A consort diagram and consort checklist is presented in Figures 1 and 2.
Figure 1.
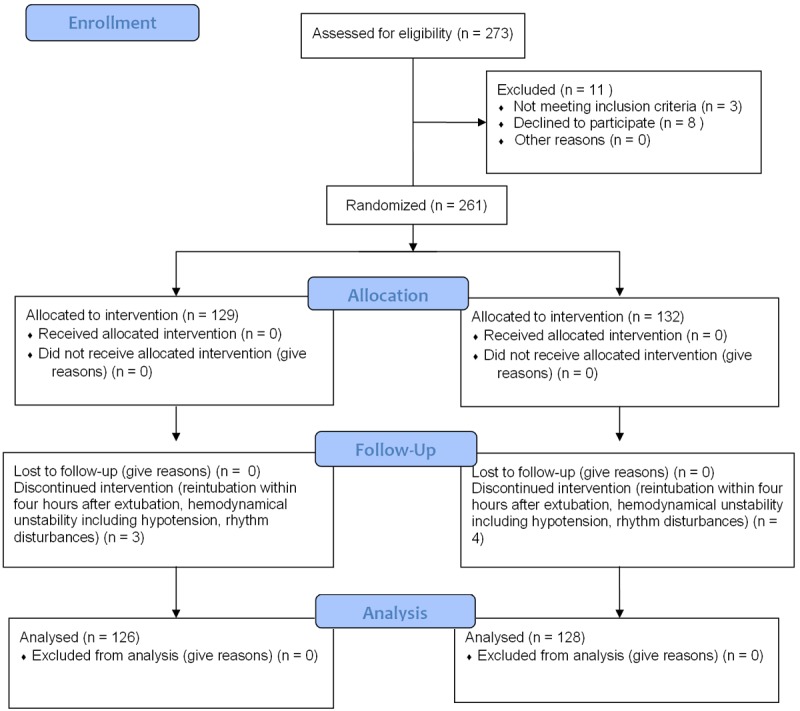
CONSORT 2010 flow diagram.
Figure 2.
CONSORT 2010 checklist of information to include when reporting a randomised trial*.
In Group 1 of 126 patients, BIPAP was applied after extubation within 48 hours after surgery following fulfilling of acute respiratory failure criterias whereas, in Group 2 of 128 patients, BIPAP was applied one hour after extubation for two episodes of 20 minute duration and 3 hours apart without an acute respiratory failure episode. BIPAP ventilatory support device (BIPAP S/T-D30 Ventilatory Support System, Respironics Inc., PA, USA) was used. BIPAP was applied with expiratory positive airway pressure (EPAP) of 4 cmH2O and inspiratory positive airway pressure (IPAP) of 8 cmH2O in a spontaneous mode. The pressures were gradually adjusted as tolerated based on continuous pulse oximetry to achieve an oxygen saturation of greater than 92%, a normal pH on arterial blood gases. Parameters collected at baseline, before and after BIPAP included; 1- Arterial blood gas values (pH, partial arterial oxygen pressure (Pa02), partial arterial carbon dioxide pressure (PaC02) and arterial oxygen saturation (Sp02)), 2- Tidal volume, respiratory and heart rate. All patients received a standard therapy protocol including diuretics and inhaled beta agonists. Patients receiving intravenous theophilline or other broncodilators were not included into the study. The primary end-point was to prevent reintubation. Other adverse events (pneumothorax, aspiration, pulmonary edema, transient ischemic attack, stroke, bronchospasm) were recorded.
The inclusion criterias to the study include; ages 50 and older, cardiac or thoracic surgery, ejection fraction equal to or greater than 50%, patients extubated in a twelve hour period postoperatively without any complications and these include; 1- Spontaneous respiratory rate (RR) < 25/min, 2- Spontaneous respiratory volume (Vt) > 0.005 L/kg of body weight, 3- Heart rate < 140/min, 4- Body temperature < 37.5°, 5- Partial arterial oxygen pressure (PaO2) > 60 mmHg with inspired oxygen fraction (FiO2) ≤ 0.4, 6- No need for vasoactive and/or inotropic support, 7- PaO2/FiO2 ratio > 200, 8- pH > 7.34, 8-No clinical signs and symptoms of acute respiratory distress (dyspnea, respiratory rate more than 24 breaths/minute, use of accessory muscles of respiration, presence of paradoxical breathing). In Group 2 of patients acute respiratory failure is diagnosed with the following criteria after extubation within fourty eight hours and these include: 1- Spontaneous respiratory rate > 25/min, 2- SpO2 < 90%, 3- Heart rate > 140/min (or more than 20% change from the initial heart rate), 4- Systolic blood pressure > 200 mmHg or < 80 mmHg, 5- PaO2 ≤ 60 mmHg, 6- pH ≤ 7.30, and 7- Restlessness.
Patients were excluded from the study if they met any of the following criteria: Patients who required immediate reintubation within four hours after extubation, history of asthma, a restrictive pattern on spirometry (FEV1 less than 80% and FEV1/FVC greater than 0.7), spirometric data providing a diagnosis of severe obstructive pattern as a FEV1 less than 50% and FEV1/FVC ratio of 0.7 or less, lack of spirometric data, history of pneumonia or acute lung injury prior to operation, medically unstable condition (hypotension, uncontrolled cardiac ischemia/arrhythmia), inability to protect airway (excess secretions, stupurous or comatose patient), neurologic or psychiatry realted disorders (agitated or uncooperative patient).
The primary outcome was failure of NIV and it is defined as; 1- the need for endotracheal intubation during the ICU stay due to inability to improve gas exchange during BIPAP, 2- failure to improve mental status after two episodes of BIPAP in patients who are lethargic from CO2 retention or agitated from hypoxemia, bradycardia (heart beat < 60 beats/minute with altered mental status), hypotension (systolic blood pressure < 90 mmHg), respiratory arrest, failure to maintain pulse oximetry (SpO2) > 90%, significant metabolic and/or respiratory acidosis (pH < 7.20) [14]. Ultimately, clinical evaluation was conducted by the anesthesiologist in the intensive care unit before a decision for intubation was made. The other parameters that were collected include; duration of mechanical ventilation before initial extubation, duration of NIV, the initial settings and final settings for IPAP and EPAP, whether the patient tolerated NIV, need for re-intubation, time to re-intubation, ICU length of stay, hospital length of stay, complications that may be related to NIV such as development of aspiration or pneumonia, mortality.
Statistical analysis
All statistical analysis was performed using SPSS Statistical Package 15.0 (SPSS Inc. California, USA). Based on the power analysis (PASS 11 (NCSS Inc. Utah, USA)), the study aimed to recruit 136 patients in order to have a clinically significant difference in the proportion of patients experiencing BIPAP trial failure for a clinically significant 3% difference in reintubation rate with a confidence interval of 95% and 80% power (α = 0.05) and this was based on a previous study sample size calculation [15]. Data are presented as mean and standard deviation (SD) or as frequencies and percentages. Differences were assessed using chi square or Fisher exact test for categorical variables. Mann Whitney U-test is used for continuous or non-parametric data. After testing for normal distribution, data were compared using a two-way analysis of variance (ANOVA) for repeated measurements. P values < 0.05 were considered statistically significant.
Results
There were no differences regarding age, sex, or weight and perioperative clinical characteristics between Groups 1 and 2 (p > 0.05) (Table 1).
Table 1.
The comparison of perioperative parameters
| Group 1 | Group 2 | p* | |
|---|---|---|---|
| Patients (n) | 126 | 128 | |
| Age, (years) ● | 60.0 (39.0-76.0) | 63.0 (29.0-78.0) | 0.770 |
| Height (cm) ● | 161.0 (148.0-183.0) | 162.0 (141.0-184.0) | 0.645 |
| Weight (kg) ● | 70.0 (57.0-110.0) | 74.50 (53.5-103) | 0.421 |
| Men (n, %) ●● | 52 (74.3) | 54 (77.1) | 0.693 |
| Women (n, %) | 18 (25.7) | 16 (22.9) | |
| Ejection fraction● (%) | 60.0 (40.0-65.0) | 60.0 (45.0-65.0) | 0.07 |
| EuroSCORE● | 2.0 (0-10.0) | 2.0 (0-8.0) | 0.469 |
| Hematocrit● (preoperative, %) | 36.5 (27.5-43.5) | 38.0 (28.5-44.5) | 0.213 |
| Thoracic surgery (n, %) | 35 (27.8) | 30 (23.4) | 0.347 |
| Cardiac surgery with CPB* (n, %) | 91 (72.2) | 98 (76.6) | 0.763 |
p < 0.05 statistically significant;
Mann Whitney U test;
Pearson chi-square test;
n: number; %: percentage; data is presented as median (minimum-maximum); CPB, cardiopulmonary bypass.
The comparison of types of cardiac surgical procedures between groups included; coronary artery bypass graft [59/126 (46.8%) versus 62/128 (48.4%); p = 0.626], mitral valve with or without tricuspid valve repair [16/126 (12.7%) versus 19/128 (14.8%); p = 0.589], aortic valve repair with or without mitral valve repair or reconstruction [9/126 (7.1%) versus 12/128 (9.4%); p = 0.789], coronary artery grafting without cardiopulmonary bypass (off-pump) [7/126 (5.6%) versus 5/128 (3.9%); p = 0.314]. The comparison of types of thoracic surgical procedures between groups included; lobectomy [20/126 (16%) versus 17/128 (13.3%); p = 0.513], bilobectomy [8/126 (6.3%) versus 3/12 (2.3%); p=0.098], pneumonectomy [7/126 (5.6%) versus 10/128 (7.8%); p = 0.192], and these were not found to be statistically different between groups (p > 0.05).
The comparison of the clinical and arterial blood gas parameters preoperatively and postoperatively in the intensive care unit are presented in Table 2. This table includes respiratory rate, heart rate, pH, PaO2, PaCO2 values before BIPAP, one and four hour after BIPAP. In comparison between groups, no significant differences were observed for arterial blood gas values of pH and PaCO2 at baseline and one and four hours after BIPAP (p > 0.05) however, the PaO2 and SpO2 (peripheral oxygen saturation) values at one and four hours after BIPAP were significantly better in Group 1 in comparison to Group 2 (p < 0.001, p < 0.001; respectively) and there was no significant difference in comparison of baseline values of PaO2 and SpO2 (p > 0.05) (Table 2). Within group comparisons revealed that, in both groups in comparison to baseline values, there were no significant differences of pH and PaCO2 values at one and four hours after BIPAP (p > 0.05). In both groups, in comparison to baseline values, the PaO2 and SpO2 values showed increase one and four hours after BIPAP in comparisons to baseline values (p < 0.001, p < 0.001; respectively). In comparison between groups, reintubation rate was 14 patients (11%) in Group 1 and 7 patients (5.5%) in Group 2 (p = 0.103).
Table 2.
Serial clinical and arterial blood gas parameters of two groups
| Group 1 (n = 126) | Group 2 (n = 128) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Parameters | Before operation | Before BIPAP (Postop. Day 1) | 1 hour after BIPAP | 4 hour after BIPAP | Before operation | Before BIPAP | 1 hour after BIPAP | 4 hour after BIPAP |
| RR* (rate/minute)* | 23.0 ± 1.3 | 24.5 ± 3.2 | 24.5 ± 3.2b | 21.7 ± 6.9c | 24.9 ± 2.3 | 26.8 ± 2.6 | 22.8 ± 2.6b | 20.4 ± 6.9c |
| HR* (rate/minute)* | 106.8 ± 14.6 | 108.3 ± 11.6 | 108.3 ± 11.6 | 101 ± 10.2c | 104.4 ± 13.3 | 105.6 ± 10.6 | 105.6 ± 10.6 | 102.1 ± 11.2c |
| pH | 7.36 ± 0.02 | 7.36 ± 0.02 | 7.36 ± 0.03 | 7.35 ± 0.05 | 7.36 ± 0.02 | 7.36 ± 0.02 | 7.37 ± 0.05 | 7.35 ± 0.06 |
| PaO2 (mmHg) | 86.29 ± 7.5 | 82.6 ± 10.2a | 93.7 ± 7.3b | 99.9 ± 8.1c | 85.6 ± 7.04 | 80.2 ± 11a | 85.4 ± 10.2b | 86.4 ± 12.8c |
| PaCO2 (mmHg) | 38.6 ± 3.5 | 39.0 ± 4.2 | 37.7 ± 3.2b | 36.9 ± 3.1 | 37.8 ± 2.3 | 38.1 ± 2.1 | 37.9 ± 7.2 | 36.7 ± 4.5 |
| SpO2 (%) | 95.9 ± 1.82 | 93.9 ± 2.4a | 97.1 ± 2.59b | 98.5 ± 3.24c | 96.0 ± 1.4 | 93.9 ± 2.5a | 94.1 ± 2.13b | 95.6 ± 1.33c,d |
p < 0.05 statistically significant;
RR, respiratory rate (breaths/minute); HR, heart rate (beat/minute); PaO2, partial pressure of arterial oxygen; PaCO2, partial pressure of arterial carbon dioxide; SpO2, periperal oxygen saturation; ICU, intensive care unit, BIPAP, Bi-level positive airway pressure;
value at 1 hour significantly different from that at baseline within the groups;
value at 4 hour significantly different from that at 1 hour within groups;
value at 1 hour significantly different from that at baseline between the groups;
value at 4 hour significantly different from that at 1 hour between the two groups;
postop.; postoperative.
Among the 254 patients that were included in the study, 6 patients (5%) from each group died within 30 days after operation (p = 0.713). In each group, four (Group 1, 4%, Group 2, 3%) of these patients had underwent cardiac surgery and 1 patient in each group had thoracic surgery (p > 0.891). The duration of intensive care unit stay and hospital stay were similar between groups. Postoperative complications related to respiratory and cardiovascular systems showed no significant differences (p > 0.05) (Table 3).
Table 3.
The comparison of risk factors for postoperative morbidity and mortality between groups
| Parameters | Group 1 | Group 2 | p* |
|---|---|---|---|
| Complications, n (%) | |||
| Cerebrovascular events | 2 (1.6) | 3 (2.3) | 0.853 |
| Atrial fibrillation | 11 (8.7) | 9 (7) | 0.682 |
| Other arrythmias | 13 (10.3) | 16 (12.5) | 0.421 |
| Pneumonia | 3 (2.4) | 1 (0.8) | 0.462 |
| Pleural effusion | 5 (4) | 6 (5) | 0.743 |
| Reintubation | 14 (11) | 7 (5.5) | 0.103 |
| Intensive care unit stay (days) (M ± SD) | 2.7 ± 1.2 | 2.9 ± 1.9 | 0.371 |
| Hospital stay (days) | 11.8 ± 6.9 | 10.8 ± 6.8 | 0.415 |
| Mortality | 6 (5) | 6 (5) | 0.857 |
p < 0.05 statistically significant;
M ± SD: mean and standard deviation; n (%): number, percentage; BIPAP, bilevel positive airway pressure.
Discussion
In postextubation respiratory failure after open heart or thoracic surgeries early treatment is mandatory as patients with normal lung functions developed respiratory related complications in the early postoperative period within eight to twelve hours after extubation [4,9-14]. We investigated the effects of BIPAP, a non-invasive method of positive pressure ventilation on early acute respiratory failure in patients who underwent open heart surgery with CPB and our main finding is that whether the BIPAP treatment is applied during acute respiratory event or as a preventive treatment after extubation in the early postoperative period, there is no significant difference in reintubation rate or other postoperative complications and this finding is in correspondance with the recent publications [16-18]. The only important finding is that the arterial partial pressure of oxygen values were significantly better in patients with acute respiratory failure after first and fourth hour BIPAP treatments (p < 0.001, p < 0.001; respectively). The reason for this is that; after one and four hour of BIPAP application, a rapid impovement in arterial blood gas PaO2 values, respiratory and heart rate are more significant in pateints with hypoxemia showing prompt physiologic responses within four hours in comparison to patients without hypoxemia and this correlated with previous reports [19]. In cardiac and thoracic surgeries, main concerns are the impairment of the pulmonary ventilation- perfusion ratio due to atelectasis, pleural effusions, diminished caused by recumbent position, temporary diaphragmatic dysfunction, impairment of pulmonary secretion clearance, and pain [20]. Several studies have shown that, in patients with postoperative hypoxemic respiratory failure, NIV improves gas exchange, minimizes atelectasis formation, and increases functional residual capacity in the early postoperative period [21].
The published literature providing evidence for the use of NIV to avoid postextubation intubation is limited. There are two randomized controlled studies that have provided information regarding use of NIV as an adjunct to weaning from mechanical ventilation and suggested that NIV permits earlier removal of the endotracheal tube [22,23]. Our study design did not provide us information regarding the use of BIPAP for early removal of the endotracheal tube. Another issue is that the published literature providing evidence for the use of NIV to avoid postextubation intubation is limited. In a recent meta-analysis, Agarwal et al. Reviewed and summarized four studies on postextubation respiratory failure and they showed that NIV can be used in the early postoperative period in patients “at risk” for developing postextubation respiratory failure to prevent re-intubation and this study design was conducted in two of these studies on patients that were randomized immediately after extubation [24]. In our study design, in one group of patients BIPAP treatment was started in acute respiratory failure within fourty eight hours after extubation however, the other group of patients received BIPAP treatment without signs of immediate postextubation respiratory failure and these patients were considered “at risk” without signs of respiratory failure and in our study design it is not possible to show a difference in reintubation rates. There is also an improvement in heart rate by the use of NIV and this was explained as to increase the intrathoracic pressure by rising the lung volume during inspiration. This physiological event leads to the consequent events reported as; 1- a reduction in right and left ventricular preload, 2- a reduction in left ventricular afterload, 3- a rise in cardiac output. During these changes a reduction in heart rate as well as mean systemic arterial pressure were reported [25]. This mechanism is related to the success of BIPAP applications in early postoperative period acute repiratory failure in open heart surgery patients. Another limitation of our study is that we were not able to include blood pressure measurements in our study design.
Our study includes several limitations and these include; 1- The sample size of our study may not be enough to detect the difference of incidence for reintubation, 2- The two groups of patients that we have enrolled into the study are equal in terms of preoperative data however, in the group of patients with acute onset of respiratory failure the hemodynamic data including heart rate may change and this may change the pathophysiological status of each group that were compared. 3- There is need for a group of patients that did not receive BIPAP treatment and it would be a better study design to observe the intubation rate in that group of patients as well, 4- This data shows that the group of patients with acute respiratory failure may have done better in terms of reintubation however, we are unable to compare with a group of patients that did not receive BIPAP treatment.
Conclusion
The early and prophylactic use of BIPAP without acute respiratory failure in the early postoperative period after extubation did not show an improvement in the rates of postoperative adverse events including reintubation.
References
- 1.Nicholson DJ, Kowalski SE, Hamilton GA, Meyers MP, Serrette C, Duke PC. Postoperative pulmonary function in coronary artery bypass graft surgery patients undergoing early tracheal extubation: a comparison between short-term mechanical ventilation and early extubation. J Cardiothorac Vasc Anesth. 2002;16:27–31. doi: 10.1053/jcan.2002.29648. [DOI] [PubMed] [Google Scholar]
- 2.Lopes CR, Brandão CM, Nozawa E, Auler JO Jr. Benefits of non-invasive ventilation after extubation in the postoperative period of heart surgery. Rev Bras Cir Cardiovasc. 2008;23:344–50. doi: 10.1590/s0102-76382008000300010. [DOI] [PubMed] [Google Scholar]
- 3.Jaber S, Michelet P, Chanques G. Role of non-invasive ventilation (NIV) in the perioperative period. Best Pract Res Clin Anaesthesiol. 2010;24:253–65. doi: 10.1016/j.bpa.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Auriant I, Jallot A, Hervé P, Cerrina J, Le Roy Ladurie F, Fournier JL, Lescot B, Parquin F. Noninvasive ventilation reduces mortality in acute respiratory failure following lung resection. Am J Respir Crit Care Med. 2001;164:1231–5. doi: 10.1164/ajrccm.164.7.2101089. [DOI] [PubMed] [Google Scholar]
- 5.Gust R, Gottschalk A, Schmidt H, Böttiger BW, Böhrer H, Martin E. Effects of continuous (CPAP) and bi-level positive airway pressure (BiPAP) on extravascular lung water after extubation of the trachea in patients following coronary artery bypass grafting. Intensive Care Med. 1996;22:1345–50. doi: 10.1007/BF01709549. [DOI] [PubMed] [Google Scholar]
- 6.Matte P, Jacquet L, Van Dyck M, Goenen M. Effects of conventional physiotherapy, continuous positive airway pressure and non-invasive ventilatory support with bilevel positive airway pressure after coronary artery bypass grafting. Acta Anaesthesiol Scand. 2000;44:75–81. doi: 10.1034/j.1399-6576.2000.440114.x. [DOI] [PubMed] [Google Scholar]
- 7.Peters JV, Moran JL, Phillips-Hughes J, Warn D. Noninvasive ventilation in acute respiratory failure-a meta-analysis up-date. Crit Care Med. 2002;30:555–62. doi: 10.1097/00003246-200203000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Lenique F, Habis M, Lofaso F, Dubois-Randé JL, Harf A, Brochard L. Ventilatory and hemodynamic effects of continuous positive airway pressure in left heart failure. Am J Resp Crit Care Med. 1997;155:500–5. doi: 10.1164/ajrccm.155.2.9032185. [DOI] [PubMed] [Google Scholar]
- 9.Keenan SP, Powers C, McCormack DG, Block G. Noninvasive positive-pressure ventilation for postextubation respiratory distress: a randomized controlled trial. JAMA. 2002;287:3238–44. doi: 10.1001/jama.287.24.3238. [DOI] [PubMed] [Google Scholar]
- 10.Esteban A, Frutos-Vivar F, Ferguson ND, Arabi Y, Apezteguía C, González M, Epstein SK, Hill NS, Nava S, Soares MA, D’Empaire G, Alía I, Anzueto A. Noninvasive positive-pressure ventilation for respiratory failure after extubation. N Engl J Med. 2004;350:2452–60. doi: 10.1056/NEJMoa032736. [DOI] [PubMed] [Google Scholar]
- 11.Nava S, Gregoretti C, Fanfulla F, Squadrone E, Grassi M, Carlucci A, Beltrame F, Navalesi P. Noninvasive ventilation to prevent respiratory failure after extubation in high-risk patients. Crit Care Med. 2005;33:2465–70. doi: 10.1097/01.ccm.0000186416.44752.72. [DOI] [PubMed] [Google Scholar]
- 12.Ferrer M, Valencia M, Nicolas JM, Bernadich O, Badia JR, Torres A. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med. 2006;173:164–70. doi: 10.1164/rccm.200505-718OC. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Role of noninvasive positive-pressure ventilation in postextubation respiratory failure: A meta-analysis. Respir Care. 2007;52:1472–79. [PubMed] [Google Scholar]
- 14.Michalopoulos A, Geroulanos S, Papadimitriou L, Papadakis E, Triantafillou K, Papadopoulos K, Palatianos G. Mild or moderate chronic obstructive pulmonary disease risk in elective coronary artery bypass grafting surgery. World J Surg. 2011;25:1507–11. doi: 10.1007/s00268-001-0161-x. [DOI] [PubMed] [Google Scholar]
- 15.Matić I, Danić D, Majerić-Kogler V, Jurjević M, Mirković I, Mrzljak Vucinić N. Chronic obstructive pulmonary disease and weaning of difficult-to-wean patients from mechanical ventilation: randomized prospective study. Croat Med J. 2007;48:51–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Spivack SD, Shinozaki T, Albertini JJ, Deane R. Preoperative prediction of postoperative respiratory outcome. Coronary artery bypass grafting. Chest. 1996;109:1222–30. doi: 10.1378/chest.109.5.1222. [DOI] [PubMed] [Google Scholar]
- 17.Michalopoulos A, Geroulanos S, Papadimitriou L, Papadakis E, Triantafillou K, Papadopoulos K, Palatianos G. Mild or moderate chronic obstructive pulmonary disease risk in elective coronary artery bypass grafting surgery. World J Surg. 2011;25:1507–11. doi: 10.1007/s00268-001-0161-x. [DOI] [PubMed] [Google Scholar]
- 18.Manganas H, Lacasse Y, Bourgeois S, Peron J, Dagenais F, Maltais F. Postoperative outcome after coronary artery bypass grafting in chronic obstructive pulmonary disease. Can Respir J. 2007;14:19–24. doi: 10.1155/2007/378963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med. 2001;163:540–577. doi: 10.1164/ajrccm.163.2.9906116. [DOI] [PubMed] [Google Scholar]
- 20.Antonelli M, Conti G, Rocco M, Bufi M, De Blasi RA, Vivino G, Gasparetto A, Meduri GU. A comparison of noninvasive positive pressure ventilation and standard mechanical ventilation in patients with acute respiratory failure. N Engl J Med. 1998;339:429–434. doi: 10.1056/NEJM199808133390703. [DOI] [PubMed] [Google Scholar]
- 21.Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, Simoneau G, Benito S, Gasparetto A, Lemaire F. Noninvasive ventilation for acute exacerbations of COPD. N Engl J Med. 1995;333:817–22. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- 22.Nava S, Ambrosino N, Clini E, Prato M, Orlando G, Vitacca M, Brigada P, Fracchia C, Rubini F. Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic pulmonary disease. A randomized, controlled trial. Ann Intern Med. 1998;128:721–8. doi: 10.7326/0003-4819-128-9-199805010-00004. [DOI] [PubMed] [Google Scholar]
- 23.Girault C, Daudenthun I, Chevron V, Tamion F, Leroy J, Bonmarchand G. Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure. A prospective randomized controlled study. Am J Respir Crit Care Med. 1999;160:86–92. doi: 10.1164/ajrccm.160.1.9802120. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Role of noninvasive positive-pressure ventilation in postextubation respiratory failure: a meta-analysis. Respir Care. 2007;52:1472–9. [PubMed] [Google Scholar]
- 25.Lenique F, Habis M, Lofaso F, Dubois-Randé JL, Harf A, Brochard L. Ventilatory and hemodynamic effects of continuous positive airway pressure in left heart failure. Am J Resp Crit Care Med. 1997;155:500–5. doi: 10.1164/ajrccm.155.2.9032185. [DOI] [PubMed] [Google Scholar]